Abstract
Congenital nephron number varies five-fold among normal humans, and individuals at the lower end of this range may have an increased lifetime risk for essential hypertension or renal insufficiency; however, the mechanisms that determine nephron number are unknown. This study tested the hypothesis that common hypomorphic variants of the RET gene, which encodes a tyrosine kinase receptor critical for renal branching morphogenesis, might account for subtle renal hypoplasia in some normal newborns. A common single-nucleotide polymorphism (rs1800860 G/A) was identified within an exonic splicing enhancer in exon 7. The adenosine variant at mRNA position 1476 reduced affinity for spliceosome proteins, enhanced the likelihood of aberrant mRNA splicing, and diminished the level of functional transcript in human cells. In vivo, normal white newborns with an rs1800860(1476A) allele had kidney volumes 10% smaller and cord blood cystatin C levels 9% higher than those with the rs1800860(1476G) allele. These findings suggest that the RET(1476A) allele, in combination with other common polymorphic developmental genes, may account for subtle renal hypoplasia in a significant proportion of the white population. Whether this gene variant affects clinical outcomes requires further study.
Human kidney development begins at approximately 5 wk gestation, when RET-bearing cells of the descending nephric duct encounter glial-derived neurotrophic factor (GDNF) released by metanephric mesenchyme at somite 24.1 In response to the trophic effects of GDNF, a ureteric bud sprouts from each nephric duct and arborizes within the lateral mesenchyme. Signals from each ureteric bud branch tip induce adjacent metanephric stem cells to form individual nephrons, which fuse to the tree-like collecting system. Because new nephron formation ends at approximately 36 wk gestation, the extent of branching nephrogenesis by this time determines nephron endowment for life.
Interestingly, nephron number varies widely (0.3 to 1.3 million nephrons per kidney) among normal humans.2 Although once dismissed as a benign reflection of human diversity, Brenner et al.3 proposed that individuals born at the low end of the nephron endowment spectrum may have increased risk for developing “essential” hypertension and renal insufficiency later in life. They hypothesized that signals driving compensatory hypertrophy of overworked nephrons cause glomerulosclerosis and a cycle of subtle, slowly progressive renal dysfunction.4
Recent evidence supports this theory; an autopsy study by Keller et al.5 showed that German adults with essential hypertension had 47% fewer nephrons per kidney than well-matched normotensive control subjects. As predicted, hypertensive patients had hypertrophic glomeruli (glomerular volume 233% of control subjects) and increased glomerulosclerosis (5.5% of glomeruli versus 0% in control subjects). Other evidence suggests that racial differences in congenital nephron number might also explain the relatively high incidence of ESRD in Aboriginal versus white populations.6,7 At autopsy, Aboriginal individuals have 23% fewer glomeruli (683,174 per kidney) than white individuals (885,318 per kidney; P < 0.04).
Little is known about the factors that set nephron number, but the GDNF/RET signaling pathway seems to play a central role. The RET gene (NM_020630) encodes a 1072 amino acid transmembrane tyrosine kinase receptor (NP_065681) expressed at the tips of the branching ureteric buds during fetal kidney development8; homozygous Ret knockout mice are anephric,9 and heterozygotes have a 22% reduction in nephron number at 15 d of age.10 In this study, we hypothesized that hypomorphic variants of the RET gene might be prevalent in “normal” humans, contributing to suboptimal nephron number and subtle renal hypoplasia in a significant portion of the population.
RESULTS
We scanned the NCBI dbSNP database (see Appendix) and identified two common variants (minor allele frequency >10%) of the RET gene coding region that might alter its tyrosine kinase receptor function during kidney development. In exon 11, an A/G substitution at mRNA position 2251 (rs1799939) occurs in approximately 16% of white alleles, changing gly691 to ser691. In exon 7, an A/G substitution at mRNA position 1476 (rs1800860) occurs in 25% of white alleles and lies within an exonic splice enhancer (ESE) sequence (Figure 1A). The critical position of this substitution suggests that it might modify pre-mRNA splicing. We used ESEfinder11 to calculate the effect of the rs1800860 polymorphism on binding to proteins (SF2/ASF, SC35) of the mRNA spliceosome (Table 1). In each case, the 1476(A) allele reduces RET affinity score below the predicted threshold for effective binding to these components of the splicing machinery.
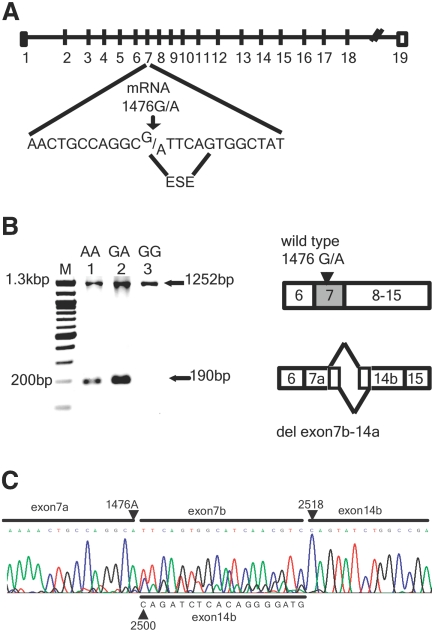
Figure 1.
1476A SNP in human RET mRNA is associated with an aberrant 190-bp transcript. (A) Genomic structure of the human RET gene, indicating the 1476(G/A) SNP within an ESE of exon 7. (B) Nested primers spanning exon 6 to exon 17 were used to amplify RET transcripts in reverse-transcribed RNA extracted from various human cell lines. Only the predicted wild-type 1252-bp transcript is evident in the homozygous (1476 G/G) Wit49 Wilms’ tumor cell line (lane 3); however, an aberrant 190-bp transcript is noted in heterozygous (1476G/A) SH-SY5Y neuroblastoma cells (lane 2) and in homozygous 1476(A/A) SK-N-BE(2) neuroblastoma cells (lane 1). The aberrant 190-bp transcript was not seen in four other 1476G/G Wilms’ tumor, renal cell carcinoma, and ovarian carcinoma cell lines (data not shown). (C) Sequence analysis of the aberrant 190-bp band demonstrates fusion of RET exon 7a to exon 14b with a heterogeneous 18-bp intervening sequence which consisted of nucleotides continuing beyond 1476(A) into exon 7b (upper sequence) or nucleotides from exon 14b upstream of mRNA position 2500 through 2518 (down sequence). This suggests aberrant splicing between sites after the ESE in exon 7 and two alternative cryptic splice sites (position 2500 and 2518) in exon 14b.
Table 1.
Predicted effect of SNP 1476G/A on affinity score for binding of SR protein
| Allele | SF2/ASF
|
SC35
|
||
|---|---|---|---|---|
| Motif | Score | Motif | Score | |
| A | CAGGCAT | 0.503 | ATTCAGTG | 2.095 |
| G | CAGGCGT | 3.081 | GTTCAGTG | 3.847 |
Predicted effect of SNP rs1800860 on the affinity score for binding of RET mRNA to core spliceosome components. We used ESEfinder software to predict the effect of an adenosine versus guanine nucleotides at position 1476 of the human RET transcript on binding to two core protein components of the spliceosome (SF2/ASF and SC35). The matrix score for SF2/ASF is reduced from 3.08 for the ancestral CAGGCGT sequence to 0.50 for the less common CAGGCAT variant (threshold for normal splicing efficiency = 1.96). Similarly, affinity for the SC35 component is reduced from 3.85 to 2.10 (threshold = 2.40). Based on these predictions, we hypothesized that the rs1800860 A allele (1476A) might lead to aberrant RET mRNA splicing.
To confirm that the minor RET1476(A) allele alters normal mRNA splicing, we studied three RET-expressing human cell lines: (1) SK-N-BE(2) neuroblastoma cells (1476A/A); (2) SH-SY5Y neuroblastoma cells (1476G/A); and (3) Wit49 Wilms’ tumor cells (1476G/G). RET transcripts were amplified with nested primers spanning exon 6 through exon 15. Only the predicted wild-type 1252-bp transcript is evident in Wit49 (1476G/G) cells, whereas an aberrant 190-bp transcript is noted in the two cell lines bearing one or more 1476A alleles (Figure 1B). The 190-bp transcript was not seen in any of four other 1476(G/G) cell lines (data not shown). Sequencing of the 1252-bp band in the heterozygous (rs1800860) SH-SY5Y cell line demonstrated that normal splicing of exon 7 can occur with either nucleotide at mRNA position 1476 (Figure 2); however, when the minor transcript in SK-N-BE(2) or SH-SY5Y cells was sequenced, we also noted aberrant splicing from 1476A to two cryptic splice sites in exon 14 (Figure 1C). This del(7b-14a) transcript eliminates part of the cadherin-like domain 4 (CLD4), the entire cysteine-rich domain (CRD), transmembrane domain, and the first tyrosine kinase domain, undoubtedly rendering the protein product nonfunctional (Figure 3). The CLD4 and CRD are the extracellular sites of interaction with RET ligand (GDNF) and co-receptor (GFRα1).12,13 To confirm that this aberrant splicing activity occurred in other cell lines, we also studied the heterozygous (1476G/A) Wilms’ tumor G401 cell line and found the same del(7b-14a) transcript (data not shown).
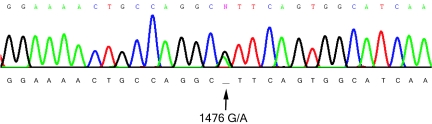
Figure 2.
Sequence of wild-type human RET exon 7 (NM_020630). After reverse transcription of mRNA from heterozygous (rs1800860) SH-SY5Y cells, exon 7 was amplified by PCR and the normal 1252-bp amplicon was sequenced. The presence of either G or A at position 1476 demonstrates that normal splicing of RET exon 7 can occur with both alleles.
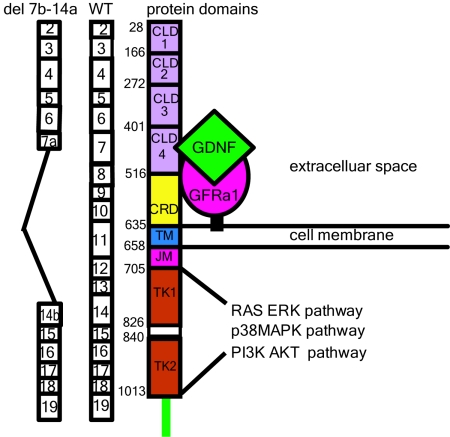
Figure 3.
The aberrant del(7b-14a) RET transcript lacks key functional domains. CLD1 through 4, CRD, transmembrane segment (TM), juxtamembrane segment (JM), and two cytoplasmic tyrosine kinase domains (TK1 and TK2) are displayed adjacent to the corresponding exon structure of the wild-type RET transcript (WT). To the left is the aberrant del(7b-14a) transcript associated with 1476(A) alleles; deletion of extracellular domains for ligand (GDNF) and co-receptor (GFRα1), transmembrane segments, and a large portion of TK1 is predicted, rendering the transcript nonfunctional.
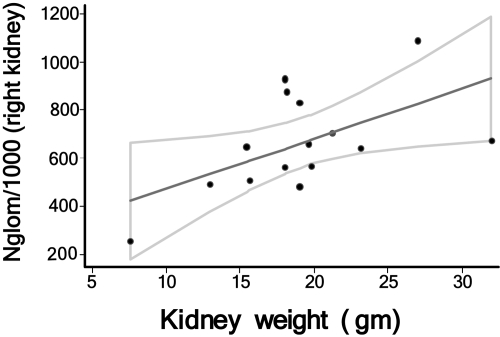
Because both the 1476A and 2251A single-nucleotide polymorphisms (SNP) are potentially hypomorphic variants of the RET gene, we hypothesized that either one could compromise nephrogenesis during kidney development, resulting in subtle renal hypoplasia in a significant fraction of newborns. To validate newborn kidney volume as a surrogate for congenital nephron number, we measured glomerular number in kidneys from 15 infants who died of congenital anomalies or sudden infant death syndrome at or before the age of 3 mo. Nephron number ranged from 246,181 to 1,106,062. A strong direct relationship (P = 0.019) between kidney mass and total nephron number in children aged ≤3 mo was identified (Figure 4). Regression analysis predicted an additional 23,459 (95% confidence interval 4590 to 42,238) glomeruli per gram of kidney mass in the range.
Figure 4.
Relationship between Nglom (with 95% confidence interval) and mass of right kidney at autopsy in infants aged ≤3 mo. Nglom was measured by the disector/fractionator method as described previously in autopsied kidneys from infants who died from various congenital anomalies or sudden infant death syndrome within the first 3 mo of life.
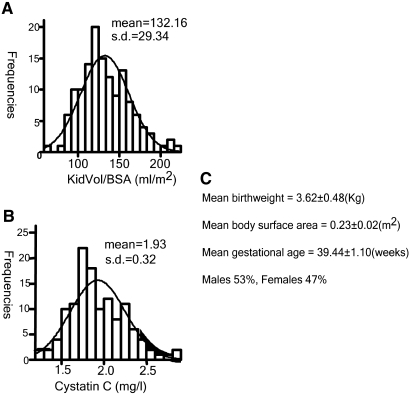
We then recruited 136 normal term newborn white infants born at the Royal Victoria Hospital in Montreal between 2004 and 2006, excluding any with low birth weight (<2500 g), renal malformation, maternal diabetes, or twin pregnancy. Total (combined) renal volume was estimated by ultrasonography within 48 h of birth and normalized for body surface; cord blood was obtained for assay of cystatin C as a surrogate of newborn GFR.14–16 In previous studies, we found that newborn kidney volume correlated directly with body surface area and inversely with cord blood cystatin C.14 Clinical characteristics of the cohort are summarized in Figure 5. The distribution of cystatin and kidney size in our cohort approached normality (skewness <2 for all parameters).
Figure 5.
Clinical characteristics of the newborns of white cohort (n = 136). (A) Total KidVol/BSA in the white newborn cohort (mean ± SD 132.16 ± 29.34 ml/m2). (B) Cord blood cystatin C (mean ± SD 1.93 ± 0.32 mg/L). (C) Clinical characteristics of the newborn cohort.
DNA extracted from cord blood was used to genotype each infant for the rs1800860 (1476G/A) and rs1799939 (2251G/A) SNP. Genotype distribution for each SNP conformed to the Hardy-Weinberg equilibrium (P = 0.39 and 0.47, respectively); SNP frequencies in our cohort (Table 2) were similar to those reported in the CAUC1 and CEU populations (NCBI dbSNP database; see Appendix). RET2251(G/A) SNP was associated with neither renal volume nor cord cystatin C; however, total kidney volume factored for body surface area (KidVol/BSA) in newborns bearing one or more 1476(A) alleles was 9.7% smaller than that in newborns with the homozygous 1476(G/G) genotype (P = 0.009). The 1476(A) allele was also associated with 9.2% increase in cord blood cystatin C concentration (P = 0.002), suggesting a comparable reduction in functional renal mass15 (Table 2).
Table 2.
Association between SNP and newborn kidney volume or cord blood cystatin C
| SNP ID | Genotype Frequencies (%) | KidVol/BSA (ml/m2) | Cystatin C (mg/L) |
|---|---|---|---|
| rs1800860 | |||
| GG | 46.3 | 139.4 ± 33.0 | 1.84 ± 0.30 |
| GA | 47.1 | 126.3 ± 24.7 | 2.02 ± 0.33 |
| AA | 6.6 | 123.0 ± 22.3 | 1.94 ± 0.14 |
| GA + AA | 53.7 | 125.9 ± 24.3a | 2.01 ± 0.32b |
| rs1799939 | |||
| GG | 56.3 | 131.3 ± 31.0 | 1.93 ± 0.32 |
| GA | 40.0 | 134.1 ± 27.5 | 1.95 ± 0.33 |
| AA | 3.7 | 132.1 ± 25.3 | 1.72 ± 0.30 |
| GA + AA | 43.7 | 133.9 ± 27.1 (NS) | 1.93 ± 0.3 (NS) |
Association between RET SNPs and newborn kidney volume or cord blood cystatin C. Normal term Caucasian newborns (136) from Montreal were genotyped for the rs1800860 (1476G/A) and rs1799939 (2251G/A) RET SNPs by sequence analysis of PCR amplicons. Genotype frequencies in our cohort are similar to those reported for the CAUC and CEU populations in the NCBI dbSNP database. Total renal volume adjusted for body surface area (KidVol/BSA) in newborns bearing one or more 1476(A) alleles was 9.7%, less than that of homozygous 1476(G/G) newborns (P = 0.009). Umbilical cordblood cystatin C concentration in newborns with one or more 1476(A) alleles was 9.2% higher than in homozygous 1476(G/G) babies (P = 0.002). The presence of a 2251(A) allele had no significant effect on KidVol/BSA (P = 0.60) or umbilical cord cystatin C (P = 0.91) compared with newborns with the homozygous 2251(G/G) genotype.
P = 0.009.
P = 0.002.
The 1476(A) allele was widely distributed in our population; combined renal volume in infants with a 1476(A) allele ranged from 16.06 to 44.48 ml versus 15.01 to 50.33 ml in infants who were homozygous for the more common 1476(G) allele. Thus, the association between 1476(A) allele and renal volume cannot be attributed to a few infants with very small kidneys. Similarly, the distribution of left/right kidney volume ratios was similar in newborns with one or more 1476(A) alleles (0.93 ± 0.22 SD) versus newborns homozygous for the 1476(G) allele (0.96 ± 0.18 SD) (P > 0.05). Thus, the association between 1476(A) and renal volume was not due to a few infants with unilateral renal hypoplasia.
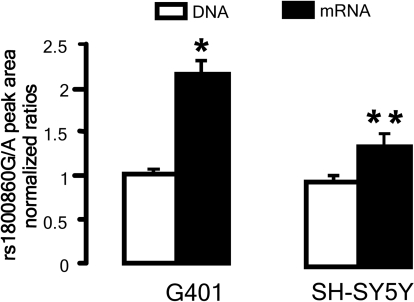
To confirm that the RET1476(A) allele compromises expression of wild-type receptor mRNA, we examined allele-specific mRNA expression in the heterozygous SH-SY5Y and G401 cell lines. RET exon 7 was amplified from genomic DNA and cellular mRNA, using high-sensitivity sequencing technology to quantify each allele-specific amplicon as described by others.17 mRNA expression from the 1476(A) allele was substantially reduced in both SH-SY5Y (by 25%) and G401 (by 50%) cells, compared with the expression level from the 1476(G) allele (Figure 6).
Figure 6.
Comparison of allele-specific mRNA expression in rs1800860(G/A) heterozygous G401 and SH-SY5Y cells. Peak area ratio of G/A alleles was measured in cDNA and genomic DNA from two cell lines heterozygous for the rs1800860 SNP. The G/A allelic ratio (2.09 ± 0.12) in cDNA was 2.1 times greater than the ratio (1.01 ± 0.02) in genomic DNA from G401 cells (*P = 0.004). Similarly, G/A ratio was 1.4 times greater in cDNA (1.41 ± 0.14) than in genomic DNA (0.98 ± 0.03) for SH-SY5Y cells (**P = 0.03).
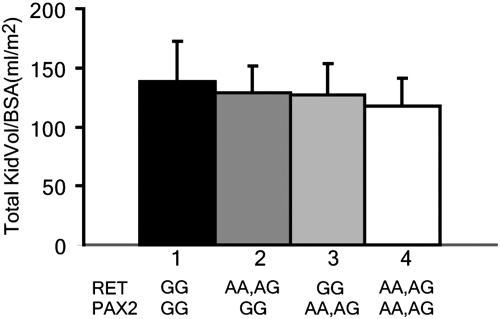
Our previous studies demonstrated an association between a common (18.5% of white individuals) human PAX2 haplotype (AAA) and a 10% reduction in newborn renal size.16 Interestingly, RET transcription is regulated by PAX2 gene dosage.10 When we analyzed our cohort for both genes, renal volume in the 17 of 136 newborns carrying both the RET1476(A) and PAX2(AAA) minor alleles was 23% lower than that in infants with the RET1476(G/G), PAX2(GGG) haplotype (Figure 7).
Figure 7.
Comparison of KidVol/BSA in newborns with various combinations of hypomorphic RET1476(A) and PAX2AAA alleles. All 136 newborns in our cohort were genotyped for both the RET(1476G/A) SNP and the hypomorphic PAX2AAA haplotype previously described by Quinlan et al.15 KidVol/BSA among newborns bearing one or more hypomorphic RET1476(A) alleles (n = 46) was 90.3% of that for infants with the major RET1476(G)/PAX2GGG alleles (n = 41; P = 0.01). Similarly, KidVol/BSA among newborns with one or more hypomorphic PAX2AAA alleles (n = 14) was 89.5% of RET1476(G)/PAX2GGG newborns (P = 0.04). In the subset of newborns bearing both hypomorphic RET1476(A) and PAX2AAA alleles (n = 17), KidVol/BSA was only 77% of that in wild-type RET1476(G)/PAX2GGG infants (P = 0.00067).
DISCUSSION
During renal development, the branching ureteric bud expresses high levels of the transcription factor PAX2.1 Brophy and colleagues10,18 recently showed that, among its many functions, PAX2 directly activates transcription of genes for both RET, a tyrosine kinase receptor, and its ligand, GDNF. Epithelial cells expressing RET receptors cluster at the tip of each ureteric bud branch as it undergoes branching morphogenesis.8 When activated by GDNF from nearby mesenchyme, the RET receptor heterodimerizes with GFRα1, stimulating cell proliferation, migration, and survival via several intracellular signals, including the RAS/mitogen-activated protein kinase, phosphatidylinositol 3-kinase/AKT, and RAC1/JUN NH(2) terminal kinase pathways.19–21 Loss of RET, GDNF, or GFRα1 results in renal agenesis, because of inhibition of ureteric bud growth and branching.9,22,23
Because RET integrity is critical for branching nephrogenesis, we hypothesized that heterozygous RET mutations might partially compromise the extent of ureteric bud branching during development, leading to suboptimal nephron number. In mutant mice, heterozygous null alleles reduce nephron number by approximately 22% at postnatal day 15.10 Thus, a heterozygous hypomorphic RET allele such as the 1476(A) variant should fit at the milder end of this spectrum and might plausibly produce the observed 10% decrease in newborn nephron number.
Mouse studies suggest a graded relationship between kidney size and the level of RET function; Ret knockout mice are anephric, whereas severely hypomorphic Ret alleles such as Tyr1062Phe24 or RetDN25 produce severe renal hypoplasia. Thus, only a subtle effect on kidney size would be expected for a RET polymorphism predicted to have only a modest effect on total RET mRNA level; however, the relationship between kidney volume and nephron number is difficult to establish in mice; nephrogenesis continues for at least 2 wk after birth and potentially overlaps with a period of postnatal compensatory hypertrophy. For example, Clarke et al.10 noted that heterozygous Ret(+/−) postnatal day 15 mice had a 22% reduction in nephron number but only a 10% reduction in kidney volume (NS). To establish that newborn kidney size was a valid surrogate for nephron number in humans, we measured glomerular number and kidney weight in newborn infants who died before 3 mo of age. This showed a strong correlation between renal mass and nephron number in the newborn period.
In this study, we identified a common SNP within the ESE of exon 7, which modifies the fidelity of mRNA splicing to the normal splice site in exon 8. An adenine nucleotide at position 1476 of this ESE increases the risk for aberrant splicing to alternative sites in exon 14. When this occurs, exons containing the crucial GFRα1 binding site, transmembrane, and first tyrosine kinase domains all are deleted, undoubtedly rendering the RET receptor dysfunctional. On the basis of our studies in cultured heterozygous human cells, the presence of a 1476A allele reduces wild-type mRNA expression by approximately 38%; in a heterozygous cell, this would amount to an overall reduction of functional RET transcript by 19%. Arguably, branching morphogenesis of the ureteric bud during fetal kidney development might be reduced in proportion to this reduction of RET expression. In our cohort of normal white infants from Montreal, the 1476(A) minor RET allele, infants with one or more 1476(A) alleles exhibited a 9.7% reduction in newborn kidney volume (normalized for body surface area) and 9.2% reduction in newborn renal function. Thus, the degree of renal hypoplasia observed in infants (9 to 10%) with one or more 1476(A) alleles is roughly commensurate with the reduction in functional RET mRNA expression (19%) measured in vitro.
To put this in a clinical perspective, the report of Keller et al.5 suggested that adults with essential hypertension are born with 47% fewer nephrons compared with those who remain normotensive. Thus, the effect of the 1476(A) allele could account for only one fifth of this clinically relevant congenital nephron deficit. Clearly, additional genes are involved in setting nephron number during development.
Heterozygous null mutations of RET have been reported in humans with Hirschprung disease. If arborization of the ureteric bud is proportional to the level of RET expression during renal development, then one might expect that congenital nephron number in Hirschprung disease should be reduced. There are reports of unilateral renal aplasia and various renal malformations in Hirschprung disease,26 but nephron number has not been carefully assessed. By the time patients with Hirschprung disease are identified, there has been ample time for compensatory renal hypertrophy to erase the initial relationship between kidney size and congenital nephron number. Thus, in the report of Keller et al.,5 describing 50% reduction in nephron number among people with essential hypertension, adult kidney mass was similar to that in normal control subjects. In contrast, we measured renal volume and function at birth before the relationship can be masked by postnatal compensatory hypertrophy.10 Indeed, our findings from autopsied newborns suggested that kidney weight correlates with congenital nephron number (adjusted for age) for up to 3 mo.
Ureteric bud cells express high levels of PAX2 during renal development; homozygous Pax2 mutant mice are anephric, and heterozygous Pax2 null mutants exhibit significant renal hypoplasia.27–29 We recently identified a fairly common (allele frequency 0.2) hypomorphic human PAX2AAA allele, which reduces PAX2 expression by 40% compared with the major wild-type PAX2GGG allele; thus, total PAX2 mRNA in a heterozygous cell is approximately 80% of normal.10 Total newborn kidney volume among infants with one or more PAX2AAA alleles was approximately 10% smaller than in PAX2GGG/ PAX2GGG infants.16 Fifteen percent of the newborn cohort in this study were compound PAX2AAA/RET1476A heterozygotes. In this subgroup, newborn KidVol/BSA was 23% smaller than in infants with homozygous wild-type RET1476G/PAX2GGG alleles. This corresponds very nicely with the observations of Clarke et al.,10 who found additive effects of compound heterozygosity for Pax2 and Ret alleles on nephron number in mutant mice. Thus, together, the two hypomorphic RET and PAX2 alleles could account for up to half of the nephron deficit (48% of control) associated with essential hypertension reported by Keller et al.5
On the basis of HapMap linkage data in the white (CEPH) population, the 1476(A) SNP is not in high genetic linkage disequilibrium with most other portions of the RET gene; however, to rule out the unlikely possibility that some other site within the RET gene is responsible for reduced kidney size, we screened for association with 22 other haplotype-tagging SNPs spanning all major linkage blocks and 10 kb to either side of the coding sequence. No other haplotype-tagging SNPs were significantly associated with either kidney size or cystatin C level (data not shown).
Interestingly, we identified a second RET SNP (rs1799939) that alters a glycine at amino acid position 691 to a serine (RET2251G/A); however, this SNP was not associated with newborn kidney size or cystatin C. Because the G/A substitution lies within a linker region between the RET transmembrane and tyrosine kinase domains, the 2251A isoform may retain sufficient signaling activity to permit normal nephrogenesis.
In conclusion, we identified a polymorphic variant (RET1476A) of the human RET receptor that increases the risk for aberrant mRNA splicing and causes decreased expression of the functional wild-type allele. The RET1476A is associated with a reduction (approximately 10%) of newborn kidney volume and an increase (approximately 9%) in umbilical cord cystatin C. Because kidney size and function were assessed at birth, before the period of postnatal hypertrophy, these measurements likely reflect congenital nephron endowment. In mice, homozygous RET mutations block ureteric bud outgrowth, and heterozygous RET null mutations have been shown to interfere with optimal nephrogenesis.10 Similarly, we previously showed that heterozygous PAX2 mutations interfere with ureteric bud branching and reduce congenital nephron number in mice; a common polymorphic variant of PAX2 causes 10% reduction in human newborn kidney volume.16 Among the 15% of normal newborns who inherit both a hypomorphic RET1476(A) and a hypomorphic PAX2AAA allele, kidney volume is reduced by 23% of wild-type controls. Clarke et al.10 also noted a synergistic effect of Pax2 and Ret mutations on ureteric bud branching and nephrogenesis in embryonic mouse kidney explants. Our observations suggest a model in which common polymorphic variants of genes involved in renal branching morphogenesis account for subtle renal hypoplasia in the normal human population.
CONCISE METHODS
Cell Culture
Human neuroblastoma cells [SK-N-BE(2) SH-SY5Y] and Wilms’ tumor cells (G401, Wit49) were obtained from ATCC (Manassas, VA). The cells were grown and maintained using standard medium and conditions according to ATCC protocols.
Reverse Transcriptase–PCR Analysis
Total RNA was isolated from cells using Qiagen RNeasy Mini-plus Kit with gDNA eliminator column (Qiagen, Mississauga, ON, Canada). Two-step reverse transcriptase–PCR was performed; first-strand cDNA was primed with random hexamers and TaqMan MultiScribe Reverse Transcriptase according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). Nested PCR was performed with the cDNA; the PCR primers are listed in Supplemental Table 1.
Study Populations
For analysis of glomerular number, we studied autopsied right kidneys from 15 infants who died of congenital anomalies within the first 3 mo of life at the University of Mississippi Medical Center. Infants with morphologic abnormalities of the kidneys were excluded. These studies were approved by the institutional review board of the University of Mississippi Medical Center. All autopsies were performed and kidney tissues were used with the permission and informed consent of county coroners and next-of-kin.
For association studies, healthy white infants (n = 136) born to women with uncomplicated pregnancies were recruited with informed parental consent at the final prenatal clinical visit to the Royal Victoria Hospital (Montreal, QC, Canada). The study (PED-04-016) was approved by the Montreal Children's Hospital Research Ethics Board. Mothers with twins, diabetes, intrauterine growth restriction, genetic abnormalities, renal malformations, hydronephrosis, or delivery at <36 wk and newborns with low birth weight (<2500 g) or low serum albumin were excluded.
Estimation of Total Glomerular (Nephron) Number
The right kidney was perfusion-fixed with 10% buffered formalin and then weighed. Kidneys were excluded from the study when the two kidneys in the one subject were unequal in size or showed macroscopic or microscopic evidence of pathology. Kidneys from 15 infants aged ≤3 mo were analyzed. Subject age, gender, race, nephron number, and kidney weight are shown in Supplemental Table 3.
After perfusion, kidneys were immersion-fixed in formalin and sent to Monash University for stereologic analysis of total nephron number (Nglom) using the physical disector/fractionator combination. This is an unbiased stereologic counting method with which all glomeruli are sampled and, thereby, counted with equal probability. Important with this method, glomeruli are counted irrespective of their size, shape, and location. Full details of this technique have been previously described in detail.30–32 The association between Nglom and kidney weight was analyzed using Pearson product moment correlation.
Kidney Volume Measurement
Left and right kidney volumes were measured by ultrasonography in newborns within the first 48 h of life using the formula kidney volume = 4/3II (length/2) (height/2) (width/2). Body surface area was calculated as the square root of [length (cm) and weight (kg)/3600] according to Mosteller.33
Renal Function Determination
Serum cystatin C was used as a surrogate of GFR.34 Cord blood cystatin C was measured by nephelometry (normal newborn range 1.17 to 3.06 ± 0.26 mg/L [SD]).15
Coding SNP Collection
The NCBI dbSNP database was screened for common coding SNP in the human RET gene with minor allele frequency of >10% in white populations. One common nonsynonymous SNP changing an amino acid was identified, and one common synonymous SNP affecting an ESE was found using the RESCU-ESE program (see Appendix).
SNP Genotyping
Genomic DNA was isolated from cord blood with the FlexiGene DNA kit (Qiagen) according to the manufacturer's protocol. For each infant, 15 ng of genomic DNA was used for multiplex genotyping, using Sequenom iPLEX PCR technology (Sequenom, San Diego, CA). This system involves extension of the PCR amplicon with modified nucleotides to distinguish SNP alleles by matrix-assisted laser desorption ionization–time of flight technology. Primers for SNP detection were designed using MassARRAY AssayDesign software (Sequenom, San Diego, CA).
Quantitative Allele Ratio Analysis
Two heterozygous cell lines (SH-SY5Y and G401) were selected for allelic expression analysis. Total RNA and genomic DNA were isolated in triplicate from the cells. PCR and reverse transcriptase–PCR amplicons were sequenced in duplicate as described by Pastinen et al.17 Sequencing primer sequences did not contain any known SNP and were used to amplify each RNA and DNA sample in duplicate (the PCR primers and sequencing primer are summarized in Supplemental Table 1). PeakPicker software (see Appendix) was used for quantitative allele ratio analysis. This program normalizes nucleotide peak amplitude for the effect of surrounding bases, and normalized ratio values were calculated in genomic DNA and mRNA (cDNA).
Statistical Analysis
Data are presented as means ± SD. Deviation from Hardy-Weinberg equilibrium was calculated by the χ2 test. Normality of data distributions for kidney volume and serum cystatin C were confirmed by tests of skewness (values of 0.501 and 0.482, respectively) and kurtosis (values of 0.216 and 0.133, respectively). Association between SNP genotypes and KidVol/BSA or cord blood cystatin C was assessed by two-tailed, independent-samples t test. The comparison of rs1800860 G/A allele expression ratios in DNA and RNA was analyzed with two-tailed t test. All data were analyzed with SPSS for Windows 11.0 (SPSS, Chicago, IL) and Microsoft Excel. The association between Nglom and kidney weight was analyzed using Pearson product moment correlation.
DISCLOSURES
None.
Acknowledgments
This work was supported by operating grants from the Canadian Institutes of Health Research (MOP 12954) and the McGill University Health Centre Research Institute, funded in part by the Fonds de Recherches en Santé du Québec. Measurements of glomerular number were funded by grants from National Institutes of Health grant 1 R01 DK065970-01, National Institutes of Health Center of Excellence in Minority Health 5P20M000534-02, and the Colonial Foundation of Australia.
Studies were conducted with informed consent from subjects and the approval of the Montreal Children's Hospital institutional review board (PED 04-016) and the institutional review board of the University of Mississippi Medical Center.
Dr. Goodyer is the recipient of a James McGill Research Chair.
APPENDIX
GenBank Accession Numbers
Human RET gene, NT_033985; human RET mRNA, NM_020630; human RET protein, NP_065681.
Web Sites
NCBI dbSNP database: http://www.ncbi.nlm.nih.gov/projects/SNP/
ESE finder software: http://rulai.cshl.edu/ESE/
RESCU-ESE program: http://genes.mit.edu/burgelab/rescue-ese/
HapMap CEU data: http://www.hapmap.org
Peakpicker software: http://genomequebec.mcgill.ca/EST-HapMap
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available at http://www.jasn.org/.
REFERENCES
- 1.Dziarmaga A, Quinlan J, Goodyer P: Renal hypoplasia: Lessons from Pax2. Pediatr Nephrol 21: 26–31, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Clark AT, Bertram JF: Molecular regulation of nephron endowment. Am J Physiol 276: F485–F497, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Garcia DL, Anderson S: Glomeruli and blood pressure: Less of one, more the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie HS, Lawler EV, Brenner BM: Congenital oligonephropathy: The fetal flaw in essential hypertension? Kidney Int Suppl 55: S30–S34, 1996 [PubMed] [Google Scholar]
- 5.Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Hoy WE, Wang Z, VanBuynder P, Baker PR, McDonald SM, Mathews JD: The natural history of renal disease in Australian Aborigines. Part 2. Albuminuria predicts natural death and renal failure. Kidney Int 60: 249–256, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Cass A, Cunningham J, Wang Z, Hoy W: Regional variation in the incidence of end-stage renal disease in Indigenous Australians. Med J Aust 175: 24–27, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Costantini F, Shakya R: GDNF/Ret signaling and the development of the kidney. Bioessays 28: 117–127, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Schuchardt A, D'Agati V, Pachnis V, Costantini F: Renal agenesis and hypodysplasia in ret-k- mutant mice result from defects in ureteric bud development. Development 122: 1919–1929, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Clarke JC, Patel SR, Raymond RM Jr, Andrew S, Robinson BG, Dressler GR, Brophy PD: Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet 15: 3420–3428, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR: ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res 31: 3568–3571, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anders J, Kjar S, Ibanez CF: Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J Biol Chem 276: 35808–35817, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Amoresano A, Incoronato M, Monti G, Pucci P, de Franciscis V, Cerchia L: Direct interactions among Ret, GDNF and GFRalpha1 molecules reveal new insights into the assembly of a functional three-protein complex. Cell Signal 17: 717–727, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Goodyer P, Kurpad A, Rekha S, Muthayya S, Dwarkanath P, Iyengar A, Philip B, Mhaskar A, Benjamin A, Maharaj S, Laforte D, Raju C, Phadke K: Effects of maternal vitamin A status on kidney development: A pilot study. Pediatr Nephrol 22: 209–214, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A: Cystatin C as a marker of GFR: History, indications, and future research. Clin Biochem 38: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Quinlan J, Lemire M, Hudson T, Qu H, Benjamin A, Roy A, Pascuet E, Goodyer M, Raju C, Zhang Z, Houghton F, Goodyer P: A common variant of the PAX2 gene is associated with reduced newborn kidney size. J Am Soc Nephrol 18: 1915–1921, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Pastinen T, Ge B, Gurd S, Gaudin T, Dore C, Lemire M, Lepage P, Harmsen E, Hudson TJ: Mapping common regulatory variants to human haplotypes. Hum Mol Genet 14: 3963–3971, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Brophy PD, Ostrom L, Lang KM, Dressler GR: Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128: 4747–4756, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Dressler GR: PTEN modulates GDNF/RET mediated chemotaxis and branching morphogenesis in the developing kidney. Dev Biol 307: 290–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plaza-Menacho I, van der Sluis T, Hollema H, Gimm O, Buys CH, Magee AI, Isacke CM, Hofstra RM, Eggen BJ: Ras/ERK1/2-mediated STAT3 Ser727 phosphorylation by familial medullary thyroid carcinoma-associated RET mutants induces full activation of STAT3 and is required for c-fos promoter activation, cell mitogenicity, and transformation. J Biol Chem 282: 6415–6424, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis V, Takahashi M, Costantini F: Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development 133: 4507–4516, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M: Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382: 70–73, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A: GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 21: 53–62, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, Ichihara M, Takahashi M: A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol 24: 8026–8036, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain S, Naughton CK, Yang M, Strickland A, Vij K, Encinas M, Golden J, Gupta A, Heuckeroth R, Johnson EM Jr, Milbrandt J: Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development 131: 5503–5513, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Sinnassamy P, Yazbeck S, Brochu P, O'Regan S: Renal anomalies and agenesis associated with total intestinal aganglionosis. Int J Pediatr Nephrol 7: 1–2, 1986 [PubMed] [Google Scholar]
- 27.Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P: Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109: 787–795, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Porteous S, Torban E, Cho NP, Cunliffe H, Chua L, McNoe L, Ward T, Souza C, Gus P, Giugliani R, Sato T, Yun K, Favor J, Sicotte M, Goodyer P, Eccles M: Primary renal hypoplasia in humans and mice with PAX2 mutations: Evidence of increased apoptosis in fetal kidneys of Pax2(1Neu) +/- mutant mice. Hum Mol Genet 9: 1–11, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Dziarmaga A, Eccles M, Goodyer P: Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol 17: 1568–1575, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Bertram JF: Analyzing renal glomeruli with the new stereology. Int Rev Cytol 161: 111–172, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int 63: 2113–2122, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Nyengaard JR: Stereologic methods and their application in kidney research. J Am Soc Nephrol 10: 1100–1123, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Mosteller RD: Simplified calculation of body-surface area. N Engl J Med 317: 1098, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Harmoinen A, Ylinen E, Ala-Houhala M, Janas M, Kaila M, Kouri T: Reference intervals for cystatin C in pre- and full-term infants and children. Pediatr Nephrol 15: 105–108, 2000 [DOI] [PubMed] [Google Scholar]