Abstract
OBJECTIVE—To evaluate whether treatment with insulin is advantageous compared with oral antidiabetes agents in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy.
RESEARCH DESIGN AND METHODS—Newly diagnosed type 2 diabetic patients with severe hyperglycemia were hospitalized and treated with intensive insulin injections for 10–14 days. The oral glucose tolerance test (OGTT) was performed after intensive insulin treatment. After discharge, the patients were randomized to receive either insulin injections or oral antidiabetes drugs (OADs) for further management. The OGTT was repeated 6 months later, and β-cell function and insulin sensitivity were evaluated again. These subjects were continually followed up for another 6 months to evaluate their long-term glycemic control.
RESULTS—At the 6th month of the study, the A1C level was significantly lower in the insulin group than in the OAD group (6.33 ± 0.70% vs. 7.50 ± 1.50%; P = 0.002). During the follow-up visit, the A1C level was still better in the insulin group (6.78 ± 1.21% vs. 7.84 ± 1.74%; P = 0.009). All parameters regarding β-cell function measured in the OGTT were improved significantly in both groups after 6 months of treatment. Compared with the OAD group, the homeostasis model assessment of β-cell function index, insulin area under the curve, and insulinogenic index were better in the insulin group.
CONCLUSIONS—A 6-month course of insulin therapy, compared with OAD treatment, could more effectively achieve adequate glycemic control and significant improvement of β-cell function in new-onset type 2 diabetic patients with severe hyperglycemia.
Insulin resistance and impaired insulin secretion are the main pathophysiological defects responsible for the development of hyperglycemia in type 2 diabetes (1,2). With the continuous presence of insulin resistance, progressive loss of β-cell function is the crucial defect. The continuous decline in β-cell function is affected by glucotoxicity generated by hyperglycemia and lipotoxicity due to lipolysis (3). Impaired β-cell function appears to be reversible, particularly in the early stage of the disease, when the limiting threshold for reversibility of decreased β-cell mass has probably not been passed (4). So the potential benefits of early, aggressive intervention with insulin treatment to counter both β-cell dysfunction and insulin resistance must be considered. Several reports (5–7) have shown that short-term intensive insulin therapy can induce long-term glycemic control in newly diagnosed type 2 diabetic patients with mild to moderate hyperglycemia. However, more than half of these patients require oral antidiabetes drug (OAD) therapy within 1 year to maintain near-euglycemia.
When a new-onset type 2 diabetic patient presents with severe hyperglycemia, there are defects in insulin secretion and action, which is optimally treated with aggressive insulin injections (8,9). After the symptoms have been relieved, it may be possible to withdraw insulin and shift to oral agents. We hypothesized that continuous insulin therapy for a few months in new-onset type 2 diabetes with severe hyperglycemia may have a prolonged glycemic control. To address this concept, we designed this 6-month study to evaluate whether treatment with insulin is advantageous compared with OADs in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy.
RESEARCH DESIGN AND METHODS
Consecutive newly diagnosed type 2 diabetic patients with severe hyperglycemia (fasting plasma glucose [FPG] >300 mg/dl or random plasma glucose >400 mg/dl) were recruited between October 2005 and December 2006. All patients were admitted to the hospital and received intensive insulin therapy. The excluding criteria included active liver disease, serum creatinine concentration >2.0 mg/dl after 5–10 days of therapy, proliferative diabetic retinopathy, definite coronary artery disease, malignancy, and pregnancy. The patients with peak C-peptide levels during the oral glucose tolerance test (OGTT) <2.0 ng/ml were also excluded to rule out type 1 diabetes and latent autoimmune diabetes in adults. The study was approved by the institutional review board of the Taipei Veterans General Hospital, and written informed consent was given before the OGTT.
During the hospitalization
The basal and premeal insulin doses were adjusted according to the preprandial and bedtime capillary blood glucose levels. The target glucose levels were preprandial blood glucose 90–130 mg/dl and bedtime blood glucose 100–160 mg/dl. After 10–14 days of intensive insulin treatment, with their fasting blood glucose levels between 100 and 140 mg/dl, subjects received a 75-g OGTT after discontinuing regular insulin for ∼12 h and NPH insulin for about 24 h. Baseline blood samples were drawn for A1C, cholesterol, triglycerides, glucose, insulin, C-peptide, and other biochemicals. Blood samples were further collected for glucose and insulin at 30, 60, 90, and 120 min and C-peptide at 120 min.
Outpatient clinic follow-up
All subjects were discharged after 10–14 days of intensive insulin therapy and then randomized into two groups: continuing with insulin treatment or shifting to OADs. Subjects were then followed up as outpatients and visited our clinic every 2 weeks during the first 2 months and then every 4 weeks for another 4 months.
In the insulin therapy group, subjects were instructed in the techniques for NPH insulin (Insulatard; Novo Nordisk, Bagsværd, Denmark) injection and home capillary glucose monitoring. Two-thirds of the daily dose was administrated before breakfast and the other was administrated at bedtime. Insulin doses were titrated every 3 days to achieve target FPG values between 90 and 130 mg/dl.
The titration of OADs in our protocol was modified from the Steno-2 Study published in 2003 (10). As the initial step, overweight or obese patients (defined as BMI >25 kg/m2) received metformin (submaximal dose 500 mg t.i.d.) and lean patients received a sulfonylurea (gliclazide-MR, submaximal dose 90 mg per day). The dosage was titrated based on the FPG on the visiting day to achieve target values between 90 and 130 mg/dl. As the second step, metformin was given to the lean patients and gliclazide-MR to overweight or obese patients. As the third step, gliclazide-MR should be uptitrated to a maximum dose of 120 mg per day and metformin to 2,550 mg per day with a splitting dose.
Clinical examination
A1C measurements were further performed at 3 and 6 months, and the OGTT was repeated after 6 months of randomization. We stopped pharmacological treatment for ∼12 h (metformin after the evening dose) and 24 h (gliclazide-MR and insulatard after the morning dose) before performing the OGTT. Area under the curve (AUC) for glucose and insulin during the OGTT were calculated by the trapezoid rule. Early-phase insulin secretion (insulinogenic index) was calculated as the ratio between incremental plasma insulin and glucose concentrations during the first 30 min of the OGTT (ΔI0–30/ΔG0–30). Total insulin secretion was calculated as the ratio between the incremental AUC of insulin and glucose during the OGTT (ΔI[AUC]/ΔG[AUC]). The Matsuda index was calculated for insulin resistance, as previously reported (11). Homeostasis model assessment (HOMA) was used to estimate insulin resistance (HOMA-IR) and β-cell function (HOMA-β) (12).
Follow-up examination
After 6 months of intervention, patients in the insulin group also shifted to OADs for further management. All of these subjects were continually followed up in our clinics for another 6 months to evaluate their long-term glycemic control.
Analytical methods
Plasma insulin levels were assayed using direct chemiluminescent technology with a two-site sandwich immunoassay (ADVIA Centaur; Bayer, Tokyo, Japan). The A1C was measured using high-performance liquid chromatography instruments (HLC-723 GHB IIIs; Tosoh, Tokyo, Japan) with a reference range of 4.5–6.2%.
Outcomes
The primary outcome was the comparison of A1C change and the proportion of subjects who reached the treatment target (A1C ≤7.0 or ≤6.5% at 6 and 12 months, respectively). The secondary outcome was the β-cell function and insulin sensitivity calculated from the OGTT, hypoglycemic rate, and weight change.
Statistical analyses
The SPSS program for Windows, version 15.0, was used for data analysis. The paired Student's t test was used to analyze the difference from baseline to the end point, and the independent Student's t test was used to compare differences between the management programs. Changes from baseline in A1C, mean self-monitored blood glucose, insulin dose, OAD dose, body weight, and hypoglycemic events were analyzed using one-way ANOVA. Data are presented as means ± SD, unless otherwise stated, and a P value of <0.05 was taken to indicate a significant difference.
RESULTS
Study design
This study was a randomized, open-label, parallel trial (online appendix figure [available at http://dx.doi.org/10.2337/dc08-0075]). Randomization was performed after 10–14 days of intensive insulin treatment. Because we suspected that some patients would refuse insulin therapy after randomization, we attempted to minimize patient dropout by randomizing in a three-to-two fashion between OADs and insulin. There were 60 patients with type 2 diabetes who were assessed for eligibility, and 50 subjects were randomized. Thirty patients were randomly assigned to receive insulin therapy and 20 patients to undergo OAD treatment.
Baseline characteristics
The baseline clinical characteristics and biochemical status of the patients in the insulin and OAD groups are shown in Table 1. The data presented in Table 1 were obtained after 10–14 days of intensive insulin therapy, except for peak fasting and random plasma glucose levels. The two treatment groups did not differ significantly in baseline clinical features.
Table 1.
Baseline demographic and clinical characteristics in the two treatment groups
| Insulin group (intention to treat) | OAD group (intention to treat) | Insulin group (A1C <7.0%) | OAD group (A1C <7.0%) | |
|---|---|---|---|---|
| n | 25 | 19 | 22 | 8 |
| Age (years) | 57.9 ± 8.5 | 59.6 ± 12.6 | 58.7 ± 16.0 | 56.5 ± 15.9 |
| Sex (male:female) | 19:6 | 13:6 | 17:5 | 5:3 |
| Body weight (kg) | 71.4 ± 10.6 | 71.7 ± 21.3 | 71.2 ± 10.3 | 71.8 ± 23.6 |
| BMI (kg/m2) | 27.55 ± 4.20 | 28.31 ± 6.20 | 27.69 ± 6.58 | 26.64 ± 8.01 |
| Peak FPG (mg/dl)* | 345.0 ± 82.2 | 329.2 ± 24.0 | 338.6 ± 66.4 | 311.3 ± 94.0 |
| Peak plasma glucose (mg/dl)* | 527.3 ± 163.8 | 483.7 ± 217.2 | 557.4 ± 160.9 | 487.6 ± 142.1 |
| A1C (%) | 11.89 ± 1.91 | 11.33 ± 1.57 | 11.73 ± 1.94 | 11.29 ± 1.46 |
| Systolic blood pressure (mmHg) | 125.4 ± 13.4 | 130.7 ± 12.9 | 129.2 ± 12.4 | 130.0 ± 15.7 |
| Diastolic blood pressure (mmHg) | 74.2 ± 10.6 | 78.5 ± 8.1 | 76.1 ± 10.0 | 79.7 ± 11.3 |
| Total cholesterol (mg/dl) | 193.1 ± 54.8 | 184.7 ± 39.5 | 202.3 ± 56.9 | 197.2 ± 25.0 |
| HDL cholesterol (mg/dl) | 45.9 ± 15.1 | 45.7 ± 12.7 | 44.4 ± 14.2 | 45.7 ± 16.0 |
| Triglycerides (mg/dl) | 135 (52–1,234) | 131 (34–1,074) | 135 (52–1,234) | 92 (34–794) |
| Urine albumin-to-creatinine ratio (mg/g) | 14.1 (3.2–293.9) | 17.3 (4.2–626.2) | 15.4 (3.2–293.9) | 17.9 (6.7–418.0) |
Data are means ± SD or means (range).
Peak FPG and plasma glucose indicate the peak glucose level before randomization. The two right columns revealed those subjects whose A1C level was <7.0%. Since the patients in the OAD group did not achieve the same glycemic target as the insulin group, we therefore only chose those with A1C level <7% to assess β-cell function and insulin resistance.
Insulin and OAD dosage
During the study period, the insulin dose was decreased from 26.4 ± 10.5 IU/day to 16.8 ± 11.0 IU/day. Eleven patients started with gliclazide-MR and eight with metformin. The OAD dosage was increased gradually and titrated to 54.5 ± 22.5 mg/day of gliclazide-MR and 884 ± 416 mg/day of metformin at the end of the intervention. There were four patients who only used gliclazide-MR (mean dose 45 mg) and four patients who only used metformin (mean dose 750 mg), and six of them reached the target A1C (<7.0%). There were 10 patients who combined both drugs (mean dose gliclazide-MR 60 mg and metformin 1,200 mg).
Glycemic control
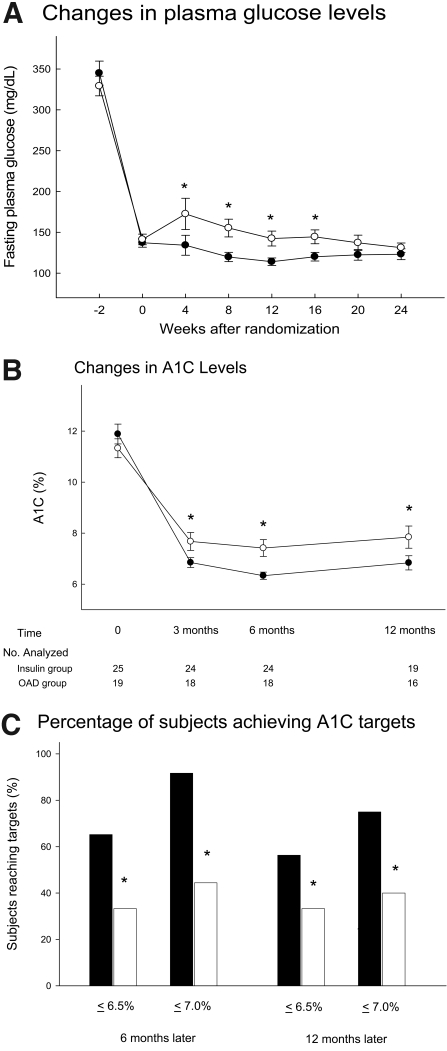
Figure 1A shows the FPG concentration in both groups in the study period. The FPG level was stable in the insulin group, while it increased in week 2 and week 4 and then decreased gradually in the OAD group. Figure 1B reveals the A1C changes in both groups during the study period and follow-up visits. At the end of the intervention, the A1C level was significantly lower in the insulin group than in the OAD group (6.33 ± 0.70% vs. 7.50 ± 1.50%; P = 0.002). During the follow-up visits, the A1C level was still better in the insulin group (6.78 ± 1.21% vs. 7.84 ± 1.74%; P = 0.009). Figure 1C shows the proportion with an A1C level <6.5 or 7.0% at 6 months and 1 year. The proportions of patients with A1C levels reaching these targets at 6 months and 1 year were significantly greater in the insulin group (P < 0.001).
Figure 1.
Glycemic control in the insulin treatment group and OAD treatment group. A: FPG concentration (mean ± SE) in both groups in the study period; −2 weeks means prerandomization. •, insulin group; ○, OAD group. B: The A1C changes (mean ± SE) in both groups during the study period and follow-up visit. •, insulin group; ○, OAD group. C: The proportion with A1C <6.5 or 7.0% at 6 months and 1 year. *P < 0.05 between groups. ▪, insulin group; □, OAD group.
β-Cell function and insulin resistance
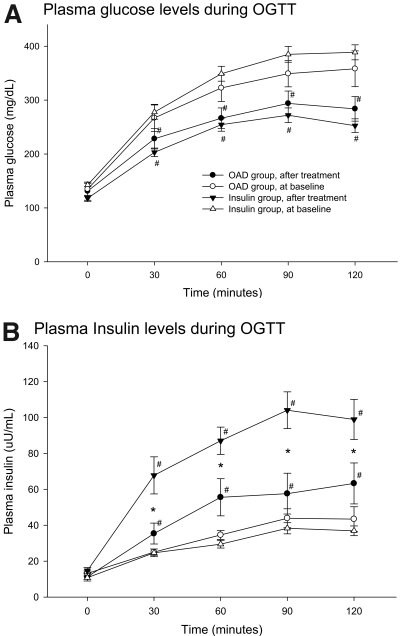
Since the patients in the OAD group did not achieve the same glycemic target as the insulin group, we therefore only chose those with A1C level <7% to assess β-cell function and insulin resistance. There was no difference between the two subgroups of the OAD group at the baseline (online appendix Table). Table 2 reveals the changes in biochemical measurements over the course of the study, and the plasma glucose and insulin excursions at each OGTT are illustrated in Fig. 2. All parameters regarding β-cell function measured in the OGTT were improved significantly in both groups after 6 months of intensive treatment. Compared with the OAD group, the HOMA-β index, insulin AUC, and insulinogenic index were significantly improved in the insulin group. The HOMA-IR and Matsuda index for insulin resistance showed no significant change from baseline to the end of the intervention and without differences between the two groups.
Table 2.
Measures of glycemia and insulin secretion during OGTT before and after intensive treatment
| Start of study period |
End of intervention period |
|||
|---|---|---|---|---|
| Insulin group | OAD group | Insulin group | OAD group | |
| n | 22 | 8 | 22 | 8 |
| Sex (male:female) | 17:5 | 5:3 | 17:5 | 5:3 |
| A1C (%) | 11.73 ± 1.94 | 11.29 ± 1.46 | 6.15 ± 0.51* | 6.40 ± 0.39* |
| HOMA-IR | 3.81 ± 1.48 | 4.33 ± 1.42 | 4.39 ± 2.85 | 3.95 ± 3.23 |
| HOMA-β (%) | 49.7 ± 19.7 | 67.0 ± 31.0 | 111.2 ± 66.7*† | 69.1 ± 33.5 |
| Glucose AUC (mg · h−1 · dl−1) | 639.1 ± 102.7 | 586.5 ± 120.8 | 457.5 ± 87.5* | 498.1 ± 107.8* |
| Insulin AUC (μU · h−1 · ml−1) | 58.3 ± 18.7 | 66.1 ± 16.9 | 158.0 ± 71.9*† | 93.04 ± 44.7* |
| Insulin0–30/Glucose0–30(μU/mg) | 1.30 ± 0.85 | 1.39 ± 1.22 | 6.48 ± 5.05*† | 2.78 ± 1.78* |
| Matsuda index | 114.9 ± 31.4 | 108.4 ± 14.2 | 86.0 ± 25.5 | 104.3 ± 22.4 |
| ΔInsulinAUC/ΔGlucoseAUC (μU/mg) | 1.17 ± 0.71 | 1.17 ± 0.71 | 6.52 ± 4.20*† | 3.33 ± 2.11* |
Data are means ± SD. We only chose those patients with A1C level <7.0% to assess β-cell function and insulin sensitivity.
P < 0.05 vs. baseline;
P < 0.05 between each group at the end of the study.
Figure 2.
Mean ± SE for plasma glucose (A) and insulin (B) concentration during the OGTT at baseline and 6 months later in both groups. *P < 0.05 between groups; #P < 0.05 baseline vs. after treatment. •, OAD group, after treatment; ○, OAD group, at baseline; ▾, insulin group, after treatment; ▵, insulin group, at baseline.
Adverse events
No severe hypoglycemia occurred in either group. The overall rate of minor hypoglycemia showed no significant difference between the two groups (1.39 ± 1.16 vs. 2.30 ± 1.87 episodes; P = 0.082). There was a small increase in body weight from baseline to the end point in the insulin group (from 71.4 ± 10.6 to 73.1 ± 11.6 kg; P = 0.028) and the OAD group (from 71.7 ± 21.3 to 72.5 ± 18.8 kg; P = 0.021), but there was no significant difference between the two groups.
CONCLUSIONS
The study showed that desired glycemic control was successfully achieved by intensive insulin therapy for 10–14 days in cases of newly diagnosed type 2 diabetes with severe hyperglycemia. However, most of these subjects required pharmacological therapy to maintain near-euglycemia in our study period. A 6-month course of further insulin therapy, compared with OAD treatment, could more effectively achieve a near-normal A1C level. We also found that parameters of the β-cell function were better improved in the insulin-treated than in the OAD-treated patients.
There has emerged evidence that short-term intensive insulin therapy in newly diagnosed type 2 diabetes could improve glycemic control associated with improved insulin secretion (5–7). Ryan et al. (6) recently reported that, in 16 newly diagnosed type 2 diabetic case subjects with moderate hyperglycemia (mean fasting blood glucose of 239 mg/dl), a 2- to 3-week course of intensive insulin therapy was able to maintain good glycemic control at 1 year in seven of the subjects. In a similar study (7), 138 newly diagnosed type 2 diabetic patients with fasting blood glucose >200 mg/dl (mean fasting blood glucose of 268 mg/dl, peak blood glucose of 390 mg/dl) were hospitalized and treated with continuous subcutaneous insulin infusion for 2 weeks. Optimal glycemic control was achieved within 6.3 ± 3.9 days in 126 patients. The remission rate at the 12th month was 47.1%. In patients with moderate hyperglycemia, a 2-week course of intensive insulin therapy achieving near-euglycemia might induce long-term glycemic control. This result may not be suitable in patients with severe hyperglycemia, such as our subjects with mean initial fasting blood glucose of 338 mg/dl and peak blood glucose of 508 mg/dl. All of our study subjects had received 10–14 days of intensive insulin therapy in hospital to make sure the glycemic control was optimal. After randomization, almost all of the patients were unable to maintain euglycemia without medication. Our data revealed that 10–14 days of intensive insulin treatment with near-normoglycemia cannot maintain good glycemic control lasting for a long period. We suggest that short-term intensive insulin therapy may induce long-term glycemic control in newly diagnosed type 2 diabetes with moderate hyperglycemia but not in patients with severe hyperglycemia. Further treatment with insulin for at least 1 year was necessary to maintain the euglycemia and improve β-cell function.
The favorable effect of insulin treatment on endogenous insulin secretion in our study could be due to better glycemic control. Glucose toxicity has been demonstrated clinically and has been investigated extensively in the laboratory (13). Defects in insulin secretion have been documented and directly related to hyperglycemia and are correctable with the establishment of euglycemia (14,15). Thus, the shorter the period of antecedent glucotoxicity, the more likely the full recovery of β-cell function. Our results do support the concept that correction of hyperglycemia can improve insulin secretion. Another possibility is that β-cell secretory capacity may have been restored by “rested” β-cells induced by insulin injection (16). In our study, most of the subjects required pharmacological therapy to maintain near-euglycemia after discontinuing insulin therapy. In the insulin-treated subjects, the fasting blood glucose levels were maintained between 90 and 130 mg/dl, with the insulin dose decreased from 26.4 to 16.8 IU per day, which means endogenous insulin secretion was increased. In the OAD-treated patients, however, the OAD doses were uptitrated in the following 6 months to reach our glycemic target. Our data provide evidence that a short-term intensive insulin therapy can shorten the period of glucotoxicity and another 6 months of insulin therapy can further improve endogenous insulin secretion.
Some reports have shown that induction of normoglycemia in type 2 diabetes results in improved insulin resistance (17–19). In our present study, the insulin resistance measured by HOMA-IR and Matsuda index showed no significant change from baseline to the end of the intervention in both groups and without significant difference between the two groups. The insulin resistance measured during the OGTT in our study was performed after intensive insulin therapy for 10–14 days. Some degree of insulin resistance might have been corrected in the 10–14 days of intensive insulin therapy. Both intensive therapy, either with OADs or insulin, could not further increase insulin sensitivity by improving glycemic control in the following 6 months. This should be confirmed by further study.
One limitation of our study is that some subjects in the OAD group were treated with different orders of gliclazide-MR and metformin. Sulfonylureas and metformin have different actions on the insulin sensitivity and secretion in type 2 diabetic patients. Our titration protocol was also inadequate to fully treat the patients randomized to OADs. It might have been more effective to use a modern OAD protocol utilizing self–blood glucose monitoring and more frequent titration to rapidly achieve a full dose and get more optimal glycemic control. Further studies will be required to intensively treat patients with insulin or one OAD to achieve the same glycemic control and then compare the β-cell function. Another limitation is that the numbers in this study were small and used indirect methods for assessing β-cell function and insulin sensitivity. The results need to confirm with a larger study and a better methodology before being considered as a routine clinical option.
In conclusion, our data demonstrated that intensive insulin therapy for 10–14 days can achieve optimal glycemic control in newly diagnosed type 2 diabetes with severe hyperglycemia but cannot induce a long-term glycemic control. A 6-month course of further insulin therapy, compared with OAD treatment, could more effectively maintain adequate glycemic control accompanied with significant improvement of β-cell function. Therefore, in the management of newly diagnosed type 2 diabetic patients with severe hyperglycemia, strong consideration should be given to early, aggressive insulin therapy for a rapid and sustained effect on glycemic control and β-cell function.
Supplementary Material
Acknowledgments
This study was supported by grants from the Taipei Veterans General Hospital (VasC1-031) and the Department of Health (DOH96-PA-1013-J) in Taiwan.
Published ahead of print at http://care.diabetesjournals.org on 12 June 2008.
Clinical trial reg. no. NCT00506194, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
See accompanying editorial, p. 2070
References
- 1.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Charles MA, Bennett PH: A two-step model for development of non-insulin-dependent diabetes. Am J Med 90:229–235, 1991 [PubMed] [Google Scholar]
- 2.Nathan DM: Clinical practice: initial management of glycemia in type 2 diabetes mellitus. N Engl J Med 347:1342–1349, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Robertson RP, Harmon J, Tran PO, Poitout V: β-Cell glucotoxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 53(Suppl. 1):S119–S124, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Wajchenberg BL: Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28:187–218, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Alvarsson M, Sundkvist G, Lager I, Henricsson M, Berntorp K, Fernqvist-Forbes E, Steen L, Westermark G, Westermark P, Orn T, Grill V: Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care 26:2231–2237, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Imes S, Wallace C: Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 27:1028–1032, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Xu W, Liao Z, Yao B, Chen X, Huang Z, Hu G, Weng J: Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of β-cell function. Diabetes Care 27:2597–2602, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B: Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 29:1963–1972, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bloomgarden ZT: Exploring treatment strategies for type 2 diabetes. Diabetes Care 30:2737–2745, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348:383–393, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic glucose clamp. Diabetes Care 22:1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R: Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 13.Yki-Järvinen H: Toxicity of hyperglycaemia in type 2 diabetes. Diabetes Metab Rev 14(Suppl. 1):S45–S50, 1998 [PubMed] [Google Scholar]
- 14.Hidaka H, Nagulesparan M, Klimes I, Clark R, Sasaki H, Aronoff SL, Vasquez B, Rubenstein AH, Unger RH: Improvement of insulin secretion but not insulin resistance after short term control of plasma glucose in obese type II diabetics. J Clin Endocrinol Metab 54:217–222, 1982 [DOI] [PubMed] [Google Scholar]
- 15.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG: The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes 34:222–234, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick ED, Robertson RP: Differentiation between glucose-induced desensitization of insulin secretion and β-cell exhaustion in the HIT-T15 cell line. Diabetes 47:606–611, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Samanta A, Burden AC, Jones GR, Clarkson L: The effect of short-term intensive insulin therapy in non-insulin-dependent diabetes who had failed on sulphonylurea therapy. Diabetes Res 3:269–271, 1986 [PubMed] [Google Scholar]
- 18.Yki-Järvinen H, Esko N, Eero H, Taskmo MR: Clinical benefits and mechanisms of sustained response to intermittent insulin therapy in type 2 diabetic patients with secondary drug failure. Am J Med 84:185–192, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Glaser B, Leibovich G, Nesher R, Hartling S, Binder C, Cerasi E: Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol 118:365–373, 1988 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.