Abstract
In secondary hyperparathyroidism, enhanced expression of TGF-α in the parathyroid leads to its own upregulation, generating a feed-forward loop for TGF-α activation of its receptor, EGFR receptor (EGFR), which promotes parathyroid hyperplasia. These studies examined the role of activator protein 2α (AP2), an inducer of TGF-α gene transcription, in the upregulation of parathyroid TGF-α in secondary hyperparathyroidism. In rat and human secondary hyperparathyroidism, parathyroid AP2 expression strongly correlated with TGF-α levels and with the rate of parathyroid growth, as expected. Furthermore, the increases in rat parathyroid content of AP2 and its binding to a consensus AP2 DNA sequence preceded the increase in TGF-α induced by high dietary phosphate. More significant, in A431 cells, which provide a model of enhanced TGF-α and TGF-α self-induction, mutating the core AP2 site of the human TGF-α promoter markedly impaired promoter activity induced by endogenous or exogenous TGF-α. Important for therapy, in five-sixths nephrectomized rats fed high-phosphate diets, inhibition of parathyroid TGF-α self-induction using erlotinib, a highly specific inhibitor of TGF-α/EGFR-driven signals, reduced AP2 expression dosage dependently. This suggests that the increases in parathyroid AP2 occur downstream of EGFR activation by TGF-α and are required for TGF-α self-induction. Indeed, in A431 cells, erlotinib inhibition of TGF-α self-induction caused parallel reductions in AP2 expression and nuclear localization, as well as TGF-α mRNA and protein levels. In summary, increased AP2 expression and transcriptional activity at the TGF-α promoter determine the severity of the hyperplasia driven by parathyroid TGF-α self-upregulation in secondary hyperparathyroidism.
In chronic kidney disease (CKD), elevated serum levels of parathyroid hormone (PTH) cause osteitis fibrosa, a high-turnover bone disease responsible for bone loss and an excess of calcium (Ca) and phosphate (P) ions in the circulation that predispose to vascular calcification, adverse cardiovascular events, and, consequently, increased morbidity and mortality rates in this patient population.1–3 The severity of parathyroid hyperplasia determines not only the elevations in serum PTH4–6 but also the reductions in parathyroid vitamin D receptor content and calcium-sensing receptor expression that render these patients’ disease refractory to therapy.
In human and experimental kidney disease, hypocalcemia, hyperphosphatemia, and 1,25-dihydroxyvitamin D (calcitriol) deficiency are the main determinants of parathyroid hyperplasia. In contrast, dietary P restriction, high Ca intake, and calcitriol therapy effectively suppress parathyroid growth.7–9 The enhanced expression of TGF-α in hyperplastic and adenomatous human parathyroid glands10 suggested a role for this potent growth promoter in the parathyroid hyperplasia of kidney disease. In fact, studies in rat secondary hyperparathyroidism (SH) have shown that elevations in parathyroid content of TGF-α and TGF-α induction of its own expression determine the severity of the parathyroid growth induced rapidly (within 1 wk) upon five-sixths nephrectomy (Nx) and aggravated by either low Ca or high P intake.11,12 Progressive increases in parathyroid TGF-α content could promote growth through autocrine, paracrine, or less characterized juxtacrine mechanisms13–15 upon activation of the TGF-α receptor, the EGF receptor (EGFR), by tyrosine phosphorylation. Juxtacrine TGF-α growth signals involve EGFR activation by the transmembrane TGF-α precursor in an adjacent cell.15,16
In rat SH, the levels of parathyroid TGF-α and TGF-α self-induction are sufficient to determine the magnitude of EGFR activation and downstream growth signaling. Indeed, the parathyroid growth arrest induced by P restriction, high dietary Ca, or prophylactic calcitriol (or analog) therapy within 1 wk of the onset of kidney disease involved prevention of uremia-induced increases in parathyroid TGF-α.11,12 More significant, suppression of TGF-α activation of the EGFR by erlotinib, a potent and highly specific EGFR-tyrosine kinase inhibitor, arrested not only parathyroid growth but also TGF-α self-upregulation.17,18 These findings underscore the importance of controlling parathyroid TGF-α expression to ameliorate the progression of SH. The goal of these studies was the identification of the pathogenesis of the elevated parathyroid TGF-α of experimental and human SH.
The demonstration that activator protein 2α (AP2) is a potent inducer of the transcription of the rat and human TGF-α gene19,20 led us to assess the contribution of AP2 to parathyroid TGF-α expression and growth rates in rat and human SH. To this end, the contribution of changes in parathyroid AP2 to TGF-α content was examined upon dietary Ca and P manipulations and/or calcitriol administration to either enhance or suppress the induction of parathyroid TGF-α and growth rates by kidney disease in rats, as well as in diffuse and nodular human parathyroid glands, two extremes as of mild or aggressive parathyroid growth.
Direct assessment of the contribution of AP2 to TGF-α induction of its own gene expression in the parathyroid glands was conducted using specific inhibition of TGF-α activation of the EGFR with erlotinib21 in five-sixths Nx rats fed high P. Because of the rapid de-differentiation of parathyroid cells in culture, further characterization of the mechanisms underlying AP2 regulation of TGF-α expression was conducted in A431 cells, a human epidermoid carcinoma cell line and a proper model of enhanced TGF-α expression and TGF-α self-induction.22,23 The results from these studies demonstrate a critical role of increases in parathyroid AP2 in mediating TGF-α self-upregulation, the main determinant of the severity of parathyroid hyperplasia in rat and human SH.
RESULTS
Initial studies were conducted in parathyroid glands from uremic rats subjected to dietary Ca and P manipulations that caused a two- to three-fold increase in parathyroid TGF-α and doubled parathyroid gland size within 1 wk after five-sixths Nx.12 Specifically, we assessed the contribution of changes in rat parathyroid expression of AP2α, a known inducer of TGF-α gene transcription, to the reported changes in parathyroid TGF-α levels.12,17
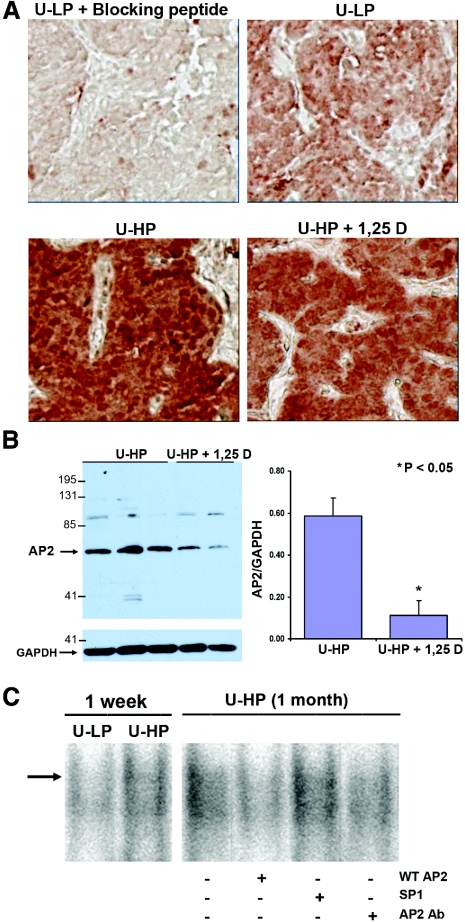
Figure 1A and Table 1 show that by day 7 after five-sixths Nx, parathyroid AP2 expression increased in rats fed either high P or low Ca diets in parallel with the reported elevations in TGF-α expression and growth rates but remained within normal levels in rats fed either low P or high Ca diets, maneuvers that prevented increases in TGF-α and, consequently, growth rates.11,12 The changes in proliferation rates in these rats could not be attributed to changes in serum levels of ionized Ca, P, total Ca, or creatinine, known modulators of parathyroid hyperplasia. Importantly, the increases in AP2 induced by high dietary P or low Ca intake reached statistical significance by day 2 after five-sixths Nx, thus preceding the increases in TGF-α that reached statistical significance only by day 5.11 Immunohistochemical quantification of parathyroid AP2 content at day 2 demonstrated either a 67.8 or a 74.5% (P < 0.05) higher AP2 expression in rats fed high-P (0.198 ± 0.066) or low-Ca (0.192 ± 0.032) diets, respectively, compared with uremic rats with dietary P restriction (0.118 ± 0.022) or fed a high-Ca diet (0.110 ± 0.025). Also, prophylactic calcitriol therapy for 1 wk in five-sixths Nx rats fed high dietary P, effective in preventing elevations in parathyroid TGF-α and gland growth,12 ameliorated the increases in parathyroid AP2, as measured by immunohistochemical quantification (Figure 1A, Table 1) and confirmed by Western blot and densitometric analysis of parathyroid AP2/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ratios in whole-cell extracts from individual parathyroid glands (Figure 1B). Table 2 depicts unchanged serum P, Ca, ionized Ca, and creatinine levels between uremic rats receiving vehicle or calcitriol treatment. Thus, calcitriol regulation of AP2 expression was unrelated to changes in these known modulators of SH.
Figure 1.
(A) Parathyroid AP2 immunostaining in response to changes in dietary P intake and/or prophylactic calcitriol administration in rat SH. Representative photomicrographs of immunohistochemical staining of AP2 in parathyroid tissue from five-sixths Nx rats undergoing the experimental conditions described in protocol 1, at day 7 after the onset of renal failure: Uremic (U) + high-P diet (U-HP); U + low-P (LP) diet (U-LP); U-HP diet + 1,25D (U-HP + 1,25D). (B) Parathyroid AP2 expression in response to prophylactic calcitriol administration in rat SH. Western blot analysis and densitometric quantification of parathyroid AP2/GAPDH ratios in parathyroid glands from five-sixths Nx rats fed high dietary P and receiving either vehicle or calcitriol (1,25D). Bars and error bars represent means ± SEM; *P < 0.05 (see Statistical Analysis section). (C) Dietary P regulation of parathyroid AP2/DNA binding. Binding to a radiolabeled AP2 consensus sequence of nuclear extracts (30 μg of total protein) from parathyroid glands from five-sixths Nx (U) rats fed either HP (0.9% P; U-HP) or LP (0.2% P; U-LP) for 1 wk. The specificity of AP2/DNA binding was studied with similar incubations of radiolabeled AP2 consensus with nuclear extracts from hyperplastic parathyroid glands from uremic rats fed high dietary P for 1 mo (U-HP [1 mo]) in the absence or presence of a 200-mol excess of radioinert AP2 consensus oligonucleotide (WT AP2) or SP1, an AP2-unrelated oligonucleotide sequence (SP1), or 1 μl of a polyclonal antibody against AP2α (AP2 Ab). Magnification, ×400 in A.
Table 1.
Parathyroid AP2 content in rat SHa
| Experimental Condition | AP2 Content (Integrated Optical Density/Area) |
|---|---|
| U + LP | 0.113 ± 0.025 |
| U + HP | 0.184 ± 0.071b |
| U + HP + 1,25D | 0.140 ± 0.042 |
| U + HCa | 0.107 ± 0.025 |
| U + LCa | 0.201 ± 0.088b |
Data are means ± SD of parathyroid AP2 content measured by quantification of AP2 immunostaining (see the Concise Methods section) in sections of parathyroid glands from seven rats per experimental condition (five-sixths Nx [U] fed high [H] or low [L] P or Ca diets and receiving either vehicle or calcitriol [1,25D]).
P < 0.05 versus the dietary counterpart.
Table 2.
Body weight, parathyroid weight, and serum chemistries in uremic ratsa
| Parameter | U-HP (n = 5) | U-HP + 1,25D (n = 4) |
|---|---|---|
| Body weight (g) | 230.0 ± 2.2 | 233.0 ± 3.0 |
| Parathyroid gland weight (μg) | 406 ± 49 (5) | 247 ± 19 (3) |
| Parathyroid gland weight/body weight | 1.750 ± 0.184 (5) | 1.060 ± 0.086 (3) |
| Serum creatinine (mg/dl) | 1.86 ± 0.12 | 1.50 ± 0.10 |
| Total Ca (mg/dl) | 10.13 ± 0.20 | 10.21 ± 0.25 |
| Ionized Ca (mg/dl) | 4.65 ± 0.06 | 4.74 ± 0.06 |
| P (mg/dl) | 5.56 ± 0.60 | 6.40 ± 0.43 |
Data are means ± SEM; n =, number of rats; (n), number of parathyroid glands harvested from five-sixths Nx rats (U) fed a high-P (HP) diet and receiving either vehicle or calcitriol (1,25D, 4 ng every other day) for 1 wk.
The increases in parathyroid AP2 driven by kidney disease and high dietary P in rats were accompanied by elevations in AP2 binding to a consensus AP2 DNA sequence. Electrophoretic mobility shift assays (Figure 1C) demonstrated that AP2 binding to DNA was higher in nuclear extracts from the parathyroid glands of rats fed high dietary P compared with P-restricted rats. The specificity of parathyroid AP2 binding to DNA was examined in nuclear extracts from the parathyroid glands of five-sixths Nx rats fed high P for 1 mo. The last four lanes in Figure 1C show that the complex of parathyroid nuclear protein that binds to the AP2 consensus does contain AP2, because binding is reduced by a polyclonal antibody against AP2α. Also, nuclear parathyroid AP2 binds specifically to the AP2 consensus sequence. The binding of nuclear parathyroid AP2 to the radiolabeled AP2 consensus sequence was competed by co-incubation with a 200 mol excess of radioinert AP2 consensus and unaffected by an excess of the SP1 consensus, a sequence unrelated to the AP2 consensus. Taken together, these results suggest that increases in parathyroid AP2 content and DNA binding activity could partially account for the induction of TGF-α expression by high-P and low-Ca diets.
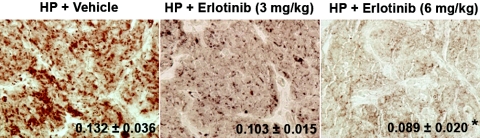
Next, we examined the contribution of increases in parathyroid AP2 levels to TGF-α induction of its own expression in the parathyroid glands. To this end, we used the parathyroid glands from five-sixths Nx rats that were fed a high-P (1.2% P) diet and also received daily either vehicle or erlotinib, a potent and highly specific inhibitor of TGF-α activation of the EGFR, at dosages of 3 and 6 mg/kg body wt. We have shown that these dosages were effective in causing a mild or complete inhibition of TGF-α self-upregulation, respectively.17Figure 2 shows that daily administration of erlotinib prevented the elevations in AP2 expression induced by five-sixths Nx in rats fed high dietary P dosage dependently. The dosage of erlotinib of 6 mg/kg body wt reduced parathyroid AP2 content by 32% (P < 0.05). As indicated in our previous report, erlotinib inhibition of increases in parathyroid TGF-α and growth rates was unrelated to erlotinib-induced changes in serum ionized Ca, P, total Ca, or creatinine levels.24
Figure 2.
Erlotinib inhibits the enhancement of parathyroid AP2 induced by kidney disease and high dietary P. Representative photomicrographs of immunohistochemical staining of AP2 in parathyroid tissue at day 7 after five-sixths Nx in rats fed HP (1.2%) receiving vehicle (HP+Vehicle) or 3 or 6 mg erlotinib/kg body wt (HP+Erlotinib 3 or 6 mg/kg, respectively). Values indicate the results of the immunohistochemical quantification of the changes in parathyroid AP2 content in response to erlotinib administration in rats treated as described. Values represent means ± SEM of parathyroid AP2 measurements from six rats; *P < 0.05 versus HP+Vehicle. Magnification, ×200.
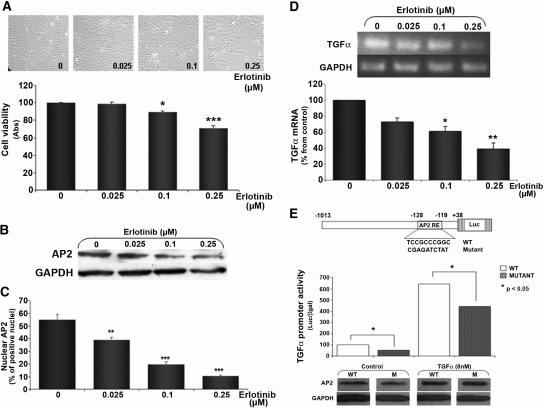
Further characterization of the mechanisms underlying AP2 regulation of TGF-α self-induction was conducted in A431 cells. First, we determined the dosages of erlotinib effective in controlling TGF-α–driven growth by measuring decreases in cell viability (MTT assays; Figure 3A). Erlotinib inhibition of EGFR-driven growth correlated with decreases in nuclear AP2 content as shown by Western blot analysis (Figure 3B) and immunofluorescence studies (Figure 3C). Indeed, the higher dosages of erlotinib of 0.10 and 0.25 μM reduced dosage dependently the number of cells staining positively for nuclear AP2. A 48.5 or 78.2% reduction in nuclear AP2 content, respectively, paralleled the decreases of TGF-α mRNA (Figure 3D). Next, we examined whether AP2 mediates TGF-α induction of the expression of its own gene. A431 cells were transiently transfected with a luciferase reporter driven by the human TGF-α promoter containing the reported AP2 binding site (Figure 3E, top). TGF-α treatment (8 nM for 24 h) of A431 cells increased AP2 protein expression and caused a six-fold induction of promoter activity. In contrast, when A431 cells were transfected with a luciferase reporter driven by a TGF-α promoter carrying a 10-bp mutation at the core AP2 site, there was a 50% reduction in basal promoter activity and a 30% decrease in the promoter activity induced by TGF-α treatment, despite similar TGF-α–driven increases in AP2 content (Figure 3E). Taken together, these results demonstrate that TGF-α induction of its own mRNA and protein levels involve increases in AP2 expression, nuclear localization, and binding to the AP2 binding site at the TGF-α promoter and induction of TGF-α gene transcription.
Figure 3.
AP2 contribution to TGF-α self-induction in A431 cells. (A) Erlotinib dosage-dependent inhibition of TGF-α/EGFR–driven growth as measured by progressive reduction in cell number (top) and MTT assay (bottom). Bars and error bars indicate means ± SEM of erlotinib-induced reduction in viable cells compared with untreated controls (untreated, erlotinib 0 μM) from four independent experiments. (B) Western blot analysis of changes in AP2 expression in response to increasing dosages of erlotinib in A431 cells treated as in A. (C) Quantification of changes in nuclear AP2 expression using immunofluorescent staining for AP2, in response to erlotinib. Bars and error bars represent means ± SEM of the number of AP2-positive nuclei per 1000 cells from three independent experiments. Results are expressed as percentage of nuclear AP2 content relative to untreated (erlotinib 0 μM) cells. (D) Erlotinib dosage-dependent inhibition of TGF-α mRNA levels in cells treated as in A. (Top) Representative RT-PCR of TGF-α mRNA expression in response to erlotinib. (Bottom) Bars and error bars represent the means ± SEM of TGF-α mRNA levels, corrected per GAPDH, from two independent experiments. Results are expressed as percentage of untreated A431 cells (0). (E, top) Partial sequence of the human TGF-α promoter containing an AP2 binding site (wild-type) and the insertion of a 10-bp mutation at the core AP2 consensus (mutant). (E, middle) Promoter activity in response to TGF-α treatment in A431 cells transfected with wild-type (WT; □) or mutant (□) TGF-α promoter/luciferase reporter. Bars and error bars indicate means ± SEM from triplicate luciferase/β-gal determinations per experimental condition from two independent experiments (P < 0.05). (E, bottom) Representative Western blot analysis of TGF-α (8 nM) induction of AP2 levels after 24 h of treatment in the cell lysates from A431 cells transfected as indicated for luciferase reporter assays.
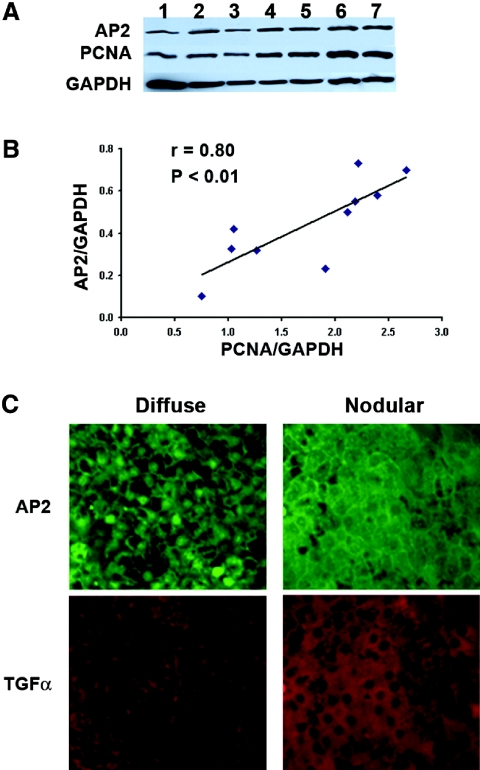
Next, the contribution of increases in AP2 to enhanced TGF-α expression and growth severity was examined in hyperplastic human parathyroid glands from patients with CKD. As expected for a mediator of TGF-α self-upregulation, parathyroid AP2 levels, in whole-cell extracts from human parathyroid glands, strongly correlated with PCNA, a marker of the severity of the hyperplasia (Figure 4A). Because parathyroid TGF-α expression and growth rates differ markedly between diffuse and nodular hyperplasia,18 we simultaneously assessed AP2 and TGF-α expression using immunofluorescence staining in areas of diffuse and nodular growth within the same parathyroid gland (Figure 4A). Histologically, diffuse hyperplasia was defined by areas with increased number of parenchymal cells with normal lobular structures. Nodular hyperplasia was defined by well circumscribed, encapsulated nodules with virtually fat cell-free accumulation.25 We found a direct association between parathyroid AP2 and TGF-α levels. Indeed, highest AP2 levels occurred in nodular areas coinciding with their higher TGF-α expression; however, AP2 subcellular localization rendered unexpected results. Nuclear AP2 levels were higher in diffuse than in nodular areas of the same gland. At the core of nodular areas eliciting the highest TGF-α, AP2 staining was mainly cytosolic (Figure 4B). This suggests that AP2 may not be the only transactivator of the TGF-α gene in nodular hyperplasia.
Figure 4.
(A) Parathyroid AP2 expression correlates directly with PCNA in human SH. (Top) Western blot analysis of AP2, PCNA, and GAPDH content in whole-cell extracts from 10 human parathyroid glands with no pathologic diagnosis of diffuse or nodular hyperplasia. (Bottom) Correlation between AP2 and PCNA content, as measured by densitometric analysis of AP2/GAPDH and PCNA/GAPDH ratios in whole-cell extracts from 10 hyperplastic human parathyroid glands depicted in the Western blot. (B) Parathyroid AP2 expression correlates directly with TGF-α content in human SH. Representative photomicrographs of immunofluorescence staining for AP2 and TGF-α in diffuse and nodular human parathyroid glands. Magnification, ×200.
DISCUSSION
Full characterization of the pathogenesis of the enhanced parathyroid expression of the powerful TGF-α/EGFR feed-forward loop for growth and vitamin D resistance in SH is critical for the design of more effective therapeutic interventions. To this end, these studies demonstrate that increases in parathyroid AP2 expression are critical contributors to the enhanced TGF-α expression that determines the severity of growth in human and experimental SH (summarized in Figure 5).
Figure 5.
Model for AP2 regulation of TGF-α self-induction. The release of the mature form of TGF-α from its cell membrane precursor binds and activates its receptor, the EGFR, thereby inducing increases in AP2 expression, nuclear translocation, and transactivation of the TGF-α gene. Inhibition of EGFR activation with erlotinib is sufficient to simultaneously arrest increases in AP2 and AP2-mediated TGF-α gene transcription.
In early kidney disease in rats, the increases in parathyroid TGF-α and growth rates induced by high-P or low-Ca diets11 were preceded by elevations in parathyroid AP2 content. Furthermore, the efficacy of high dietary Ca or P restriction and that of prophylactic calcitriol administration in rats fed high-P diets in ameliorating the increases in parathyroid TGF-α and growth rates were associated with unchanged parathyroid AP2 content. This strong direct correlation between parathyroid AP2 and TGF-α expression supported a role for AP2 in TGF-α gene transcription. Furthermore, as expected for a transactivator of the TGF-α gene, the increases in parathyroid AP2 expression induced by high dietary P concurred with elevations in nuclear AP2 immunostaining and AP2 binding to an AP2 consensus DNA sequence. Unfortunately, the rapid de-differentiation of hyperplastic parathyroid cells in culture26,27 impeded a direct assessment of the contribution of AP2 binding to the AP2 binding site at the rat/human TGF-α gene promoter to induce parathyroid TGF-α gene transcription. To overcome this limitation, we used A431 cells, a validated model of TGF-α self-induction.18 Similar to hyperplastic parathyroid cells, TGF-α overexpression drives A431 cell growth.22 In these cells, a 10-bp mutation at the core AP2 binding site of the human TGF-α promoter was sufficient to reduced markedly TGF-α induction of its own gene despite TGF-α–induced increases in AP2. This renders AP2 a bona fide transactivator of the TGF-α gene in A431 cells. Thus, full characterization of the mechanism underlying the increases in AP2 is critical to treat SH effectively. To this end, we assessed the role of AP2 in TGF-α self-upregulation in rat SH through administration of erlotinib, a highly specific inhibitor of TGF-α activation of EGFR.28 In early experimental uremia, dosages of erlotinib that effectively prevented uremia and high P–induced TGF-α self-upregulation24 and, consequently, increases in parathyroid TGF-α and growth rates17 also caused a dosage-dependent reduction of parathyroid AP2 expression. Erlotinib-mediated prevention of the increases in parathyroid AP2 conclusively demonstrated that the elevations in AP2 occurred downstream from TGF-α activation of its receptor and mediated TGF-α self-induction. Studies in A431 cells corroborated the critical contribution of increases in AP2 to TGF-α self-induction. Similar to rat SH, erlotinib inhibition of TGF-α self-induction, both mRNA and protein, involved a dosage-dependent reduction of AP2 expression and nuclear localization. More significant, mutation of the core AP2 binding site of the human TGF-α promoter markedly reduced the induction of TGF-α gene expression by exogenous TGF-α. If TGF-α activation of the EGFR causes the increases in AP2, then how could the increases in parathyroid AP2 expression in five-sixths Nx rats precede the elevations in TGF-α content? We postulate that an underestimation of parathyroid TGF-α levels at day 2 after five-sixths Nx accounts for this apparent discrepancy. Our immunostaining for TGF-α quantification can detect only intracellular or membrane-bound TGF-α and not the mature, soluble isoform of the TGF-α molecule, which is released from its transmembrane precursor by cell-specific proteinases29; therefore, autocrine/paracrine TGF-α activation of the EGFR by the mature, no longer cell-bound TGF-α could trigger the rapid increases in AP2 necessary to induce TGF-α gene transcription and TGF-α self-induction in rat SH. If confirmed, then this provides relevant translational insights into the pathogenesis of the onset of TGF-α/EGFR signaling in SH. Effective inhibition of either the expression or the activity of the proteinase(s) that releases parathyroid TGF-α could arrest the progression of parathyroid hyperplasia. This is not mere speculation. The TGF-α sheddase ADAM 17 is expressed in the parathyroid gland and required for parathyroid gland development.30
These findings also suggest that calcitriol efficacy in preventing high P–induced increases in parathyroid AP2 in rat SH may not result from direct inhibition of AP2 expression. Instead, calcitriol inhibition of TGF-α/EGFR-driven signaling, a mechanism demonstrated by our group in A431 cells,23 by attenuating TGF-α self-induction could simultaneously ameliorate the increases in TGF-α and TGF-α–driven increases in AP2. Alternatively, calcitriol could inhibit the expression and/or activity of parathyroid TGF-α sheddase. In the course of CKD, however, progressive reductions in parathyroid vitamin D receptor, induced by increasing TGF-α/EGFR-driven signaling, antagonize calcitriol actions in the parathyroid glands, generating a feed-forward loop for enhanced AP2/TGF-α levels and calcitriol resistance.18
The identification of a similar association between AP2 and TGF-α levels in human hyperplastic glands from patients with CKD suggests that AP2-mediated TGF-α self-induction also contributes to the progression of human SH. Indeed, parathyroid AP2 levels strongly correlated with the severity of hyperplastic growth. Importantly, the direct correlation between nuclear AP2 and TGF-α induction of its own expression found in rat SH and A431 cells only occurs in diffuse hyperplasia. Despite the higher AP2/TGF-α levels, at the core of the aggressively growing nodules, there is lower nuclear AP2 than in diffuse areas of the same gland. Thus, at the core of the nodules, AP2 may no longer be the main regulator of TGF-α induction of the transcription of its own gene. The recent demonstration that hypoxia inducible factor 2α (HIF2α) is a potent transactivator of the TGF-α gene31 suggested that mild hypoxia at the nodule core could increase HIF2α levels to substitute for AP2 transactivation of the TGF-α gene. Indeed, preliminary studies suggested that the reduction in nuclear parathyroid AP2 coincides with progressive increases in HIF2α from diffuse areas to the core of an adjacent nodular area (data not shown). In the most severe forms of human SH, increases in HIF2α could not only aggravate the TGF-α/EGFR feed-forward loop for enhanced TGF-α expression and aggressive growth signaling but also sustain proper nodule vascularization through HIF2α-mediated activation of vascular endothelial growth factor,32 a hallmark of tumorigenesis. Importantly, EGFR activation maintains constitutive HIF signaling even upon re-establishment of normoxia33; however, de-differentiation of hyperplastic human parathyroid cells in culture and the lack of nodule formation in the rat model of SH impede accurate assessment of the relative contribution of AP2 and HIF2α to enhance parathyroid TGF-α gene transcription or tumoral expansion in human SH.
The AP2/TGF-α cross-talk delineated in A431 cells and calcitriol efficacy in inhibiting their aggressive TGF-α/EGFR-driven growth23 suggest that a similar inhibition of TGF-α–driven AP2 expression by calcitriol or its analogs in human carcinomas including cervical cancer, ovarian cancer, and colorectal adenomas could attenuate AP2-induction of HER-2/neu oncogene,34 which is overexpressed in approximately one fourth of human breast cancers, and also reduce AP2 contribution to melanoma progression and metastasis.35
In summary, TGF-α induction of AP2 expression, nuclear localization, binding to the AP2 binding site, and transactivation of the TGF-α promoter contributes to TGF-α upregulation of its own levels in the parathyroid hyperplasia secondary to kidney disease and also in the aggressive human squamous carcinoma (A431 cell line), thereby aggravating the severity of TGF-α/EGFR driven hyperplasia.
CONCISE METHODS
Rat Parathyroid Glands
These studies used the parathyroid glands from five-sixths Nx rats subjected to one of the following protocols described by Cozzolino et al.12,17 Briefly, in protocol 1, rats (female Sprague-Dawley, 220 to 240 g body wt) were fed (1) high-P diet (0.9% P) and treated with either vehicle or 4 ng of 1,25-dihydroxyvitamin D (calcitriol, 1,25D) intraperitoneally, every other day in 100 μl of propylene glycol, for 1 wk (this dosage of 1,25D controls parathyroid hyperplasia while avoiding hypercalcemia and hyperphosphatemia12); (2) low-P diet (0.2% P; 0.5% Ca); (3) high-Ca diet (1.25% P; 2.0% Ca); or (4) low-Ca diet (0.6% P; 0.1% Ca). A similar protocol including only dietary P and Ca manipulations was conducted for 2 d after five-sixths Nx. In protocol 2, rats (160 to 180 g body wt) were fed either a low-P diet (0.2% P; 0.5% Ca) or high-P diet (1.2% P; 0.4% Ca) and treated with either DMSO as vehicle or 3 or 6 mg/kg body wt erlotinib (provided by Genentech, San Francisco, CA) for 1 wk. Parathyroid glands were surgically removed, and their weight was measured as described previously.11,12,17 All diets were purchased from Dyets, Inc. (Bethlehem, PA). All experimental protocols were approved by the Animal Study Committee at Washington University School of Medicine.
Human Parathyroid Glands
All parathyroid glands used in the immunohistochemical studies were obtained from patients who underwent total parathyroidectomy from 2001 to 2003 at Miulli Hospital, Acquaviva delle Fonti (Bari, Italy). Sections of human parathyroid glands with no histologic diagnosis of diffuse or nodular hyperplasia were provided by Dr. Alex Brown (Washington University School of Medicine).
Immunohistochemistry
Immediately after surgical resection, parathyroid tissue was kept in 10% formaldehyde overnight and then transferred to 70% ethanol in water before paraffin embedding. Immunohistochemical staining for AP2 was performed on formalin-fixed, paraffin-embedded parathyroid glands (both human and rat) using rabbit polyclonal anti-AP2α antibody (SC-184; Santa Cruz Biotechnology, Santa Cruz, CA). Parathyroid tissue was deparaffinized and rehydrated. Endogenous peroxidases were quenched using 0.6% hydrogen peroxide in methanol. For antigen retrieval, parathyroid tissue was then pretreated with 0.05% saponin for 30 min at room temperature, blocked with 10% preimmune goat serum, and incubated with 4 μg/ml AP2α antibody followed by biotinylated secondary antibody and streptavidin–horseradish peroxidase conjugate (Histostain-plus, rabbit). Immune complexes were visualized with aminoethyl carbazole substrate-chromagen. The specificity of the primary antibody for immunohistochemical staining was tested by replacing the primary antibody with preimmune IgG1 or the supernatant of immunoabsorption of the AP2 antibody. Briefly, primary antibody and its blocking peptide were incubated for 8 h at 4°C, followed by a 15-min centrifugation at 10,000 × g. Immunohistochemical staining of AP2 proteins was quantified using a Nikon Diaphot-TMD microscope coupled to a camera and an image analysis system as described previously.11 The average OD per section of tissue was calculated by dividing the sum integrated OD by the sum area. At least six different slides were analyzed for each experimental condition.
Electrophoretic Mobility Shift Assays
Nuclear extracts from freshly excised rat parathyroid glands were prepared using the Nuclear Extract Kit (Active Motif, Carlsbad, CA). The AP2-Gel Shift Assay System (Promega, Madison, WI) was used according to the manufacturer's recommendations to measure AP2 binding to DNA in nuclear extracts (30 μg of total protein) from parathyroid glands from five-sixths Nx rats fed either a high-P diet for 1 wk or 1 mo or a low-P diet for 1 wk. The specificity of AP2/DNA binding was studied with similar incubations of radiolabeled-oligonucleotide of the AP2 consensus sequence with nuclear extracts of hyperplastic parathyroid glands from uremic rats fed high dietary P for 1 mo, in the absence or presence of a 200-mol excess of either a radioinert AP2 consensus oligonucleotide or an SP1 consensus (an AP2-unrelated oligonucleotide sequence) or 1 μg of a polyclonal antibody against AP2α (SC184; Santa Cruz Biotechnology).
Cell Culture and Proliferation Assays
A431 (ATCC, Manassas, VA) cells were grown in 10% FBS DMEM (Invitrogen, Carlsbad, CA) containing 4 mM l-glutamine, 4.5 g/L glucose, and 1.5 g/L sodium bicarbonate at 37°C in 5% CO2. Cells, plated at a concentration of either 106 cells in 10-cm plates or 104 cells in 96-well plates, were synchronized at G0 by serum deprivation (serum-free DMEM) for 8 h and treated with erlotinib (0.000, 0.025, 0.100, and 0.250 μM) in 2% FBS DMEM for 60 h followed by fresh treatment in 1% BSA DMEM up to 84 h. At the end of treatment, cell viability was measured using the Colorimetric Assay for cell survival and proliferation (MTT assay; Chemicon International, Temecula, CA) following the manufacturer's specifications.
Immunofluorescence
A431 cells (5 × 104 in 24-well plate) were cultured as described in the previous section. After treatment, cells were washed with PBS and fixed with PFA 2% for 15 min. After three PBS washes, 0.1% sodium borohydride/PBS was added for 10 min. Cells were then washed with PBS (three times), permeabilized with 0.2% Triton X-100/PBS, blocked with 3% skim milk, and incubated overnight at 4°C with the primary antibody against AP2α (SC184, 1:1000 dilution; Santa Cruz Biotechnology). Secondary antibody with a fluorophore was applied for 1 h at room temperature (Alexa 488, 1:300 dilution; Invitrogen). Positive nuclei were counted in >1000 cells per experimental condition from three independent experiments.
Sections from human parathyroid glands were incubated overnight at 4°C simultaneously with primary antibodies against AP2 (SC184, 1:1000 dilution; Santa Cruz Biotechnology) and TGF-α (GF10, 1:20 dilution; Calbiochem, San Diego, CA). After washing, secondary antibody was applied for 1 h at room temperature (Alexa 488 and Alexa 594, respectively, 1:300 dilution; Invitrogen).
Western Blotting
For protein extraction for A431 whole-cell extracts, cells washed twice with PBS were added to 500 μl of RIPA buffer 1% (2% RIPA buffer: 50 mM Tris-HCl [pH 7.4]; 1% NP-40; 0.25% Na-deoxycholate, 150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 μg/ml each aprotinin, leupeptin, and pepstatin; 1 mM Na3VO4; and 1 mM NaF) with 40 μl/ml fresh protease inhibitor cocktail (Roche, Indianapolis, IN), sonicated on ice for 40 s, and centrifuged for 20 min at 14,000 rpm at 4°C to collect supernatants. For protein extraction from individual rat parathyroid glands, 4.5 μl of 1% RIPA buffer was added per 100 μg of parathyroid tissue. Tissue was then frozen in dry ice and thawed on ice three times, followed by a centrifugation at 14,000 rpm for 20 min at 4°C. Human parathyroid glands were homogenized in hypotonic conditions (300 nM sucrose, 25 mM Tris [pH 7.4], 0.4 mM EDTA, 0.4 mM PMSF, 1 mM benzamidine, and 1 mM NaN), and total protein was obtained from the homogenates using the Active Motif Kit. Protein concentrations were measured using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein from whole-cell extracts of rat parathyroid glands (15 μg), A431 cells (40 μg), or human parathyroid glands (40 μg) with no pathologic diagnosis of diffuse or nodular hyperplasia were resolved by 12% SDS-PAGE and electroblotted onto polyvinylidene difluoride (Millipore, Bedford, MA) membrane. Membranes were blocked with 3% skim milk (Merck, Darmstadt, Germany) in Tris-buffered saline containing 0.1% Tween-20 (T-TBS) at room temperature for 1 h and probed with an anti-AP2α antibody (SC184, 1:200 dilution; Santa Cruz Biotechnology) overnight at 4°C. Blots were washed three times (10 min each) with T-TBS and incubated for 1 h at room temperature with anti-rabbit secondary antibody (#7040, 1:2000 dilution; Cell Signaling, Danvers, MA). Blots were visualized using chemiluminescent substrate (SuperSignal West Pico; Pierce, Rockford, IL) and analyzed by Gel Pro Analyzer (Media Cybernetics, Silver Spring, MD).
RT-PCR
Two micrograms of total RNA, collected using RNA-Bee (TEL-TEST, Friendswood, TX) were used. First-strand cDNA synthesis was obtained using Omniscript Reverse Transcription reagents (Qiagen, Valencia, CA). PCR for TGF-α and GAPDH was performed on 1 μL of experimental template using the AmpliTaq (Applied Biosystems, Foster City, CA) kit and 4 pmol of each primer. Cycling conditions were 3 min at 94°C, 35 or 30 cycles, respectively, of 30 s at 94°C, 30 s at 56°C, 45 s at 72°C, with the final extension of 2 min at 72°C. RT-PCR products were electrophoresed in 1% agarose gels and visualized using a transilluminator (Sigma T1202; Sigma Chemical, St. Louis, MO). Primer sequences were as follows: TGF-α forward 5′-ATGGTCCCCTCGGCTGGACAG-3′ and reverse 5′-TCCTCCTCTGGGCTCTTCAG-3′; and GAPDH forward 5′-ATGGGGAAGGTGAAGGTCGG-3′ and reverse: 5′-GGTGGTGAAGACGCCAGTGG-3′.
Promoter-Reporter Analysis
Luciferase reporters driven by either the wild-type human TGF-α promoter (−1013 to 38) containing the AP2 binding site or the same length promoter carrying a 10-bp mutation at the core AP2 binding site, from the TCCGCCCGGC in wild-type to CGAGATCTAT, were generated in our laboratory using PCR amplification techniques and final insertion of wild-type and mutant AP2 TGF-α promoters into pGL2 basic vectors. The β-galactosidase expression plasmid was obtained from Dr. Michael Rauchman (St. Louis University). A431 cells, plated in six-well plates at a concentration of 3 × 105 cells/ml of medium per well, were transfected using Myrus Transfection reagents (Myrus, Madison, WI) following the manufacturer's protocols using 4 μl of transfection reagent per 1 μg of DNA. One microgram of the wild-type or AP2 mutant reporters and 0.1 μg of β-galactosidase expression plasmids were transfected per well. Upon a 48 h incubation, medium was replaced by serum-free medium, and transfected cells were treated with either vehicle or TGF-α (8 nM) for 24 h. Cells were then lysed, and luciferase and β-galactosidase activities were measured using Luciferase reporter system (Promega) and Galacto-Light (Applied Biosystems), respectively. Western blot analysis of pooled cell lysates for each experimental condition evaluated the changes in AP2 content induced by vehicle or TGF-α treatment in A431 cells transfected with either wild-type or the AP2 mutant (−1013, 38) human TGF-α promoter.
Statistical Analysis
ANOVA was used to assess statistical differences among all experimental groups under study. Multiple comparisons using the stringent Bonferroni test, unpaired t test analysis, or 95% confidence intervals measured the statistical significance of the differences between two experimental groups.
DISCLOSURES
E.S. is a consultant/speaker for Genzyme and Abbott.
Acknowledgments
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant DK062713 (to A.S.D.); grants from Research in Renal Diseases, Washington University, and Abbott Pharmaceuticals (to E.S.); and Core C of the O'Brien Center for renal chemistries, grant P3ODK079333. T.S. is the recipient of an award by Nagono Medical Foundation (Nagoya, Japan).
These findings were presented as a free communication (abstract F-FC118) at the annual meeting of the American Society of Nephrology; November 1 through 5, 2007; San Francisco, CA (J Am Soc Nephrol 18: 25A, 2007).
The authors greatly acknowledge Genetech's support in supplying erlotinib.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Cozzolino M, Dusso AS, Slatopolsky E: Role of calcium-phosphate product and bone-associated proteins on vascular calcification in renal failure. J Am Soc Nephrol 12: 2511–2516, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez EA, Martin KJ: Renal osteodystrophy: Pathogenesis and management. Nephrol Dial Transplant 10[Suppl 3]: 13–21, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Parfitt AM: The hyperparathyroidism of chronic renal failure: A disorder of growth. Kidney Int 52: 3–9, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Slatopolsky E, Finch J, Denda M, Ritter C, Zhong M, Dusso A, MacDonald PN, Brown AJ: Phosphorus restriction prevents parathyroid gland growth: High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest 97: 2534–2540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silver J, Sela SB, Naveh-Many T: Regulation of parathyroid cell proliferation. Curr Opin Nephrol Hypertens 6: 321–326, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Naveh-Many T, Rahamimov R, Livni N, Silver J: Parathyroid cell proliferation in normal and chronic renal failure rats: The effects of calcium, phosphate, and vitamin D. J Clin Invest 96: 1786–1793, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slatopolsky E, Brown A, Dusso A: Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl 73: S14–S19, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Denda M, Finch J, Slatopolsky E: Phosphorus accelerates the development of parathyroid hyperplasia and secondary hyperparathyroidism in rats with renal failure. Am J Kidney Dis 28: 596–602, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Gogusev J, Duchambon P, Stoermann-Chopard C, Giovannini M, Sarfati E, Drueke TB: De novo expression of transforming growth factor-alpha in parathyroid gland tissue of patients with primary or secondary uraemic hyperparathyroidism. Nephrol Dial Transplant 11: 2155–2162, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Dusso AS, Pavlopoulos T, Naumovich L, Lu Y, Finch J, Brown AJ, Morrissey J, Slatopolsky E: p21(WAF1) and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth. Kidney Int 59: 855–865, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Cozzolino M, Lu Y, Finch J, Slatopolsky E, Dusso AS: p21WAF1 and TGF-alpha mediate parathyroid growth arrest by vitamin D and high calcium. Kidney Int 60: 2109–2117, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Bustin SA, McKay IA: Transforming growth factor alpha. Cell Biol Int 19: 373–388, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Driman DK, Kobrin MS, Kudlow JE, Asa SL: Transforming growth factor-alpha in normal and neoplastic human endocrine tissues. Hum Pathol 23: 1360–1365, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Shum L, Reeves SA, Kuo AC, Fromer ES, Derynck R: Association of the transmembrane TGF-alpha precursor with a protein kinase complex. J Cell Biol 125: 903–916, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong ST, Winchell LF, McCune BK, Earp HS, Teixido J, Massague J, Herman B, Lee DC: The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell 56: 495–506, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Cozzolino M, Lu Y, Sato T, Yang J, Suarez IG, Brancaccio D, Slatopolsky E, Dusso AS: A critical role for enhanced TGF-alpha and EGFR expression in the initiation of parathyroid hyperplasia in experimental kidney disease. Am J Physiol Renal Physiol 289: F1096–F1102, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Arcidiacono MV, Sato T, Alvarez-Hernandez D, Yang J, Tokumoto M, Gonzalez-Suarez I, Lu Y, Tominaga Y, Cannata-Andia J, Slatopolsky E, Dusso AS: EGFR activation increases parathyroid hyperplasia and calcitriol resistance in kidney disease. J Am Soc Nephrol 19: 310–320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson LG, Thompson KL, Xu J, Gill GN: Identification and characterization of a regulated promoter element in the epidermal growth factor receptor gene. Proc Natl Acad Sci U S A 87: 7536–7540, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Kudlow JE: Purification and characterization of TEF1, a transcription factor that controls the human transforming growth factor-alpha promoter. Biochim Biophys Acta 1449: 50–62, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, Barbacci EG, Pustilnik LR, Smolarek TA, Davis JA, Vaidya MP, Arnold LD, Doty JL, Iwata KK, Morin MJ: Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: Dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther 291: 739–748, 1999 [PubMed] [Google Scholar]
- 22.Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Williams RD, Bringman TS, Berger WH: Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res 47: 707–712, 1987 [PubMed] [Google Scholar]
- 23.Cordero JB, Cozzolino M, Lu Y, Vidal M, Slatopolsky E, Stahl PD, Barbieri MA, Dusso A: 1,25-Dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. J Biol Chem 277: 38965–38971, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Cozzolino M, Lu Y, Sato T, Yang J, Suarez IG, Brancaccio D, Slatopolsky E, Dusso AS: A critical role for enhanced TGF-alpha and EGFR expression in the initiation of parathyroid hyperplasia in experimental kidney disease. Am J Physiol Renal Physiol 289: F1096–F1102, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Lomonte C, Vernaglione L, Chimienti D, Bruno A, Cocola S, Teutonico A, Cazzato F, Basile C: Does vitamin D receptor and calcium receptor activation therapy play a role in the histopathologic alterations of parathyroid glands in refractory uremic hyperparathyroidism? Clin J Am Soc Nephrol 3: 794–799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Hernandez D, Gonzalez-Suarez I, Naves M, Carrillo-Lopez N, Fdez-Coto T, Fernandez-Martin JL, Cannata-Andia JB: Long-term response of cultured rat parathyroid glands to calcium and calcitriol: The effect of cryopreservation. J Nephrol 18: 141–147, 2005 [PubMed] [Google Scholar]
- 27.Brown AJ, Zhong M, Ritter C, Brown EM, Slatopolsky E: Loss of calcium responsiveness in cultured bovine parathyroid cells is associated with decreased calcium receptor expression. Biochem Biophys Res Commun 212: 861–867, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Ranson M, Mansoor W, Jayson G: ZD1839 (IRESSA): A selective EGFR-TK inhibitor. Expert Rev Anticancer Ther 2: 161–168, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Hinkle CL, Sunnarborg SW, Loiselle D, Parker CE, Stevenson M, Russell WE, Lee DC: Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family: The juxtamembrane stalk determines cleavage efficiency. J Biol Chem 279: 24179–24188, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA: An essential role for ectodomain shedding in mammalian development. Science 282: 1281–1284, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Gunaratnam L, Morley M, Franovic A, de Paulsen N, Mekhail K, Parolin DA, Nakamura E, Lorimer IA, Lee S: Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL(-/-) renal cell carcinoma cells. J Biol Chem 278: 44966–44974, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Carroll VA, Ashcroft M: Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: Implications for targeting the HIF pathway. Cancer Res 66: 6264–6270, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Garcia JA: HIFing the brakes: Therapeutic opportunities for treatment of human malignancies. Sci STKE pe25, 2006 [DOI] [PubMed]
- 34.Li M, Wang Y, Hung MC, Kannan P: Inefficient proteasomal-degradation pathway stabilizes AP-2alpha and activates HER-2/neu gene in breast cancer. Int J Cancer 118: 802–811, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Nyormoi O, Bar-Eli M: Transcriptional regulation of metastasis-related genes in human melanoma. Clin Exp Metastasis 20: 251–263, 2003 [DOI] [PubMed] [Google Scholar]