Abstract
Excessive glucocorticoid hormone, as occurs with Cushing syndrome, is known to be associated with altered body water homeostasis, but the molecular mechanisms are unknown. In this study, rats treated with daily dexamethasone (Dex) for 14 d provided a model of Cushing syndrome. Compared with control rats, Dex-treated rats demonstrated increased mean arterial pressure, urine flow rate, and urinary excretion of both sodium and urea. Dex-treated rats had increased abundance of aquaporin 1 (AQP1), AQP3, and Na-K-2Cl co-transporter proteins and a marked reduction of the urea transporters UT-A1 and UT-A3. In response to an acute water load, Dex-treated rats increased water excretion more than control rats, but both groups exhibited similar AQP2 expression. In response to fluid deprivation, Dex-treated rats demonstrated an impaired urinary concentrating capacity: Urine flow rate was higher and urine osmolality was lower than control rats despite an increase in AQP1, AQP3, and Na-K-2Cl co-transporter expression. AQP2 expression was similar between the two groups, but UT-A1 and UT-A3 were decreased and urinary urea excretion was increased in Dex-treated rats. Because Dex-treated rats ingested less food and water compared with controls, paired food and water studies were performed; these substantiated the previous results. In summary, the alterations in body water observed with glucocorticoid excess may be a result, in part, of impaired urinary concentrating capacity; downregulation of UT-A1 and UT-A3 and increased urea excretion may contribute to this impairment.
Glucocorticoid hormones are known to exert in vivo effects on body water homeostasis.1 Glucocorticoid deficiency is associated with impaired urinary dilution and water retention, leading to hyponatremia.2 With experimental glucocorticoid deficiency, increased plasma vasopressin and aquaporin 2 (AQP2) water channel expression were associated with water retention and were reversed by a vasopressin V2 receptor antagonist.2 Experimental glucocorticoid deficiency also exhibited impaired maximal urinary concentration, which was associated with a downregulation of AQP2.3
The role of the excessive glucocorticoid hormone, as occurs with Cushing syndrome, is also known to be associated with alteration in body water homeostasis, but the molecular mechanisms have not been studied. It has been shown, however, that patients as well as experimental animals demonstrated polyuria in the presence of excess of glucocorticoid hormone.1,4,5
Experimental studies were therefore undertaken to examine urinary dilution and concentration in the presence of glucocorticoid excess. These studies involved the molecular analysis of water channels and ion and urea transporters in control and excess glucocorticoid state.
RESULTS
Systemic and Renal Hemodynamics and Urinary Excretion with Glucocorticoid Excess
Compared with control rats, rats given dexamethasone (Dex; 10 μg/100 g body wt) for 14 d exhibited an increased mean arterial pressure (MAP; 119 ± 5 versus 105 ± 3 mmHg), but serum or urinary glucose concentrations were not different (Table 1). In preliminary studies, Dex administered at a dosage of 100 μg/100 g body wt by subcutaneous injection for 5 d caused hyperglycemia and glucosuria (data not shown). Because hyperglycemia and glucosuria are known to impair urinary dilution and concentration, the lower dosage of Dex (10 μg/100 g body wt) was used in these studies. Renal blood flow (RBF) and estimated clearance of creatinine were also increased in Dex-treated rats. Dex-treated rats also exhibited significant increased urine flow rate, urinary sodium excretion, and urinary urea excretion after 14 d of chronic Dex treatment. Solute-free water reabsorption (TCH2O) was increased significantly in Dex-treated rats as compared with controls. Serum and urinary osmolality and urea concentrations and fractional excretion of water and sodium were not different between Dex-treated and control groups (Table 1).
Table 1.
Characteristics of Dex-treated rats and control rats on day 14 of study (protocol 1)a
| Characteristic | CTR | Dex | P |
|---|---|---|---|
| n | 6 | 6 | |
| MAP (mmHg) | 105 ± 3 | 119 ± 5 | <0.05 |
| RBF (ml/min) | 4.4 ± 0.1 | 5.6 ± 0.3 | <0.01 |
| ClCrea (ml/min per kg body wt) | 6.4 ± 0.4 | 12.6 ± 1.7 | <0.01 |
| SOsm (mOsm/kgH2O) | 308 ± 1 | 307 ± 1 | NS |
| SUrea (mg/dl) | 12 ± 1 | 13 ± 1 | NS |
| SGlucose (mg/dl) | 169 ± 17 | 155 ± 6 | NS |
| UGlucose (mg/dl) | 13 ± 4 | 18 ± 8 | NS |
| Urine flow rate (μl/min per kg body wt) | 15 ± 1 | 27 ± 3 | <0.001 |
| UOsm (mOsm/kgH2O) | 2410 ± 206 | 2170 ± 154 | NS |
| UUrea (mg/dl) | 2533 ± 262 | 2950 ± 267 | NS |
| TCH2O (μl/min per kg body wt) | 99 ± 7 | 157 ± 15 | <0.05 |
| FEH2O (%) | 18 ± 1 | 16 ± 1 | NS |
| UNaUVol (μmol/min per kg body wt) | 3.0 ± 0.1 | 5.5 ± 1.1 | <0.05 |
| FENa (%) | 0.34 ± 0.02 | 0.35 ± 0.09 | NS |
| UUreaUVol (mg/min per kg body wt) | 0.37 ± 0.03 | 0.77 ± 0.05 | <0.0001 |
Data are mean ± SEM. ClCrea, creatinine clearance; CTR, control; FEH2O, fractional water excretion; FENa, fractional excretion of sodium; SGlucose and UGlucose, serum and urine glucose concentrations; SOsm, serum osmolality; SUrea, serum urea; UNaUVol, urinary sodium excretion; UOsm, urine osmolality; UUrea, urine urea; UUreaUVol, urinary urea excretion.
Water Channels and Ion and Urea Transporters with Glucocorticoid Excess
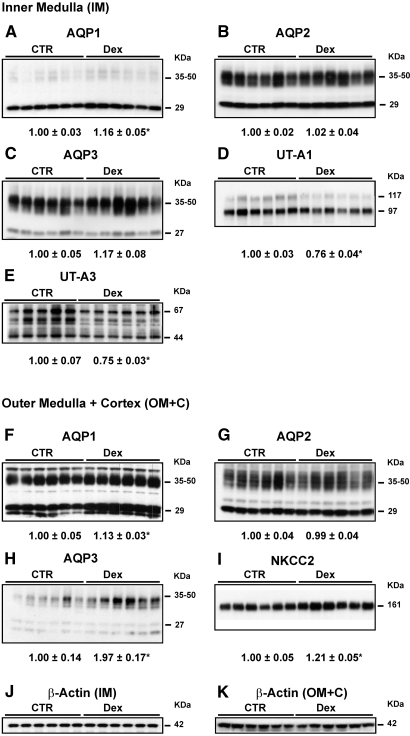
The protein abundance of AQP1 in the inner medulla (IM; Figure 1A) and outer medulla plus cortex (OM+C; Figure 1F), AQP3 in the OM+C (Figure 1H), and Na-K-2Cl co-transporter (NKCC2) in the OM+C (Figure 1I) was significantly increased and the abundance of UT-A1 (Figure 1D) and UT-A3 (Figure 1E) was significantly decreased in the IM of Dex-treated rats. There was no difference of AQP2 in the IM (Figure 1B) and OM+C (Figure 1G), of AQP3 in the IM (Figure 1C), of β-actin in the IM (Figure 1J), and of OM+C (Figure 1K) as loading control between Dex-treated and control rats.
Figure 1.
Semiquantitative immunoblots of AQP1, 2, and 3; urea transporters UT-A1 and UT-A3; and NKCC2 using kidney proteins (same protein amounts were loaded) prepared from IM and OM+C of both Dex-treated and controls (CTR; protocol 1). Dex-treated rats showed a significant increase in protein abundance of renal AQP1 (29 kD, 35 to 50 kD) in the IM (A) and OM+C (F) and of AQP3 (27 kD, 35 to 50 kD) in the OM+C (H) and NKCC2 (161 kD) in the OM+C (I) and reduced expression levels of UT-A1 (97 kD, 117 kD) in the IM (D) and UT-A3 (a protein “smear” between 44 and 67 kD) in the IM (E) compared with CTR rats. β-Actin (42 kD) in the IM (J) and OM+C (K) did not show any difference between CTR and Dex-treated rats. Western immunoblots shown were representative of blots performed with a total sample size of n = 6 rats in CTR and n = 6 rats in Dex group. *P < 0.05 versus controls.
Urinary Water Excretion during Glucocorticoid Excess
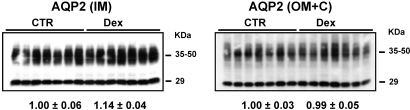
To investigate the urinary diluting ability in the glucocorticoid excess state, Dex-treated and control rats were challenged with an acute oral water load (40 ml/kg). With oral water loading, Dex-treated rats had a significantly increased percentage of water excretion at 1 h (58 ± 4 versus 36 ± 7%; P < 0.05) as compared with controls 1 h after completion of the water load. The protein levels of AQP2 in the IM and OM+C were no different between control and Dex-treated rats (Figure 2). Similar to the baseline results, chronic Dex-treated rats exhibited higher MAP, creatinine clearance, RBF, and urinary sodium and urea excretion. Similarly, the abundance levels of AQP1, AQP3, and NKCC2 were increased, and the expression of UT-A1 and UT-A3 was decreased as compared with control rats (data not shown).
Figure 2.
Semiquantitative immunoblots of AQP2 using kidney proteins prepared from IM and OM+C of both Dex-treated and CTR rats after acute water load (40 ml/kg). The protein levels of AQP2 in the IM and OM+C were no different in CTR and Dex-treated rats.
Urinary Concentration Capacity during Glucocorticoid Excess
After chronic Dex injection for 14 d, all rats then were subjected to a 36-h period of fluid deprivation (rats had free access to food, but no water allowed). Dex-treated rats demonstrated a significantly higher percentage of body weight loss and urine flow rate than control rats. Urine osmolality was also significantly decreased in Dex-treated rats in response to water deprivation as compared with control rats. Urinary sodium excretion did not change and urinary urea excretion was significantly increased in Dex-treated rats after water deprivation as compared with control rats. Serum osmolality and glucose concentrations, creatinine clearance, and fractional excretion of sodium were not different between the two study groups (Table 2).
Table 2.
For Dex-treated rats, 36 h of water deprivation resulted in an increase in urine volume and urinary urea excretion and a decrease in maximal urine osmolalitya
| Parameter | CTR | Dex | P |
|---|---|---|---|
| n | 7 | 7 | |
| % loss in body weight (%) | 6.3 ± 0.9 | 10.4 ± 1.1 | <0.001 |
| SOsm (mOsm/kgH2O) | 313 ± 3 | 314 ± 2 | NS |
| UVol before dehydration (μl/min per kg body wt) | 21 ± 2 | 35 ± 4 | <0.0001 |
| UVol after dehydration (μl/min per kg body wt) | 7 ± 1 | 11 ± 1 | <0.05 |
| UOsm after dehydration (mOsm/kgH2O) | 4017 ± 158 | 3580 ± 122 | <0.05 |
| UNaUVol (μmol/min per kg body wt) | 3.3 ± 0.2 | 2.9 ± 0.1 | NS |
| FENa (%) | 0.57 ± 0.06 | 0.48 ± 0.05 | NS |
| UUreaUVol (mg/min per kg body wt) | 0.24 ± 0.01 | 0.45 ± 0.03 | <0.0001 |
Data are means ± SEM.
Water Channels and Ion and Urea Transporters during Fluid Deprivation
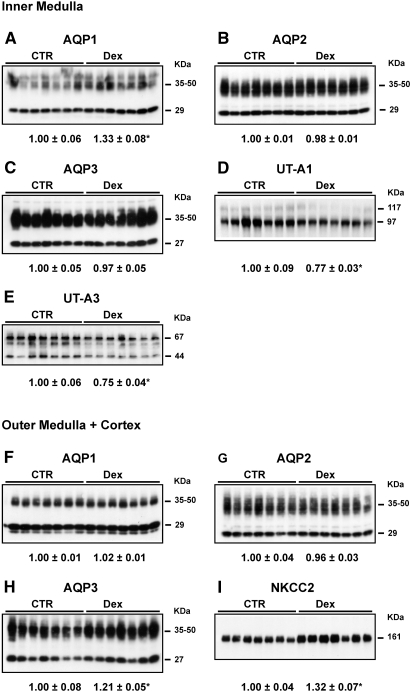
After fluid deprivation, AQP1 in the IM, AQP3 in the OM+C, and NKCC2 in the OM+C were significantly increased in Dex-treated versus control rats. The protein expression levels of UT-A1 and UT-A3 in the IM, however, were significantly decreased in Dex-treated rats. There was no difference in the protein expression of AQP1 in the OM+C, AQP2 in the IM and OM+C, AQP3 in the IM (Figure 3), and β-actin levels in the IM and OM+C (data not shown) between Dex-treated and control rats after water deprivation.
Figure 3.
Semiquantitative immunoblots of AQP1, 2, and 3; UT-A1; UT-A3; and NKCC2 using kidney proteins prepared from IM and OM+C of both Dex-treated and CTR rats after 36 h of water deprivation (protocol 3). Dex-treated rats showed a significant increase in protein abundance of renal AQP1 (A) and AQP3 in the OM+C (H) and of NKCC2 in the OM+C (I) and decreased expression levels of UT-A1 in the IM (D) and of UT-A3 in the IM (E) compared with CTR rats. Western immunoblots shown were representative of blots performed with a total sample size of n = 7 rats in CTR and n = 7 rats in Dex group. *P < 0.05 versus controls.
Paired Food and Water Intake Study in Glucocorticoid Excess
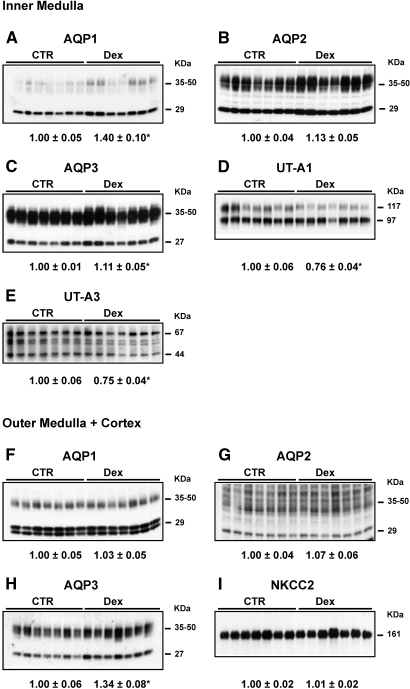
Because Dex-treated rats weighed less (192 ± 6 versus 273 ± 6 g), ate less food (14.5 ± 0.4 versus 19.8 ± 0.3 g/d), and drank less water (18 ± 1 versus 23 ± 1 ml/d) compared with controls at day 14, paired food and water studies were performed. The Dex-treated groups had free access to water and food for 14 d. The control rats received the same food and water as ingested by the Dex-treated rats. In these paired studies, Dex-treated rats still exhibited increased MAP, urine flow rate, urinary sodium and urea excretion, TCH2O, and fractional excretion of sodium (Table 3). As with the nonpaired studies, immunoblots demonstrated increased expression levels of AQP1 in the IM and AQP3 in the IM and OM+C, whereas decreased abundance of UT-A1 and UT-A3 protein occurred in the IM (Figure 4) in the Dex-treated rats as compared with pair-fed controls; however, AQP2 protein levels in the IM and OM+C and NKCC2 protein levels in the OM+C were not different between Dex-treated and control rats (Figure 4). β-Actin levels in the IM and OM+C did not change between control and Dex-treated rats (data not shown).
Table 3.
Characteristics of Dex-treated rats and control rats on day 14 of study after pair food and water intakea
| Parameter | CTR | Dex | P |
|---|---|---|---|
| n | 8 | 8 | |
| Body weight (g) | 230 ± 4 | 188 ± 5 | <0.0001 |
| Food intake (g) | 15 | 15 | NS |
| Water intake (ml) | 20 ± 1 | 20 ± 1 | NS |
| MAP (mmHg) | 97 ± 5 | 110 ± 2 | <0.05 |
| ClCrea (ml/min per kg body wt) | 9.5 ± 0.9 | 9.4 ± 1.6 | NS |
| SOsm (mOsm/kgH2O) | 310 ± 1 | 313 ± 2 | NS |
| SUrea (mg/dl) | 18 ± 1 | 16 ± 1 | NS |
| Urine flow rate (μl/min per kg body wt) | 27 ± 2 | 39 ± 4 | <0.05 |
| UUrea (mg/dl) | 1425 ± 182 | 2163 ± 240 | <0.05 |
| TCH2O (μl/min per kg body wt) | 95 ± 9 | 149 ± 7 | <0.01 |
| FEH2O (%) | 0.17 ± 0.02 | 0.28 ± 0.03 | <0.05 |
| UNaUVol (μmol/min per kg body wt) | 3.3 ± 0.2 | 4.5 ± 0.3 | <0.01 |
| FENa (%) | 0.25 ± 0.02 | 0.49 ± 0.04 | <0.0001 |
| UUreaV (mg/min per kg body wt) | 0.38 ± 0.06 | 0.79 ± 0.06 | <0.0001 |
Data are mean ± SEM.
Figure 4.
Semiquantitative immunoblots of AQP1, 2, and 3; UT-A1; UT-A3; and NKCC2 using kidney proteins prepared from IM and OM+C of both Dex-treated and CTR rats after pair food and water intake (protocol 4). As compared with CTR, AQP1 in the IM and AQP3 in the IM and OM+C were increased, UT-A1 and UT-A3 were decreased, and there were no changes in AQP2 and NKCC2 expression in Dex-treated rats. Western immunoblots shown were representative of blots performed with a total sample size of n = 7 rats in CTR and n = 7 rats in Dex group. *P < 0.05 versus controls.
DISCUSSION
As with Cushing syndrome, glucocorticoid excess in this study was associated with hypertension and increased renal function and urine flow as compared with controls.1,4 The greater urine output in the glucocorticoid-treated rats persisted during 36 h of fluid deprivation, thus excluding an effect of increased thirst and fluid intake as a cause of the increased urine output. There were, however, several other possibilities to explain the increased urine output associated with glucocorticoid excess. Elegant knockout studies in mice have shown that nephrogenic diabetes insipidus occurs in AQP16 and AQP37 knockout mice and renal collecting duct–selective AQP2 conditional knockout mice.8 Thus, downregulation of these water channels during excess glucocorticoid hormone could have contributed to the increased urine flow rate. Our results, however, demonstrated an upregulation of AQP1 and AQP3 in the glucocorticoid excess. Most important, the protein expression of AQP2 water channel in the principal cells of the collecting duct are vasopressin dependent and are therefore the primary regulator of renal water excretion.9 In this study, however, the increased urine output of glucocorticoid excess was not associated with any alteration in AQP2 expression. Thus, the increased urine output could not be explained by alterations in renal AQP1, 2, or 3 water channels.
Of importance, however, the UT-A1 urea transporter expression was downregulated in the glucocorticoid excess state. This finding is compatible with previous studies by Sands et al.,10 in which glucocorticoid replacement (Dex) to adrenalectomized rats for 7 d downregulated the expression of the vasopressin-regulated urea transporter UT-A1 in rat terminal IM collecting ducts. Previous studies of UT-A1/3 knockout mice also exhibited impaired urinary concentration.11 In this study, the downregulation of the urea transporter UT-A1 and UT-A3 with 14 d of glucocorticoid excess was supported by the associated increase in urea excretion. The catabolic effect of glucocorticoid excess also could have contributed to the increase in urinary urea excretion. With the same food including protein intake in the paired studies, the downregulation of the urea transporters and increased urea excretion in the Dex-treated rats is compatible with a decrease in IM urea concentration. Although extracellular fluid expansion has been shown to decrease UT-A1 abundance,12 the persistent downregulation of this transporter in the paired food and water studies makes this possibility less likely.
The results of this study also demonstrated that glucocorticoid excess was associated with an increased response to an acute water load compared with control rats. This increase in water excretion occurred despite no difference in renal AQP2 expression in the glucocorticoid excess as compared with the control animals. Thus, this increase in response to an acute water load was likely related to the increase in MAP and thus renal perfusion pressure. This effect of increased renal arterial pressure to increase urine flow was originally described as a “druck diuresis,” or pressure diuresis.13,14 An increase in estimated GFR in the Dex-treated rats as compared with control rats undoubtedly also contributed to the increased urine flow rate. The comparable fractional water excretion in glucocorticoid excess versus controls supports this possibility. A resultant increase in tubular flow also may have contributed to the upregulation of AQP1 and NKCC2 in the glucocorticoid excess state.
As already noted, studies were then undertaken to examine the effect of glucocorticoid excess on maximal urinary concentration after 36 h of fluid deprivation. The increased percentage of body weight loss and increased urine volume as well as decreased maximal urine osmolality during fluid deprivation indicated impaired urinary concentration in the glucocorticoid excess as compared with control rats; however, the concentrating defect was modest and would become clinically relevant only with a prolonged period (e.g., 36 h) of absence of fluid intake, as was the case in this study. The associated increase in TCH2O in the Dex-treated rats was compatible with a decrease in proximal fluid reabsorption with increase distal sodium and water delivery as occurs with an increase in renal perfusion pressure.13 It should also be noted that an increase in collecting duct flow can decrease maximal urinary concentration.15
The molecular analysis of the potential mechanisms of the impaired maximal urinary concentration with glucocorticoid excess demonstrated no difference in AQP2 water channel as compared with control rats. Moreover, AQP1 and AQP3 water channels were significantly higher in the glucocorticoid excess state. The results were consistent in both the ad libitum and paired studies in demonstrating downregulation of urea transporters. Specifically, after 14 d of glucocorticoid excess, the impaired urinary concentration was associated with a decrease in UT-A1 and UT-A3 expression and an increase in urinary urea excretion. It is known that urea accumulation in the IM is a major contributor to maximal interstitial osmolality and thus would be diminished with decreased urea transporters in the medullary collecting duct. Increased mean arterial pressure and potential response of steroid receptor with glucocorticoid is in need of further study with respect to the downregulation of urea transporters UT-A1 and UT-A3.
In summary, glucocorticoid excess was associated with increased urine flow rate in the absence of downregulation of renal water channels, including AQP1, 2, and 3. The increased response to an acute water load in glucocorticoid excess as compared with control rats was independent of differences in water channels but was associated with increased renal arterial pressure and GFR, estimated by creatinine clearance. The impaired urinary concentration after 36 h of fluid deprivation during glucocorticoid excess occurred despite no downregulation of renal water channels or NKCC2 co-transporter, the initiator of the countercurrent concentrating mechanism. There was, however, a consistent downregulation of UT-A1 and UT-A3, known important transporters for maximal urinary concentration.
CONCISE METHODS
Experimental Animals
The study protocol was approved by the University of Colorado Institutional Animal Care and Use Committee. Male Sprague-Dawley (SD) rats weighing 150 to 175 g were allowed to acclimate to Denver's altitude (1500 m) for 4 d before any experimental protocols. All rats underwent acclimation to metabolic cages for a continuous 2-d period before initiation of study. The rats were housed individually in metabolic cages and exposed to a 12-h light-dark cycle and constant ambient temperature.
Protocol 1.
Twelve rats were divided randomly into two study groups: Dex-treated group (n = 6) and control (n = 6). SD rats were treated with dexamethasone (Research Plus, Manasquan, NJ; 10 μg/100 g body wt, dissolved in peanut oil) by subcutaneous injection for 14 d.
Protocol 2.
The rats underwent the same Dex treatment as described for protocol 1. On day 14, the control (n = 7) and Dex-treated (n = 7) rats were subjected to an acute oral water load (40 ml/kg) for determining the effect of Dex on urinary diluting ability.
Protocol 3.
The rats underwent the same Dex treatment as described for protocol 1. On day 14, the control (n = 7) and Dex-treated (n = 7) rats were subjected to a 36-h period of water deprivation to examine the effect of Dex on urinary concentrating ability.
Protocol 4.
The rats underwent the same Dex treatment as described for protocol 1. The Dex-treated groups had free access to water and food for 14 d. For exclusion of the effects of less food and water intake in Dex-treated rats, all rats were pair-fed, and the amount of water given to control rats (n = 8) was restricted to the same daily consumption of Dex-treated rats (n = 8).
Measurement of MAP and RBF
For protocol 1, rats underwent MAP and RBF measurements.16 The rats were anesthetized with ketamine (40 mg/kg body wt intraperitoneally) and xylazine (5 mg/kg body wt intraperitoneally). MAP was measured via a carotid artery catheter connected to a Transpac IV transducer and monitored continuously using Windaq Waveform recording software (Dataq Instruments, Akron, OH). For RBF measurement, the kidney was exposed by a left subcostal incision and was dissected free from perirenal tissue, and renal arteries were isolated for the determination using a blood flowmeter and probe (0.5v; Transonic Systems, Ithaca, NY).
Biochemical Measurements
Urine was collected for all protocols, and urine osmolality and creatinine were measured. Rats were killed by decapitation. Trunk blood was collected for serum osmolality and serum sodium and creatinine concentrations. Serum and urine osmolality were measured by freezing-point depression (Advanced Instruments, Norwood, MA). Serum and urine creatinine were measured by using a Creatinine Analyzer 2 (Beckman Instruments, Fullerton, CA). Serum and urine sodium, urea, and glucose concentrations were measured by flame photometry.
Protein Isolation
After decapitation, kidneys were removed and placed in ice-cold isolation solution containing 250 mM sucrose, 25 mM imidazole, and 1 mM EDTA (pH 7.2) with 0.1 vol% protease inhibitors (0.7 μg/ml pepstatin, 0.5 μg/ml leupeptin, and 1 μg/ml aprotinin) and 200 μM PMSF.17 Kidneys were dissected on ice into OM+C and IM regions. Tissue samples were immediately homogenized in a glass homogenizer at 4°C. This homogenate was centrifuged in a Hermle Labnet Z323K centrifuge (Labnet International, Woodbridge, NJ) at 4000 × g for 15 min at 4°C, and the supernatant was pipetted off and solubilized at 65°C for 15 min in Laemmli sample buffer containing 2% SDS and then stored at −20°C. Protein concentration was determined for each sample by the Bradford method (Bio-Rad, Richmond, CA). Tissue protein was used for immunoblotting for aquaporin water channels and sodium and urea transporters.
Electrophoresis and Immunoblotting
Samples of membrane fractions were run on 4 to 12% or 12% acrylamide gels using methods described previously in detail.18 For each gel, an identical gel was run in parallel and subjected to Coomassie staining for confirming equal loading of protein. The other gel was subjected to Western blotting analysis. Protein samples loaded in each lane were the same according to protein concentration. After transfer by electroelution to nitrocellulose membranes, blots were blocked with 5% milk in PBS-T (80 mM Na2HPO4, 20 NaH2PO4, 100 mM NaCl, and 0.1% Tween 20 [pH 7.5]) for 1 h and incubated with primary antibodies (see next section) overnight at 4°C. After washing with PBS-T, the blots were incubated with horseradish peroxidase–conjugated secondary antibody (P448; Dako A/S, Glostrup, Denmark; diluted 1:3000). After a final washing as already detailed, antibody binding was visualized using the ECL (enhanced chemiluminescence) system (Amersham Int., Buckinghamshire, UK).
Primary Antibodies
For semiquantitative immunoblotting and immunocytochemistry, antibodies to AQP2 and AQP3 have been previously characterized.19,20 Anti-AQP1 was obtained from BD Biosciences Pharmingen (San Jose, CA). Anti–β-actin was obtained from Sigma-Aldrich Corp. (St. Louis, MO). Anti–Na-K-2Cl antibody was provided by Dr. Mark A. Knepper (Bethesda, MD). Anti–UT-A110 and UT-A3 (NH2-terminal UT-A1 and UT-A3)21 antibodies were provided by Dr. Jeff Sands (Atlanta, GA).
Statistical Analyses
Values are presented as means ± SE. Comparisons between two groups were made by unpaired t test. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by the National Institutes of Health (DK19928).
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ferrari P: Cortisol and the renal handling of electrolytes: Role in glucocorticoid-induced hypertension and bone disease. Best Pract Res Clin Endocrinol Metab 17: 575–589, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Li C, Summer SN, Falk S, Cadnapaphornchai MA, Chen YC, Schrier RW: Molecular analysis of impaired urinary diluting capacity in glucocorticoid deficiency. Am J Physiol Renal Physiol 290: F1135–F1142, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Chen YC, Cadnapaphornchai MA, Summer SN, Falk S, Li C, Wang W, Schrier RW: Molecular mechanisms of impaired urinary concentrating ability in glucocorticoid-deficient rats. J Am Soc Nephrol 16: 2864–2871, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kalimi M: Role of antiglucocorticoid RU 486 on dexamethasone-induced hypertension in rats. Am J Physiol 256: E682–E685, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Thunhorst RL, Beltz TG, Johnson AK: Glucocorticoids increase salt appetite by promoting water and sodium excretion. Am J Physiol Regul Integr Comp Physiol 293: R1444–R1451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS: Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem 273: 4296–4299, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS: Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci U S A 97: 4386–4391, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojek A, Fuchtbauer EM, Kwon TH, Frokiaer J, Nielsen S: Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci U S A 103: 6037–6042, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrier RW: Body water homeostasis: Clinical disorders of urinary dilution and concentration. J Am Soc Nephrol 17: 1820–1832, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Naruse M, Klein JD, Ashkar ZM, Jacobs JD, Sands JM: Glucocorticoids downregulate the vasopressin-regulated urea transporter in rat terminal inner medullary collecting ducts. J Am Soc Nephrol 8: 517–523, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA: Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci U S A 101: 7469–7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XY, Beutler K, Nielsen J, Nielsen S, Knepper MA, Masilamani S: Decreased abundance of collecting duct urea transporters UT-A1 and UT- A3 with ECF volume expansion. Am J Physiol Renal Physiol 282: F577–F584, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Schrier RW, De Wardener HE: Tubular reabsorption of sodium ion: Influence of factors other than aldosterone and glomerular filtration rate. 1. N Engl J Med 285: 1231–1243, 1971 [DOI] [PubMed] [Google Scholar]
- 14.Selkurt EE, Womack I, Dailey WN: Mechanism of natriuresis and diuresis during elevated renal arterial pressure. Am J Physiol 209: 5–99, 1965 [DOI] [PubMed] [Google Scholar]
- 15.Stephenson JL: Countercurrent transport in the kidney. Annu Rev Biophys Bioeng 7: 315–339, 1978 [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Li C, Summer SN, Falk S, Schrier RW: Polyuria of thyrotoxicosis: Downregulation of aquaporin water channels and increased solute excretion. Kidney Int 72: 1088–1094, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Li C, Wang W, Summer SN, Westfall TD, Brooks DP, Falk S, Schrier RW: Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol 19: 225–232, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Wang W, Summer SN, Cadnapaphornchai MA, Falk S, Umenishi F, Schrier RW: Hyperosmolality in vivo upregulates aquaporin 2 water channel and Na-K-2Cl co-transporter in Brattleboro rats. J Am Soc Nephrol 17: 1657–1664, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Terris J, Ecelbarger CA, Nielsen S, Knepper MA: Long-term regulation of four renal aquaporins in rats. Am J Physiol 271: F414–F422, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Xu DL, Martin PY, Ohara M, St. John J, Pattison T, Meng X, Morris K, Kim JK, Schrier RW: Upregulation of aquaporin-2 water channel expression in chronic heart failure rat. J Clin Invest 99: 1500–1505, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM: Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007 [DOI] [PubMed] [Google Scholar]