Abstract
Na,K-ATPase is ubiquitously expressed and is essential for maintaining electrochemical and osmotic gradients. The α subunit of Na,K-ATPase is the receptor for cardiotonic steroids, which act through the ouabain-binding site and are important in cardiovascular regulation. Interestingly, the presence of endogenous Na,K-ATPase ligands has been implicated in the natriuretic response to perturbations such as hypertension and salt loading; therefore, it is important to characterize the role of the ouabain-binding sites in this context. Because the α1 isoform of mice and rats is relatively ouabain resistant, gene-targeting strategies were used to produce mice with reversed responses of the α1 and/or α2 isoforms to ouabain to assess for altered natriuretic responses to acute salt loading. Regardless of the sensitivity of the α2 isoform to ouabain, conferring ouabain sensitivity to α1 augmented the natriuretic response to an acute salt load. In addition, when endogenous Na,K-ATPase inhibitors were sequestered with an anti-digoxin antibody fragment, the sodium excretion rates in the ouabain-sensitive α1 isoform mice were equivalent to the ouabain-resistant α1 isoform mice. These data suggest that the ouabain-binding site of the α1 Na,K-ATPase can participate in the natriuretic response to a salt load by responding to endogenous Na,K-ATPase ligands.
Na,K-ATPase, a heteromeric membrane protein complex consisting of α, β, and FXYD subunits, is essential for maintenance of electrochemical and osmotic gradients.1 The α subunit is responsible for the cation transporting activities of the enzyme as well as the ATPase activity, which generates the energy required for active transport. There are four known α isoforms: α1 is found ubiquitously; α2 is expressed in the brain as well as cardiac, skeletal, and vascular smooth muscle; α3 is expressed in neurons and ovaries; and α4 is expressed in the testis.2–4 Because of the tissue-specific distribution of the α isoforms, it is thought that they may have differential physiologic roles determined in part by their cellular distribution, regulation, and catalytic activities.3,5,6
The Na,K-ATPase α isoforms contain a highly conserved ouabain-binding site that mediates sensitivity to inhibition by cardiotonic steroids, such as ouabain and digoxin. In most mammals, all of the α subunits are sensitive to ouabain inhibition, a notable exception being the α1 isoform in rats and mice, which is relatively resistant.7–9 Remarkably, the ouabain sensitivity of the α subunit is determined primarily by two amino acids located at positions 111 and 122 in the first extracellular loop.10 Our laboratory has developed mouse models in which the sensitivities of the α1 and α2 isoforms to ouabain have been reversed through the substitution of these amino acids. These animals make it possible to explore the relevance of the ouabain-binding site in physiologic situations.11–15
It is well established that changes in Na,K-ATPase activity can importantly influence cardiovascular function, because inhibition of the α isoform increases contractility in both cardiac and vascular smooth muscle.6,16–18 In addition, a variety of studies indicate that endogenous Na,K-ATPase inhibitors, or endogenous cardiotonic steroids that interact directly with the α subunit, can play an important role in regulating cardiovascular function and BP.6,11–13,19 For example, we previously showed that mutation of the ouabain-binding site of the α2 subunit, from sensitive to resistant, results in mice that are resistant to ACTH-induced hypertension.11,14 This observation is most easily understood in terms of an endogenous ligand hormone being released during ACTH treatment that then interacts with the high-affinity ouabain-binding site of the native α2 isoform to increase BP.
Although our previous studies explored the contribution of the ouabain-binding site in regulating cardiac and vascular function, the critical role of the kidney in long-term BP regulation has not been studied in these mice. Epithelial solute reabsorption in the kidney is ultimately dependent on the activity of the Na,K-ATPase α1 isoform, and endogenous cardiotonic steroids have been reported to be released in a variety of conditions involving altered renal Na+ excretion.20–25 Our genetically modified animal models can contribute to our understanding of this regulatory mechanism by acting as a detection system to verify the existence of endogenous Na,K-ATPase ligands; therefore, it is important to examine the role of the α1 isoform ouabain-binding site in the renal response to salt challenge. To accomplish this, we monitored kidney function in three groups of mice with various ouabain sensitivities in the α isoforms: α1-resistant/α2-sensitive (wild type; α1R/Rα2S/S), double-mutant α1-sensitive/α2-resistant (α1S/Sα2R/R), and, as controls, single-mutant α1-resistant/α2-resistant (α1R/Rα2R/R). Our data indicate that α1S/Sα2R/R mice are able to excrete more readily an acute saline load, suggesting that the interaction of endogenous cardiotonic steroids with a high-affinity α1 isoform can importantly influence salt and water homeostasis.
RESULTS
Ouabain Infusion Generates an Augmented Natriuretic Response in α1S/Sα2R/R Animals
To evaluate the effects of exogenous cardiac glycosides on Na+ excretion in animals expressing the various forms of the α Na,K-ATPase isoforms, we monitored renal function before and during a constant infusion of ouabain. We hypothesized that ouabain treatment would inhibit renal epithelial transport to a greater extent in α1S/Sα2R/R mice compared with control mice and thereby produce a greater natriuresis. Basic physiologic variables are shown in Table 1. Although basal BP seemed to be somewhat lower in α1R/Rα2R/R mice, the differences were not statistically significant (P = 0.24, by Tukey post hoc analysis) and there were no consistent differences in the other experiments (Tables 2 and 3). Ouabain infusion caused a significant increase in BP in α1S/Sα2R/R mice but had no effect on BP in α1R/Rα2S/S or α1R/Rα2R/R mice, a finding that reflects an important role for the α1 isoform in maintaining cardiovascular function. Heart rate increased significantly in each of the groups. Ouabain treatment also markedly increased plasma K+ concentration in α1R/Rα2S/S (P < 0.001) and α1S/Sα2R/R (P < 0.001) mice but not in α1R/Rα2R/R mice (P = 0.981). Although the reason for this is not clear, it is possible that ouabain treatment may disrupt K+ stores in skeletal muscle expressing either of the sensitive α isoforms.26 Basal GFR was not different among genotypes in this experiment (Table 1) or in the other experiments in this study (Tables 2 and 3). By the end of the second treatment period, ouabain infusion caused a substantial decrease in GFR in only the α1S/Sα2R/R mice, likely as a result of the accumulation of circulating levels of ouabain.
Table 1.
Physiologic measurements before and during ouabain infusiona
| Parameter | MAP (mmHg) | HR (bpm) | Hct (%) | PNa (mEq/L) | PK (mEq/L) | GFR (μl/min per g) |
|---|---|---|---|---|---|---|
| α1R/Rα2R/R (n = 7) | ||||||
| basal | 85 ± 3 | 489 ± 15 | 48 ± 2 | 155 ± 2 | 4.6 ± 0.4 | 1045 ± 125 |
| saline 1 | 85 ± 3 | 559 ± 16 | 48 ± 2 | 160 ± 1 | 4.6 ± 0.5 | 1156 ± 124 |
| saline 2 | 81 ± 4 | 563 ± 19 | 48 ± 2 | 156 ± 1 | 4.7 ± 0.4 | 1129 ± 156 |
| α1S/Sα2R/R (n = 9) | ||||||
| basal | 95 ± 5 | 458 ± 12 | 49 ± 1 | 163 ± 1 | 5.0 ± 0.2 | 956 ± 82 |
| saline 1 | 110 ± 5b,c | 496 ± 14 | 51 ± 1 | 161 ± 1 | 6.3 ± 0.3b,c | 1033 ± 85 |
| saline 2 | 107 ± 4b,c | 476 ± 10 | 51 ± 1 | 160 ± 3 | 7.2 ± 0.4b,c | 528 ± 136b,c |
| α1R/Rα2S/S (n = 8) | ||||||
| basal | 93 ± 4 | 438 ± 25 | 49 ± 1 | 158 ± 2 | 4.5 ± 0.1 | 1224 ± 68 |
| saline 1 | 99 ± 4 | 500 ± 37 | 52 ± 1 | 160 ± 2 | 4.9 ± 0.2 | 1165 ± 81 |
| saline 2 | 94 ± 5 | 506 ± 47 | 52 ± 1 | 157 ± 2 | 6.7 ± 0.4b,c | 1164 ± 112 |
| Two-way ANOVA | ||||||
| group | 0.005 | 0.140 | 0.180 | 0.160 | 0.003 | 0.037 |
| treatment | 0.005 | <0.001 | 0.024 | 0.022 | <0.001 | 0.018 |
| group × treatment | 0.038 | 0.200 | 0.340 | 0.110 | <0.001 | 0.003 |
Hct, hematocrit; HR, heart rate; PNa and PK, plasma concentration of Na+ and K+.
Post-hoc analyses:
P < 0.05 versus basal level in same group;
P < 0.05 versus corresponding level in α1R/Rα2R/R.
Table 2.
Physiologic measurements before and during intravenous saline load
| Parameter | MAP (mmHg) | HR (bpm) | Hct (%) | PNa (mEq/L) | PK (mEq/L) | GFR (μl/min per g) |
|---|---|---|---|---|---|---|
| α1R/Rα2R/R (n = 7) | ||||||
| basal | 82 ± 1 | 421 ± 28 | 51 ± 1 | 159 ± 1 | 4.3 ± 0.3 | 1259 ± 162 |
| saline 1 | 83 ± 2 | 452 ± 24 | 48 ± 1 | 158 ± 1 | 4.6 ± 0.3 | 1267 ± 183 |
| saline 2 | 86 ± 2 | 459 ± 20 | 47 ± 1 | 158 ± 2 | 4.8 ± 0.2 | 1315 ± 251 |
| α1S/Sα2R/R (n = 8) | ||||||
| basal | 85 ± 2 | 385 ± 11 | 49 ± 0 | 156 ± 1 | 4.4 ± 0.2 | 1115 ± 79 |
| saline 1 | 88 ± 2 | 438 ± 13 | 48 ± 1 | 157 ± 1 | 4.7 ± 0.3 | 1179 ± 64 |
| saline 2 | 91 ± 3 | 446 ± 17 | 47 ± 1 | 155 ± 1 | 5.0 ± 0.3 | 1334 ± 83 |
| α1R/Rα2S/S (n = 11) | ||||||
| basal | 81 ± 2 | 409 ± 11 | 51 ± 1 | 157 ± 1 | 4.8 ± 0.2 | 1337 ± 46 |
| saline 1 | 79 ± 2 | 441 ± 15 | 47 ± 1 | 157 ± 1 | 4.8 ± 0.2 | 1347 ± 117 |
| saline 2 | 81 ± 2 | 449 ± 14 | 45 ± 1 | 157 ± 1 | 5.0 ± 0.2 | 1357 ± 135 |
| Two-way ANOVA | ||||||
| group | 0.011 | 0.670 | 0.510 | 0.440 | 0.730 | 0.700 |
| treatment | 0.070 | <0.001 | <0.001 | 0.270 | 0.003 | 0.012 |
| group × treatment | 0.433 | 0.370 | 0.180 | 0.470 | 0.590 | 0.190 |
Table 3.
Physiologic measurements before and during saline load in the presence of Digibind
| Parameter | MAP (mmHg) | HR (bpm) | Hct (%) | PNa (mEq/L) | PK (mEq/L) | GFR (μl/min per g) |
|---|---|---|---|---|---|---|
| α1R/Rα2R/R (n = 7) | ||||||
| basal | 90 ± 3 | 459 ± 24 | 50 ± 1 | 159 ± 4 | 4.2 ± 0.2 | 1259 ± 85 |
| saline 1 | 86 ± 3 | 491 ± 27 | 46 ± 1 | 160 ± 4 | 3.9 ± 0.2 | 1402 ± 95 |
| saline 2 | 87 ± 3 | 495 ± 25 | 44 ± 1 | 163 ± 4 | 4.4 ± 0.2 | 1219 ± 92 |
| α1S/Sα2R/R (n = 9) | ||||||
| basal | 88 ± 4 | 412 ± 23 | 52 ± 2 | 155 ± 4 | 4.1 ± 0.2 | 1155 ± 77 |
| saline 1 | 86 ± 2 | 437 ± 23 | 47 ± 2 | 157 ± 4 | 4.0 ± 0.2 | 1347 ± 82 |
| saline 2 | 81 ± 3 | 436 ± 24 | 45 ± 2 | 155 ± 3 | 4.1 ± 0.2 | 1358 ± 77 |
| α1R/Rα2S/S (n = 9) | ||||||
| basal | 83 ± 2 | 439 ± 23 | 50 ± 1 | 156 ± 4 | 4.1 ± 0.2 | 1337 ± 46 |
| saline 1 | 84 ± 3 | 468 ± 20 | 47 ± 1 | 158 ± 4 | 4.0 ± 0.2 | 1347 ± 117 |
| saline 2 | 84 ± 2 | 470 ± 18 | 45 ± 1 | 159 ± 3 | 4.4 ± 0.2 | 1357 ± 135 |
| Two-way ANOVA | ||||||
| group | 0.420 | 0.270 | 0.890 | 0.630 | 0.840 | 0.810 |
| treatment | 0.140 | <0.001 | <0.001 | 0.008 | <0.001 | 0.140 |
| group × treatment | 0.110 | 0.940 | 0.840 | 0.070 | 0.090 | 0.260 |
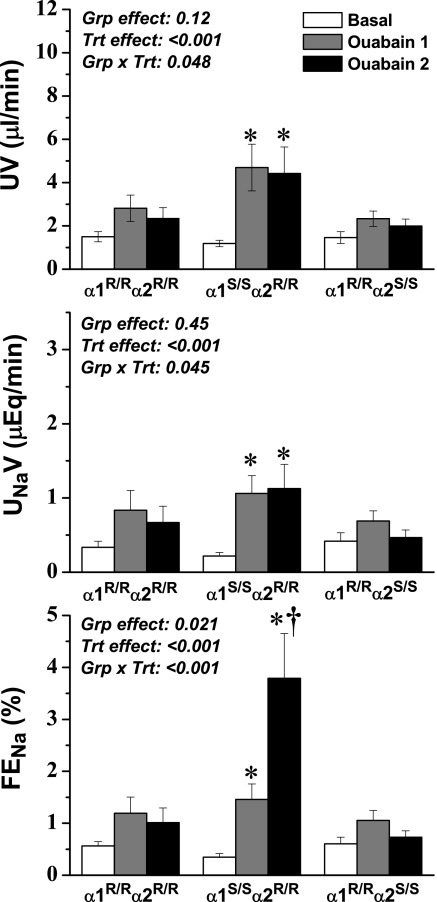
In terms of renal excretory function, ouabain treatment in α1S/Sα2R/R mice resulted in a significant increase in absolute and fractional Na+ excretion, as well as urine flow rate, whereas there was no significant effect in α1R/Rα2S/S and α1R/R α2R/R mice (Figure 1). Furthermore, although absolute Na+ excretion did not continue to increase through the second ouabain treatment period, fractional Na+ excretion increased dramatically in α1S/Sα2R/R mice. These data suggest that tubular Na+ reabsorption continues to decrease markedly with accumulating levels of circulating ouabain but that the expected increase in absolute Na+ excretion is offset by corresponding decreases in GFR. Nonetheless, these results indicate that the expression of an ouabain-sensitive α1 isoform in renal epithelial cells indeed renders them highly sensitive to circulating cardiotonic steroids.
Figure 1.
Urine flow rate (UV; top), absolute Na+ excretion rate (UNaV; middle), and fractional Na+ excretion (FENa; bottom) before and during ouabain infusion at 6.6 ng/min/per g body wt. Two steady-state control periods were averaged and are represented as one value (Basal); two subsequent ouabain treatment periods are represented individually because steady state was not achieved (Ouabain 1 and Ouabain 2). ANOVA results are shown in each panel, and post hoc results are indicated by symbols: *P < 0.05 versus basal value in the same group; †P < 0.05 versus corresponding value from the α1R/Rα2R/R group.
Saline Loading Produces Greater Natriuresis in α1S/Sα2R/R Mice
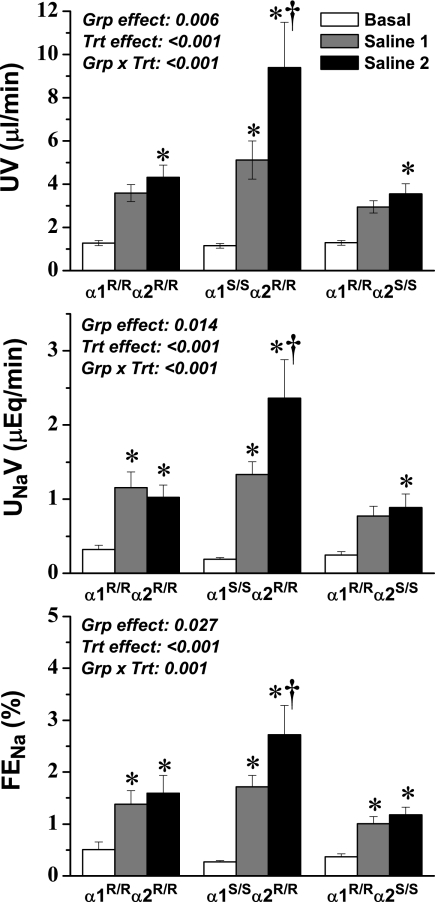
Salt loading has been shown to elevate the release of endogenous cardiotonic steroids,20–25 which would potentially act on the ouabain-binding site of the α1 isoform of Na,K-ATPase in renal tubular cells to decrease reabsorption and thereby contribute to the compensatory increase in renal salt and water excretion; therefore, we hypothesized that saline loading would produce a greater natriuretic response in α1S/Sα2R/R mice than in other groups. Cardiovascular and renal functions were monitored before and during saline infusion (Table 2, Figure 2). There was a small but significant difference in BP among the three groups, but because these changes were not consistently observed among the three protocols, it seems that the differences are not meaningful. Predictably, saline infusion increased heart rate and decreased hematocrit in each group, reflecting the expected increase in extracellular and vascular fluid volume. Although there were no changes in plasma Na+ levels, saline loading slightly but significantly (P = 0.003) increased plasma K+ levels in all three groups. GFR increased moderately and to the same extent in each group.
Figure 2.
UV (top), UNaV (middle), and FENa (bottom) before and during saline loading at 1 μl/min per g body wt. Two steady-state control periods were averaged and are represented as one value (Basal); two subsequent saline infusion periods are represented individually because steady state was not achieved (Saline 1 and Saline 2). ANOVA results are shown in each panel, and post hoc results are indicated by symbols: *P < 0.05 versus basal value in the same group; †P < 0.05 versus corresponding value from the α1R/Rα2R/R group.
As predicted, absolute and fractional Na+ excretion and urine flow rate increased progressively in all three groups in response to saline loading, and although the initial response was not different among the genotypes, by the end of the second treatment period, there was a marked increase in Na+ and water excretion in α1S/Sα2R/R mice compared with other groups (Figure 2). Because the enhanced excretion rate observed in α1S/Sα2R/R mice was paralleled by fractional excretion but was not associated with differences in the GFR response among the groups, the data suggest that differences in excretion rate are likely of tubular origin. These data are consistent with the hypothesis that an endogenous cardiotonic steroid is released in response to saline loading, which can interact with the α1 isoform ouabain-binding site in the renal epithelium of α1S/Sα2R/R mutants and thereby enhance the natriuretic and diuretic response.
Sequestration of Cardiotonic Steroids in the Circulation Prevents Enhanced Natriuresis in α1S/Sα2R/R Mice
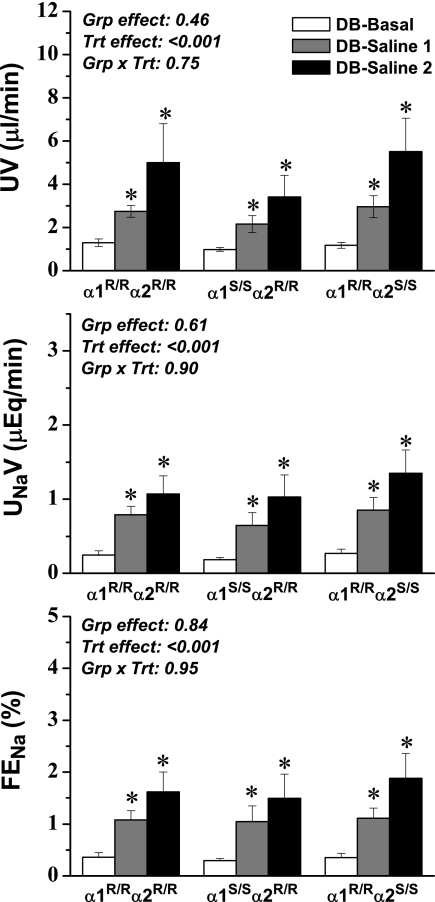
To evaluate specifically the potential contribution of endogenous cardiotonic steroids in the enhanced response to saline loading in the α1S/Sα2R/R mice, mice were treated before administration of a saline load with Digibind, an Fab antibody fragment that binds to digoxin-like molecules14,27 and is commonly used therapeutically to treat digoxin toxicity. We hypothesized that if endogenous steroid hormones were sequestered as they were being released, then they would not be available to promote enhanced natriuresis and diuresis in α1S/Sα2R/R mice, and therefore the response would be similar in all three genotypes. Importantly, we did not observe any effects of Digibind treatment alone on basal physiologic variables (Table 3). There were no statistically significant changes in BP in response to saline loading, and there was a small but significant increase in heart rate in each of the three groups. Hematocrit decreased equivalently in each group, indicating that the same degree of volume expansion was achieved, and the effects on plasma Na+ and K+ concentration were also similar among the groups. There were no significant changes in GFR in any of the groups.
In striking contrast to saline loading alone, saline combined with Digibind treatment produced nearly identical changes in absolute and fractional Na+ excretion and in the urine flow rate in the three groups (Figure 3). Furthermore, the enhanced natriuresis and diuresis observed during the second saline infusion period in untreated α1S/Sα2R/R (without Digibind) was conspicuously absent. Altogether, these data provide compelling evidence that the presence or absence of a sensitive α1 ouabain-binding site can alter the natriuretic response and also that an endogenous cardiotonic steroid is released during salt loading conditions that can act through this sensitive α1 isoform to enhance natriuresis.
Figure 3.
UV (top), UNaV (middle), and FENa (bottom) before and during saline loading (see Figure 2) in mice that were pretreated with Digibind (DB; 60 ng/g body wt). Two steady-state control periods were averaged and are represented as one value (DB-Basal); two subsequent saline infusion periods are represented individually because steady state was not achieved (DB-Saline 1 and DB-Saline 2). ANOVA results are shown in each panel, and post hoc results are indicated by symbol: *P < 0.05 versus basal value in the same group.
DISCUSSION
A major goal of this study was to investigate the potential contribution of the ouabain-binding site of Na,K-ATPase to the regulation of renal salt and water balance. We found that the α1S/Sα2R/R mice responded to salt load with a greater level of natriuresis than mice expressing the resistant α1 isoform. Treatment with a cardiac glycoside antibody, Digibind, before salt load equalized the natriuretic response in the three groups of mice. These data indicate that ouabain binding can play a role in determining salt and water balance and demonstrate that an endogenous cardiotonic steroid is released during salt loading and contributes to the enhanced response seen in the α1S/Sα2R/R mice. One possible explanation for the response in these mice is that the α1 subunit expressed in the basolateral membrane in renal tubular epithelial cells is a primary target of the cardiotonic steroid. Our results provide additional evidence not only for the presence of endogenous cardiotonic steroids but also for a potential physiologic role for these hormones in modulating Na+ excretion under conditions of salt excess through the ouabain-binding site of Na,K-ATPase.
Although a vast majority of animals express a sensitive α1 isoform, mice and rats naturally express a variation of the α1 isoform that is relatively resistant to ouabain inhibition.7 This species heterogeneity has served as a major impediment to our understanding of the putative actions of endogenous cardiotonic steroids, because rats and mice are among the most commonly studied physiologic models used to explore such phenomena and because the α1 isoform is ubiquitously expressed.3 The availability of our genetically altered mice allows for the investigation of the potential role of a sensitive α1 isoform, as is expressed in humans, in a frequently used rodent model. Furthermore, these mice permit direct evaluation of the participation of a specific isoform, because mice are also available with a ouabain-resistant α2 isoform (i.e., α1R/Rα2R/R). These advantages are balanced, however, with the caveat that mice and rats have evolved, presumably to some benefit, a ouabain-resistant α1 subunit and that targeted mutation of this isoform does not ideally recapitulate the situation in the human.
Several well-characterized natriuretic hormones inhibit Na+ transporters in the kidney through a variety of signal transduction pathways28; however, de Wardener29 hypothesized that an additional hormone acts directly through the α1 isoform during high salt conditions and that this hormone contributes to the resolution of excessive salt by inhibiting α1 isoform activity. Because the ouabain-binding site is so highly conserved, an excellent candidate for this hormone would be an endogenous cardiotonic steroid. Much work has been done to identify and characterize this proposed natriuretic factor, including the isolation of endogenous steroids from salt-loaded animals.30,31 Our work continues these efforts from a different perspective and supports the hypothesis that such a hormone can contribute to the resolution of Na+ imbalance selectively through the ouabain-binding site of the α1S/S isoform. These studies also provide an additional important contribution in that the mutant ouabain-sensitive α1 isoform serves to detect endogenous ligands. Because the α1S/S mice show increased natriuresis compared with the α1R/R mice, some ligand must be present to modify the activity of Na,K-ATPase during salt loading. Although integrating a sensitive α1 isoform into mice may not represent an exact correlate to the human cellular environment, our findings do suggest that the activity of a sensitive α1 subunit can be modulated differently than the resistant form by endogenous ligands. Also, although these experiments provide strong evidence for a role for endogenous cardiotonic steroids in natriuresis, they do not define which specific molecules might be involved, because Digibind cross-reacts with a variety of cardiotonic steroids.27
Although these data demonstrate influence of endogenous cardiotonic steroids on renal excretory function, the potential targets and pathways are yet to be elucidated. The most obvious possibility is that these substances interact directly with the renal tubule to decrease overall salt and water reabsorption and promote excretion. It is also intriguing to speculate that the effects may also be mediated indirectly through the alteration of other salt and water regulatory systems. Salt loading decreases the plasma levels of Na+-retaining hormones such as renin, angiotensin II, and aldosterone32–34 while at the same time increases the levels of natriuretic hormones, such as atrial natriuretic peptide (ANP) and dopamine.33,35 It is possible, for example, that endogenous cardiotonic steroids, interacting with an ouabain-sensitive α1 Na,K-ATPase on atrial myocytes, might enhance the release of ANP and thereby promote salt and water excretion. It is further possible that elevated circulating cardiotonic steroids may enhance the sensitivity of the renal tubule to ANP, as suggested by Fedorova et al.36 In any case, whether the observed effects are related to a direct effect on the renal tubule or indirectly through the alteration of the sensitivity of other Na+ regulatory systems does not alter our overall hypothesis that the interaction of endogenous cardiotonic steroids with a sensitive α1 binding site can play a role in the response to salt loading.
It is important to note that the natriuresis and diuresis measured under basal conditions were not different among the three genotypes. This indicates that during resting states, the ouabain-binding site of the α1 isoform plays no observable role in the salt-handling mechanisms of the kidney. This correlates well with the fact that cardiotonic steroids are maintained at low levels in the bloodstream until the animal experiences some form of stress, such as salt loading.14,25,37–41
Interestingly, we observed that α1R/Rα2R/R mice seemed to respond to ouabain infusion with a modest natriuresis (P = 0.06), despite the lack of an ouabain-sensitive α1 or α2 subunit. One explanation for this is that these mice still express an ouabain-sensitive α3 subunit in neuronal tissue. Because neural pathways and reflexes are of central importance in regulating vascular tone and kidney function, it may be that ouabain-induced decreases in α3 Na,K-ATPase activity modified renal excretory function by influencing renal nerve traffic or by influencing the release of other natriuretic hormones such as ANP and dopamine.42 We are currently developing animals that express an ouabain-resistant α3 isoform that may help further explore this interesting finding.
Because the α2 isoform is expressed in smooth muscle throughout the vasculature, we had originally hypothesized that the α2 isoform binding site may play a role in regulating vascular tone of the afferent arterioles and thereby influence GFR; however, there were no significant differences between the GFR responses to either ouabain infusion or saline loading between the α1R/Rα2S/S and α1R/Rα2R/R mice or between the natriuresis and diuresis observed in these groups. These findings suggest that the sensitivity of the α2 isoform ouabain-binding site does not play a major role in regulating overall kidney function, whether through vascular tone of the afferent arterioles or through Na+ reabsorption mechanisms. Furthermore, ouabain infusion caused a significant drop in the GFR of α1S/Sα2R/R mice during the second period of ouabain infusion but not in α1R/Rα2S/S or α1R/Rα2R/R mice. Because α1 is the more abundantly expressed isoform in vascular smooth muscle, it may be that ouabain treatment caused significant vasoconstriction in the afferent arterioles of these mice, thereby decreasing GFR.
Perspectives
The typical Western diet contains high Na+ levels, and a correlation between salt intake and the development of hypertension has been established.16,30 This study contributes to the understanding of how the body might resolve Na+ conditions that rise above the normal homeostatic set point. Our work suggests that endogenous cardiotonic steroids released during high salt conditions may help to augment the excretion of excessive salt and therefore contribute to the maintenance of normal BP.
There is increasing evidence that endogenous cardiotonic steroid levels are higher in patients with certain forms of hypertension.16,19,30 The ability of cardiotonic steroids to cause vasoconstriction has led many to postulate that these hormones contribute to the development of the hypertensive state, despite their natriuretic properties. The study from Fedorova et al.36 may somewhat resolve this conflict: Marinobufagenin and ANP may function synergistically in the kidney to induce natriuresis but in opposition to each other in the vasculature, because ANP seems to counteract the vasoconstrictive effects of marinobufagenin; therefore, endogenous cardiotonic steroids may in fact be a positive factor in the body's defense against hypertension. Further work is necessary to solidify the role of endogenous cardiotonic steroids in the resolution of hypertensive states.
CONCISE METHODS
Mice
Mice were obtained from two established colonies at the University of Cincinnati, both of which were on a mixed 129SvJ and Black Swiss background. The colony of single-mutant mice expressing the ouabain-resistant α2 Na,K-ATPase isoform (α1R/Rα2R/R) were maintained by mating heterozygous male and female mice (α1R/Rα2S/R× α1R/Rα2S/R).11 The colony of double-mutant mice expressing the ouabain-sensitive α1 Na,K-ATPase isoform and the ouabain-resistant α2 Na,K-ATPase isoform (α1S/Sα2R/R) was maintained by mating homologous double-mutant mice13; breeding pairs were periodically backcrossed to a subcolony of wild-type mice to sustain a reasonably consistent genetic background among mutant and wild-type mice. The wild-type mice used in these studies were obtained from both colonies and showed no differences in any observed measurements. Genotypes were determined by PCR analysis of DNA from tail biopsies, as described previously.11,13 Experiments were performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Renal Function Measurements
Female adult mice were fasted overnight and prepared for clearance measurements. Mice were anesthetized with ketamine (50 μg/g body weight, intraperitoneally) and thiobutabarbital (Inactin, 100 μg/g body wt intraperitoneally; Sigma-Aldrich, St. Louis, MO) and placed on a temperature-controlled table. After tracheotomy, the right femoral artery and vein were cannulated for the measurement of arterial pressure and the administration of solutions. Arterial pressure was monitored using an Argon model CDXIII transducer (Maxxim Medical, Clearwater, FL) connected to a PowerLab data acquisition system (AD Instruments, Colorado Springs, CO). The bladder was cannulated for the collection of urine. A 3-μl/g body weight bolus of 1.5% FITC-inulin (Sigma-Aldrich) in isotonic saline was administered, followed by a maintenance infusion of the same solution at 0.1 μl/min per g body wt, and the mice were allowed to equilibrate for 30 min. In each protocol, two 30-min control clearance periods were followed by two 30-min treatment clearance periods. At the midpoint of each period, an 80-μl blood sample was obtained for determination of plasma electrolytes and inulin concentration. Inulin concentration in plasma and urine was measured fluorometrically as described previously,43 and Na+ and K+ concentration was determined by flame photometer (Cole Parmer, Vernon Hills, IL; Model 2655-10).
Ouabain-Induced Natriuresis
For direct evaluation of the effects of ouabain on Na+ excretion in mice expressing the various forms of the α1 and α2 Na,K-ATPase isoforms, ouabain was infused during the treatment periods at a dosage of 6.6 ng/min per g body wt. The mice were allowed to equilibrate for 30 min after initiation of the ouabain infusion before beginning the urine collections.
Salt Loading
To determine whether the ouabain-binding sites of the α1 and α2 isoforms play a role in the natriuretic response to salt loading, we infused isotonic saline to expand acutely their extracellular fluid volume. After the control period, saline loading was initiated by increasing the infusion rate of the saline/FITC solution to 1 μl/min per g body wt. The concentration of FITC-inulin was diluted 1:10 to maintain constant infusion of inulin. The mice were allowed to equilibrate for 30 min, after which two additional urine collections were performed.
Digibind + Saline Loading
For further evaluation of whether differences in renal function between mice with different sensitivities of the α1 isoform were indeed related to altered circulating levels of endogenous cardiotonic steroids, a solution of the anti-digoxin Fab fragment Digibind (GlaxoSmithKline; 60 ng/g body wt bolus injection) was administered at the beginning of the experiment and the saline infusion protocol was repeated.
Statistical Analysis
Because mice were in a steady state during the two control periods, the values from these two periods were averaged and used in the analysis. The mice did not reach a steady state during ouabain infusion or saline loading, so these periods were analyzed as separate treatment periods. Statistical analysis was performed by ANOVA using a mixed two-factor design (group × treatment), with repeated measures on the treatment factor. Where appropriate, post hoc analyses of main effects and interactions were made using the Tukey test. Differences were regarded as significant at P < 0.05.
DISCLOSURES
None.
Acknowledgments
This research was supported by National Institutes of Health Grants R01 HL28573 (to J.B.L.), R01 HL66062 (to J.B.L.), and R01 DK57552 (to J.N.L.) and 5T32 HL007382 (to A. Schwartz).
We thank Michelle L. Nieman, Valerie Lasko, and Naomi Oshiro for technical assistance and Maureen L. Bender for animal husbandry.
Published online ahead of print. Publication date available at www.jasn.org.
J.N.L. and J.B.L. contributed equally to this work.
See related editorial, “A Natriuretic Hormone–Binding Site on the Sodium Pump,” on pages 1839–1840.
References
- 1.Lingrel JB, Kuntzweiler T: Na+,K(+)-ATPase. J Biol Chem 269: 19659–19662, 1994 [PubMed] [Google Scholar]
- 2.Lingrel JB, Young RM, Shull MM: Multiple forms of the Na,K-ATPase: Their genes and tissue specific expression. Prog Clin Biol Res 268B: 105–112, 1988 [PubMed] [Google Scholar]
- 3.Lingrel J, Moseley A, Dostanic I, Cougnon M, He S, James P, Woo A, O'Connor K, Neumann J: Functional roles of the alpha isoforms of the Na,K-ATPase. Ann N Y Acad Sci 986: 354–359, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Sweadner KJ: Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta 988: 185–220, 1989 [DOI] [PubMed] [Google Scholar]
- 5.James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB: Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol Cell 3: 555–563, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Dostanic-Larson I, Lorenz JN, Van Huysse JW, Neumann JC, Moseley AE, Lingrel JB: Physiological role of the alpha1- and alpha2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol 290: R524–R528, 2006 [DOI] [PubMed] [Google Scholar]
- 7.O'Brien WJ, Lingrel JB, Wallick ET: Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch Biochem Biophys 310: 32–39, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Wallick ET, Schwartz A: Interaction of cardiac glycosides with Na+,K+-ATPase. Methods Enzymol 156: 201–213, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Velotta JB, McDonough AA, Farley RA: All human Na(+)-K(+)-ATPase alpha-subunit isoforms have a similar affinity for cardiac glycosides. Am J Physiol Cell Physiol 281: C1336–C1343, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Price EM, Lingrel JB: Structure-function relationships in the Na,K-ATPase alpha subunit: Site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry 27: 8400–8408, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, Lingrel JB: The alpha2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem 278: 53026–53034, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB: The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB: The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem 279: 54053–54061, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB: The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci U S A 102: 15845–15850, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz JN, Dostanic-Larson I, Shull GE, Lingrel JB: Ouabain inhibits tubuloglomerular feedback in mutant mice with ouabain-sensitive alpha1 Na,K-ATPase. J Am Soc Nephrol 17: 2457–2463, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Blaustein MP, Zhang J, Chen L, Hamilton BP: How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol 290: R514–R523, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Akera T, Brody TM: The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev 29: 187–220, 1977 [PubMed] [Google Scholar]
- 18.Bagrov AY, Roukoyatkina NI, Pinaev AG, Dmitrieva RI, Fedorova OV: Effects of two endogenous Na+,K(+)-ATPase inhibitors, marinobufagenin and ouabain, on isolated rat aorta. Eur J Pharmacol 274: 151–158, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Schoner W, Scheiner-Bobis G: Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs 7: 173–189, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Bagrov AY, Fedorova OV, Dmitrieva RI, French AW, Anderson DE: Plasma marinobufagenin-like and ouabain-like immunoreactivity during saline volume expansion in anesthetized dogs. Cardiovasc Res 31: 296–305, 1996 [PubMed] [Google Scholar]
- 21.Graves SW, Glatter KA, Lazarus JM, Williams GH, Hollenberg NK: Volume expansion in renal failure patients: A paradigm for a clinically relevant [Na,K]ATPase inhibitor. J Cardiovasc Pharmacol 22[Suppl 2]: S54–S57, 1993 [PubMed] [Google Scholar]
- 22.McKinnon W, Lord GA, Forni LG, Hilton PJ: Circulating sodium pump inhibitors in five volume-expanded humans. J Hypertens 21: 2315–2321, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Pamnani MB, Burris JF, Jemionek JF, Huot SJ, Price M, Freis ED, Haddy FJ: Humoral Na+-K+ pump inhibitory activity in essential hypertension and in normotensive subjects after acute volume expansion. Am J Hypertens 2: 524–531, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Price MB, Pamnani MB, Burris JF, Link WT, Freis ED, Haddy FJ: Acute volume expansion in humans releases a factor which inhibits the vascular Na+-K+ pump. J Hypertens Suppl 2: S471–S472, 1984 [PubMed] [Google Scholar]
- 25.Yamada K, Goto A, Nagoshi H, Terano Y, Omata M: Elevation of ouabainlike compound levels with hypertonic sodium chloride load in rat plasma and tissues. Hypertension 30: 94–98, 1997 [DOI] [PubMed] [Google Scholar]
- 26.McDonough AA, Thompson CB, Youn JH: Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol 282: F967–F974, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Pullen MA, Brooks DP, Edwards RM: Characterization of the neutralizing activity of digoxin-specific Fab toward ouabain-like steroids. J Pharmacol Exp Ther 310: 319–325, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Hansell P, Ande'n NE, Grabowska-Ande'n M, Ulfendahl HR: Atrial natriuretic factor, urinary catechol compounds and electrolyte excretion in rats during normal hydration and isotonic volume expansion: Influence of dopamine receptor blockade. Acta Physiol Scand 134: 421–428, 1988 [DOI] [PubMed] [Google Scholar]
- 29.de Wardener HE: The concept of the natriuretic hormone and its relation to hypertension. Clin Exp Hypertens A 7: 647–662, 1985 [DOI] [PubMed] [Google Scholar]
- 30.Haddy FJ: Role of dietary salt in hypertension. Life Sci 79: 1585–1592, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Goto A, Yamada K: Ouabain-like factor. Curr Opin Nephrol Hypertens 7: 189–196, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Tuck ML, Dluhy RG, Williams GH: A specific role for saline or the sodium ion in the regulation of renin and aldosterone secretion. J Clin Invest 53: 988–995, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckley JW, Hedner T, Masotto C, Posvar E, Negro-Villar A, Cubeddu LX: Comparative effects of verapamil and volume overload on atrial natriuretic factors and the renin-angiotensin aldosterone-vasopressin system. J Clin Pharmacol 32: 1120–1127, 1992 [PubMed] [Google Scholar]
- 34.Carvalho AC, Botelho LM, Greene LJ, Santos RA: Effect of acute volume expansion associated with salt load on the profile of plasma angiotensins in rats. Immunopharmacology 33: 143–145, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Hegde SS, Jadhav AL, Lokhandwala MF: Role of kidney dopamine in the natriuretic response to volume expansion in rats. Hypertension 13: 828–834, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Fedorova OV, Agalakova NI, Morrell CH, Lakatta EG, Bagrov AY: ANP differentially modulates marinobufagenin-induced sodium pump inhibition in kidney and aorta. Hypertension 48: 1160–1168, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Haddy FJ: Potassium, Na+-K+ pump inhibitor and low-renin hypertension. Clin Invest Med 10: 547–554, 1987 [PubMed] [Google Scholar]
- 38.de Wardener HE, Clarkson EM: Concept of natriuretic hormone. Physiol Rev 65: 658–759, 1985 [DOI] [PubMed] [Google Scholar]
- 39.Graves SW, Williams GH: Endogenous digitalis-like natriuretic factors. Annu Rev Med 38: 433–444, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Schoner W: Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem 269: 2440–2448, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Gottlieb SS, Rogowski AC, Weinberg M, Krichten CM, Hamilton BP, Hamlyn JM: Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation 86: 420–425, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Wongmekiat O, Johns E: Role of nitric oxide and renal nerves in the renal responses to acute volume expansion in anaesthetized rats. Exp Physiol 86: 47–54, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Lorenz JN, Gruenstein E: A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol 276: F172–F177, 1999 [DOI] [PubMed] [Google Scholar]