Abstract
Higher levels of albumin excretion within the normal range are associated with cardiovascular disease in high-risk individuals. Whether incremental increases in urinary albumin excretion, even within the normal range, are associated with the development of hypertension in low-risk individuals is unknown. This study included 1065 postmenopausal women from the first Nurses’ Health Study and 1114 premenopausal women from the second Nurses’ Health Study who had an albumin/creatinine ratio <25 mg/g and who did not have diabetes or hypertension. Among the older women, 271 incident cases of hypertension occurred during 4 yr of follow-up, and among the younger women, 296 incident cases of hypertension occurred during 8 yr of follow-up. Cox proportional hazards regression was used to examine prospectively the association between the albumin/creatinine ratio and incident hypertension after adjustment for age, body mass index, estimated GFR, baseline BP, physical activity, smoking, and family history of hypertension. Participants who had an albumin/creatinine ratio in the highest quartile (4.34 to 24.17 mg/g for older women and 3.68 to 23.84 mg/g for younger women) were more likely to develop hypertension than those who had an albumin/creatinine ratio in the lowest quartile (hazard ratio 1.76 [95% confidence interval 1.21 to 2.56] and hazard ratio 1.35 [95% confidence interval 0.97 to 1.91] for older and younger women, respectively). Higher albumin/creatinine ratios, even within the normal range, are independently associated with increased risk for development of hypertension among women without diabetes. The definition of normal albumin excretion should be reevaluated.
For decades, microalbuminuria has been recognized as a risk factor for progression of kidney disease, diabetic complications, and cardiovascular outcomes.1–4 Microalbuminuria has been proposed as a generalized marker of vascular damage in individuals both with and without diabetes, reflective of vascular endothelial dysfunction and abnormal vascular permeability.5,6 Urinary albumin excretion is typically categorized into normoalbuminuria (0 to 20 μg/min, or <30 mg/d), microalbuminuria (20 to 200 μg/min, or 30 to 300 mg/d), and overt albuminuria (>200 μg/min, or >300 mg/d) using fairly arbitrary cut points. The upper limit of normal was defined in 1985 as the 95th percentile of albumin excretion rate among a cohort of Italians without diabetes; the upper limit of microalbuminuria was the limit of standard dipsticks to detect albumin in 1981.7
More recent studies have demonstrated that increasing levels of albumin excretion, even within the normal range, are associated with increasing risk for cardiovascular end points among individuals who do and do not have diabetes and are at high baseline cardiovascular risk, such as individuals with multiple cardiovascular risk factors and those with left ventricular hypertrophy.8,9 Data from the Framingham study suggested that higher albumin/creatinine ratios (ACR), even within the normal range, may be associated with the risk for hypertension; however, a sizable proportion of individuals in the highest quartile (which was associated with elevated risk) had microalbuminuria.10
We examined prospectively the association between the baseline ACR and risk for development of hypertension among 1065 older women without hypertension and diabetes and with normoalbuminuria from the first Nurses’ Health Study (NHS I) and among 1114 younger women without hypertension and diabetes and with normoalbuminuria from the second Nurses’ Health Study (NHS II).
RESULTS
In NHS I, the median ACR was 2.7 mg/g (interquartile range [IQR] 1.7 to 4.3). The median age was 65 yr (IQR 60 to 70), and the median body mass index (BMI) was 24.2 kg/m2 (IQR 21.9 to 27.5). In NHS II, the median ACR was 2.4 mg/g (IQR 1.6 to 3.7). The median age was 44 yr (IQR 41 to 47), and the median BMI was 24.5 kg/m2 (IQR 21.9 to 28.3). Baseline characteristics are shown in Table 1 stratified by quartile of ACR. With increasing quartile of ACR in both cohorts, median BMI values were lower and median estimated GFR (eGFR) values were higher. In NHS I but not NHS II, median age was higher with higher ACR. No other baseline covariates seemed to differ consistently with differences in ACR.
Table 1.
Baseline characteristics according to quartile of ACRa
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P |
|---|---|---|---|---|---|
| NHS I | |||||
| ACR (mg/g; median [range]) | 0.97 (0.00 to 1.69) | 2.22 (1.70 to 2.73) | 3.42 (2.74 to 4.33) | 6.46 (4.34 to 24.17) | |
| urine albumin (mg/L; median [IQR]) | 0.66 (0.00 to 1.16) | 1.61 (1.14 to 2.21) | 2.09 (1.52 to 3.07) | 3.99 (2.60 to 7.12) | <0.001 |
| urine creatinine (mg/dl; median [IQR]) | 76.9 (52.9 to 112.4) | 73.9 (54.1 to 101.3) | 60.7 (42.7 to 92.3) | 55.8 (41.8 to 84.6) | <0.001 |
| age (yr; median [IQR]) | 64 (59 to 69) | 64 (60 to 70) | 65 (59 to 70) | 67 (61 to 72) | 0.008 |
| BMI (kg/m2; median [IQR]) | 24.9 (22.3 to 28.2) | 24.6 (22.3 to 27.7) | 23.8 (21.6 to 26.9) | 23.6 (21.6 to 27.4) | 0.030 |
| physical activity (METS/wk; median [IQR]) | 15.2 (8.4 to 29.4) | 17.8 (8.6 to 34.2) | 15.2 (6.2 to 28.9) | 13.2 (5.1 to 27.7) | 0.004 |
| SBP (mmHg; median [IQR]) | 120 (120 to 130) | 120 (110 to 130) | 120 (110 to 130) | 120 (120 to 130) | 0.070 |
| DBP (mmHg; median [IQR]) | 80 (70 to 80) | 80 (70 to 80) | 80 (70 to 80) | 80 (70 to 80) | 0.400 |
| MDRD eGFR (ml/min per 1.73 m2; median [IQR]) | 76 (65 to 85) | 77 (69 to 87) | 79 (69 to 90) | 80 (70 to 91) | 0.004 |
| serum creatinine (mg/dl; median [IQR]) | 0.81 (0.73 to 0.92) | 0.80 (0.72 to 0.88) | 0.78 (0.70 to 0.88) | 0.77 (0.68 to 0.86) | 0.001 |
| alcohol intake (g/d; median [IQR]) | 1.1 (0.0 to 9.7) | 2.4 (0.0 to 9.5) | 1.5 (0.0 to 7.2) | 1.5 (0.0 to 6.9) | 0.340 |
| sodium intake (g/d; median [IQR]) | 1.9 (1.6 to 2.1) | 1.9 (1.7 to 2.1) | 1.8 (1.6 to 2.1) | 1.9 (1.6 to 2.1) | 0.650 |
| current smoking (%) | 7.3 | 5.9 | 8.0 | 6.5 | 0.970 |
| past smoking (%) | 43.1 | 43.4 | 47.2 | 45.2 | 0.460 |
| white race (%) | 85.8 | 88.6 | 87.6 | 88.3 | 0.510 |
| family history of hypertension (%) | 39.4 | 43.8 | 40.1 | 35.6 | 0.290 |
| NHS II | |||||
| ACR (mg/g; median [range]) | 1.06 (0.00 to 1.62) | 2.04 (1.63 to 2.43) | 2.93 (2.44 to 3.67) | 5.40 (3.68 to 23.84) | |
| urine albumin (mg/L; median [IQR]) | 1.07 (0.24 to 1.91) | 2.60 (1.82 to 3.58) | 3.23 (2.24 to 4.79) | 6.48 (3.98 to 10.67) | <0.001 |
| urine creatinine (mg/dl; median [IQR]) | 124.9 (88.7 to 175.3) | 131.1 (96.8 to 176.4) | 111.4 (72.9 to 162.2) | 116.4 (75.2 to 162.9) | <0.001 |
| age (yr; median [IQR]) | 44 (41 to 47) | 44 (41 to 47) | 43 (41 to 46) | 44 (40 to 47) | 0.280 |
| BMI (kg/m2; median [IQR]) | 25.0 (22.0 to 28.2) | 24.8 (22.0 to 28.3) | 24.3 (22.3 to 28.3) | 24.0 (21.6 to 28.1) | 0.260 |
| physical activity (METS/wk; median [IQR]) | 12.5 (5.1 to 25.5) | 12.1 (5.0 to 25.2) | 14.2 (5.6 to 27.1) | 11.5 (5.0 to 22.5) | 0.620 |
| SBP (mmHg; median [IQR]) | 120 (110 to 120) | 110 (110 to 120) | 120 (110 to 120) | 120 (110 to 120) | 0.570 |
| DBP (mmHg; median [IQR]) | 70 (70 to 80) | 70 (70 to 80) | 70 (70 to 80) | 70 (70 to 80) | 0.730 |
| MDRD eGFR (ml/min per 1.73 m2; median [IQR]) | 86 (75 to 98) | 94 (82 to 100) | 96 (83 to 105) | 96 (84 to 113) | <0.001 |
| serum creatinine (mg/dl; median [IQR]) | 0.80 (0.70 to 0.88) | 0.72 (0.70 to 0.80) | 0.70 (0.66 to 0.80) | 0.70 (0.60 to 0.80) | <0.001 |
| alcohol intake (g/d; median [IQR]) | 1.5 (0.0 to 6.0) | 1.1 (0.0 to 3.85) | 1.0 (0.0 to 5.7) | 0.9 (0.0 to 4.7) | 0.370 |
| sodium intake (g/d; median [IQR]) | 2.0 (1.7 to 2.2) | 2.0 (1.7 to 2.2) | 2.0 (1.7 to 2.2) | 2.0 (1.7 to 2.2) | 0.720 |
| current smoking (%) | 9.1 | 7.0 | 8.1 | 8.3 | 0.910 |
| past smoking (%) | 30.2 | 23.0 | 24.1 | 28.3 | 0.780 |
| white race (%) | 95.4 | 96.0 | 95.8 | 94.0 | 0.410 |
| family history of hypertension (%) | 55.0 | 48.0 | 50.2 | 56.2 | 0.610 |
DBP, diastolic BP; METS, metabolic equivalent task score; SBP, systolic BP.
Among 1065 women in NHS I without prevalent hypertension, diabetes, or microalbuminuria at the time of urine collection, there were 271 cases of incident hypertension through 4 yr of follow-up. Compared with women whose ACR was in the lowest quartile, the hazard ratio (HR) for incident hypertension among those in the highest quartile was 1.76 (95% confidence interval [CI] 1.21 to 2.56; P = 0.004 for trend; Table 2) after multivariable adjustment for age, BMI, eGFR, baseline systolic and diastolic BP, physical activity, smoking, and family history of hypertension.
Table 2.
Quartiles of ACR (mg/g) and risk for incident hypertensiona
| Parameter | Quartiles (Median [Range])
|
P (Trend) | |||
|---|---|---|---|---|---|
| 1.0 (0.0 to 1.7) | 2.2 (1.7 to 2.7) | 3.4 (2.7 to 4.3) | 6.5 (4.3 to 24.2) | ||
| NHS I | |||||
| no. of participants | 246 | 290 | 299 | 230 | |
| no. of cases | 50 | 70 | 80 | 71 | |
| multivariable RR (95% CI) | 1.00 (reference) | 1.31 (0.90 to 1.89) | 1.37 (0.95 to 1.97) | 1.76 (1.21 to 2.56) | 0.004 |
| 1.1 (0.0 to 1.6) | 2.0 (1.6 to 2.4) | 2.9 (2.4 to 3.7) | 5.4 (3.7 to 23.8) | ||
| NHS II | |||||
| no. of participants | 242 | 300 | 307 | 265 | |
| no. of cases | 63 | 75 | 79 | 79 | |
| multivariable RR (95% CI) | 1.00 (reference) | 1.03 (0.73 to 1.45) | 1.13 (0.80 to 1.59) | 1.35 (0.97 to 1.91) | 0.060 |
Adjusted for age, BMI, eGFR, baseline BP, physical activity, smoking, and family history of hypertension. RR, relative risk.
Among 1114 women in NHS II without prevalent hypertension, diabetes, or microalbuminuria at the time of urine collection, 296 cases of incident hypertension were identified through 8 yr of follow-up. Compared with women whose ACR was in the lowest quartile, the multivariable HR for incident hypertension among those in the highest quartile was 1.35 (95% CI 0.97 to 1.91; P = 0.06 for trend; Table 2).
Because 10 women (eight from NHS I and two from NHS II) did not report having clinician examinations after submission of the urine specimen and during the period of follow-up, we repeated our multivariable analyses after excluding these 10 women; the results were essentially unchanged. Comparing women in the highest with lowest quartile of ACR, the HR were 1.76 (95% CI 1.21 to 2.55; P = 0.04 for trend) in NHS I and 1.34 (95% CI 0.96 to 1.90; P = 0.06 for trend) in NHS II. We also analyzed our data after (1) adjusting for alcohol and sodium intake; (2) including use of aspirin, acetaminophen, and nonsteroidal anti-inflammatory drugs in the multivariable models; and (3) excluding not only women with diabetes at baseline but also women who developed diabetes during follow-up. None of these secondary analyses affected the results.
DISCUSSION
Among 2179 women without hypertension and diabetes and with normoalbuminuria, we observed that increasing ACR, even within the range considered normal, is independently associated with an increased risk for development of hypertension. There are several potential mechanisms to explain this observation. Physiologic abnormalities of glomerular endothelial cells, the glomerular basement membrane, or podocytes could ultimately lead to increased filtration of albumin and, hence, albuminuria. The relative contributions of these three barriers to albumin filtration are a topic of active debate.11 The extent to which albumin is normally filtered and reabsorbed is also an area of rich debate, because some investigators believe that a defect in tubular reabsorption is the primary factor contributing to albuminuria.12
What seems to be clear is that the glomerular endothelial cell does play some role, however large, in the filtration barrier, and dysfunction of these endothelial cells may therefore lead to increased albumin excretion.11 Dysfunction of glomerular endothelial cells may reflect more widespread endothelial dysfunction. For example, endothelial-dependent vasodilation of the brachial artery, a surrogate marker of endothelial function, is inversely related to albumin excretion.5,13 Furthermore, albumin excretion is positively associated with some circulating biomarkers secreted by activated endothelial cells, such as von Willebrand factor.14 Taken together, higher levels of urine albumin excretion may reflect systemic endothelial dysfunction, which in turn may be a precursor to hypertension.15
Glomerular hyperfiltration may be a second mechanism linking higher levels of albumin excretion with hypertension. Because the glomerular filtration barrier is incomplete, some albumin does enter the proximal tubular fluid; however, under normal circumstances, this filtered albumin is completely reabsorbed by proximal tubular cells.16 A recent study by Lazzara and Deen16 suggested that increases in single-nephron GFR (snGFR), with concomitant increases in proximal tubular flow, can overwhelm the reabsorptive capacity of the proximal tubule; for example, a 50% increase in snGFR was predicted to cause a four- to five-fold increase in albumin excretion. Thus, states of glomerular hyperfiltration (i.e., increased snGFR), such as reduced nephron number and/or increased activity of the kidney renin-angiotensin system, may provide a link between higher levels of albumin excretion and the development of hypertension.17
It is well established that, among individuals who are at high baseline risk for cardiovascular events, high-normal compared with low-normal levels of ACR are associated with a greater risk for adverse cardiovascular outcomes. In a post hoc analysis of the Heart Outcomes Prevention Evaluation (HOPE) trial, the risk for a composite end point of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction began to increase as the ACR increased above 1.9 mg/g.8 The risk was 6% higher for every 4-mg/g increase in the ACR, and the findings were similar among individuals with and without diabetes.8 Similar results were observed in the Losartan Intervention For Endpoint reduction in hypertension (LIFE) trial. The risk of a composite cardiovascular end point began rising with ACR values above 2.2 mg/g; individuals whose ACR was 14 to 22 mg/g had a 60% increased risk compared with those whose ACR was <2.2 mg/g.9 Both of these studies enrolled high-risk individuals; HOPE included participants with known vascular disease or with diabetes plus one other risk factor, and LIFE included individuals with hypertension and left ventricular hypertrophy.8,9 Multiple other investigations have confirmed these findings among individuals with hypertension18 and among elderly individuals with a mixture of baseline hypertension, diabetes, and ischemic heart disease.19
In contrast, few studies have examined low-risk populations without hypertension and without diabetes. Wang et al.10 first reported an association between low-level albumin excretion and progression to hypertension in the Framingham Heart Study. Men and women in the highest compared with lowest quartile of ACR had a 1.93-fold (95% CI 1.18 to 3.10) higher risk for incident hypertension; however, the ACR values in the highest quartile in women ranged from 15.2 to 297.2 mg/g, and 35% of the women in this highest quartile had an ACR >30 mg/g. Although the proportion of women who had an ACR ≥25 mg/g (the female-specific cut point) in that study's highest quartile was not given, it was certainly at least 35%. Although only 10% of the highest quartile of men had an ACR >30 mg/g, the male-specific cut point for defining microalbuminuria is ≥17 mg/g (reflecting a higher urine creatinine excretion in men than in women).20,21
Our study has limitations that deserve mention. First, each participant submitted a single morning urine specimen rather than multiple specimens or a 24-h collection. Because of day-to-day within-person variation in albumin excretion,22,23 some participants in our study likely had their ACR status misclassified; however, this type of misclassification, if it occurred, would likely be random and therefore tend to bias the results toward an underestimation of the true association. Second, we did not directly measure our participants’ BP. Nevertheless, all participants are trained health professionals, and self-reporting of hypertension has been validated in these cohorts. Third, the NHS I and NHS II cohorts are predominately white and entirely female, so the results may not be generalizable to other racial groups or to men.
In conclusion, variations within the normal range of albumin excretion are associated with the development of hypertension, which is a major cause of cardiovascular morbidity and mortality. The findings of this study, in conjunction with the findings of numerous others, including the Framingham Heart Study, HOPE, and LIFE, suggest that it may be time to reevaluate our current concept of “normal” albumin excretion.
CONCISE METHODS
Nurses’ Health Studies
The NHS I cohort was assembled in 1976, when 121,700 female registered nurses 30 to 55 yr of age returned a mailed questionnaire. Subsequent questionnaires have been mailed every 2 yr to update information on health-related behaviors and medical events. NHS II is an independent cohort of 116,671 female registered nurses who were 25 to 42 yr of age when they returned an initial questionnaire in 1989. These women have also been followed with similar biennial questionnaires.
Study Populations
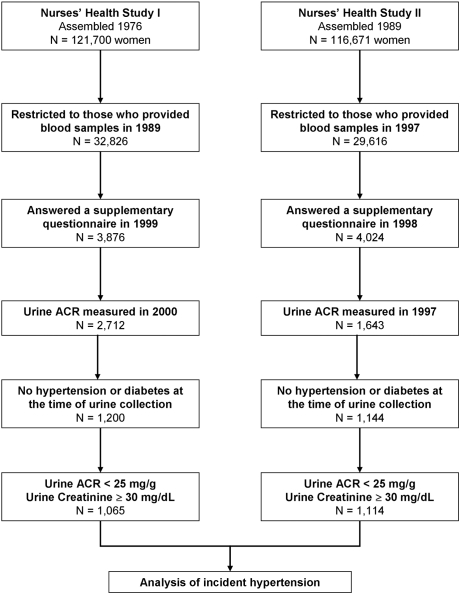
The study populations analyzed here are subcohorts of NHS I and NHS II. The derivation of these subcohorts for the analysis of incident hypertension is detailed in Figure 1. With the original intent of examining the association between analgesic intake and decline in kidney function, the populations were first limited to those who provided blood samples in 1989 (NHS I) or 1997 (NHS II), then restricted to those who returned supplementary questionnaires about analgesic use, and finally to those indicating a willingness to provide additional future blood samples. Urine samples were submitted by participants of NHS I in 2000 and by participants of NHS II between the years 1996 and 1998. Women with prevalent hypertension or those reporting use of antihypertensive medications at the time of urine collection were excluded, as were women with diabetes. We used an accepted gender-specific cut point of ≥25 mg/g to define microalbuminuria20,21; therefore, for this study, women whose ACR were ≥25 mg albumin/g creatinine were also excluded (i.e., those with microalbuminuria). Finally, because the ACR is highly denominator dependent (i.e., ACR values may be spuriously inflated if urinary creatinine excretion is low),24 we further excluded women with very low urinary creatinine concentrations (<30 mg/dl).24 A spot urine with <30 mg/dl of creatinine is currently defined by the World Health Organization as too dilute for adequate analysis.24 The institutional review board at Brigham and Women's Hospital reviewed and approved this study.
Figure 1.
Derivation of the study populations.
Ascertainment of ACR
Urine albumin was measured by immunoassay using the Hitachi 911 analyzer and Roche Diagnostics reagents (Indianapolis, IN). Urine albumin concentrations as low as 0.1 mg/L were reported; values <0.1 mg/L were defined as 0. Urine samples for NHS I and NHS II were measured in the same laboratory during the same time frame. Using blinded quality control samples, the coefficient of variation for this assay was 8%. Urine creatinine was measured using a modified Jaffe method (coefficient of variation 2%). For each participant, urine albumin was divided by urine creatinine to obtain the ACR and expressed as mg/g.
Ascertainment of Hypertension
Hypertension was self-reported in these cohorts of health professionals on biennial questionnaires; self-reported hypertension has been shown to be highly reliable. In a subset of women who reported hypertension, medical chart review confirmed a documented BP >140/90 mmHg in 100%; in addition, self-reported hypertension was predictive of subsequent cardiovascular events.25 Women were considered to have prevalent hypertension at the time of urine collection when they reported a diagnosis of hypertension on any previous biennial questionnaire or reported use of antihypertensive medications on the questionnaire that preceded urine collection. To analyze incident hypertension, women with prevalent hypertension were excluded. Among those without prevalent hypertension at baseline, women were considered to have incident hypertension when they reported, after the date of urine collection, an initial diagnosis of hypertension or new use of antihypertensive medications.
Ascertainment of Other Factors
Age, BMI (kg/m2), smoking status, and physical activity (metabolic equivalent task scores) were ascertained from the main biennial questionnaires returned in 2000 (NHS I) or 1997 (NHS II). Information on history of hypertension in a first-degree relative was available on the 1992 (NHS I) and 1989 (NHS II) questionnaires. We obtained self-reported baseline BP in NHS I from the 1998 questionnaire (this was not ascertained in 2000) and in NHS II from the 1999 questionnaire (this was not ascertained in 1997). Systolic BP was reported in nine categories (<105, 105 to 114, 115 to 124, 125 to 134, 135 to 144, 145 to 154, 155 to 164, 165 to 174, and ≥175 mmHg), and diastolic BP was reported in seven categories (<65, 65 to 74, 75 to 84, 85 to 89, 90 to 94, 95 to 104, and ≥105 mmHg). A participant's BP was defined as the middle systolic and middle diastolic value of the reported category. Classification of self-reported BP in this manner is highly predictive of subsequent cardiovascular events in these cohorts.26 Alcohol and sodium intake were ascertained from food frequency questionnaires that participants answered in 1998 (NHS I) or 1995 (NHS II).
Creatinine was measured by a modified Jaffe method. eGFR was estimated using the Modification of Diet in Renal Disease (MDRD) equation: 186 × [creatinine]−1.154× age−0.203× 0.742 × 1.21 (if black).27
Statistical Analysis
Trends among baseline covariates across quartiles of ACR were examined using the Kruskal-Wallis test (for continuous variables) or the Mantel-Haenszel χ2 test of trend (for categorical variables). For the analysis of incident hypertension, we examined the ACR in quartiles using the lowest quartile as the reference group. Person-time was counted from the date of urine collection to the date the last biennial questionnaire was returned (2004 in NHS I and 2005 in NHS II) and allocated according to exposure status. Person-time was truncated when an event occurred. Participants were censored at the date of death, or, when they did not return a subsequent questionnaire, they were censored at the date the subsequent questionnaire was mailed. Associations with incident hypertension were analyzed using Cox proportional hazards regression. We computed HR for age-adjusted models, as well as multivariable-adjusted models that included age (continuous), BMI (continuous), physical activity (quintiles), smoking (never, past, current), family history of hypertension (yes/no), MDRD eGFR (continuous), and baseline BP. In secondary analyses, we also adjusted for alcohol and sodium intake, as well as baseline use of aspirin, acetominophen, and non-steroidal anti-inflammatory drugs. The proportional hazards assumption was tested by plotting the hazards function for quartiles of ACR using log-log curves (proc lifetest). There were no departures from the proportional hazards assumption in the NHS I cohort and only minor departures in NHS II. Tests for linear trend across quartiles were assessed using the median ACR within each quartile.
For all HR, we calculated 95% CI. All P values are two-tailed. Statistical tests were performed using SAS statistical software, version 9 (SAS Institute, Cary, NC).
DISCLOSURES
None.
Acknowledgments
This research was supported by National Institutes of Health grants CA87969, CA50385, DK66574, DK07791, and HL079929-01A2 and American Heart Association grant 0535401T.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H: Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1: 1430–1432, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Mogensen CE: Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310: 356–360, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Yudkin JS, Forrest RD, Jackson CA: Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Islington Diabetes Survey. Lancet 2: 530–533, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Damsgaard EM, Froland A, Jorgensen OD, Mogensen CE: Microalbuminuria as predictor of increased mortality in elderly people. BMJ 300: 297–300, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B: Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation 103: 1869–1874, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Garg JP, Bakris GL: Microalbuminuria: Marker of vascular dysfunction, risk factor for cardiovascular disease. Vasc Med 7: 35–43, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Forman JP, Brenner BM: ‘Hypertension’ and ‘microalbuminuria’: The bell tolls for thee. Kidney Int 69: 22–28, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Wachtell K, Ibsen H, Olsen MH, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristianson K, Lederballe-Pedersen O, Nieminen MS, Okin PM, Omvik P, Oparil S, Wedel H, Snapinn SM, Aurup P: Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: The LIFE study. Ann Intern Med 139: 901–906, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D'Agostino RB, Levy D, Vasan RS: Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 111: 1370–1376, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ballermann BJ, Stan RV: Resolved: Capillary endothelium is a major contributor to the glomerular filtration barrier. J Am Soc Nephrol 18: 2432–2438, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Dogra G, Rich L, Stanton K, Watts GF: Endothelium-dependent and independent vasodilation studies at normoglycaemia in type I diabetes mellitus with and without microalbuminuria. Diabetologia 44: 593–601, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Clausen P, Feldt-Rasmussen B, Jensen G, Jensen JS: Endothelial haemostatic factors are associated with progression of urinary albumin excretion in clinically healthy subjects: A 4-year prospective study. Clin Sci (Lond) 97: 37–43, 1999 [PubMed] [Google Scholar]
- 15.Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG: Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol 44: 1636–1640, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Lazzara MJ, Deen WM: Model of albumin reabsorption in the proximal tubule. Am J Physiol Renal Physiol 292: F430–F439, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Klausen KP, Scharling H, Jensen G, Jensen JS: New definition of microalbuminuria in hypertensive subjects: Association with incident coronary heart disease and death. Hypertension 46: 33–37, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P: N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 293: 1609–1616, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Mattix HJ, Hsu CY, Shaykevich S, Curhan G: Use of the albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J Am Soc Nephrol 13: 1034–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Warram JH, Gearin G, Laffel L, Krolewski AS: Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 7: 930–937, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Cohen DL, Close CF, Viberti GC: The variability of overnight urinary albumin excretion in insulin-dependent diabetic and normal subjects. Diabet Med 4: 437–440, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Jensen JS: Intra-individual variation of overnight urinary albumin excretion in clinically healthy middle-aged individuals. Clin Chim Acta 243: 95–99, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL: Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ Health Perspect 113: 192–200, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE: Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 123: 894–900, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Fiebach NH, Hebert PR, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH: A prospective study of high blood pressure and cardiovascular disease in women. Am J Epidemiol 130: 646–654, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]