Abstract
OBJECTIVE—β-Cell function in type 1 diabetes clinical trials is commonly measured by C-peptide response to a secretagogue in either a mixed-meal tolerance test (MMTT) or a glucagon stimulation test (GST). The Type 1 Diabetes TrialNet Research Group and the European C-peptide Trial (ECPT) Study Group conducted parallel randomized studies to compare the sensitivity, reproducibility, and tolerability of these procedures.
RESEARCH DESIGN AND METHODS—In randomized sequences, 148 TrialNet subjects completed 549 tests with up to 2 MMTT and 2 GST tests on separate days, and 118 ECPT subjects completed 348 tests (up to 3 each) with either two MMTTs or two GSTs.
RESULTS—Among individuals with up to 4 years’ duration of type 1 diabetes, >85% had measurable stimulated C-peptide values. The MMTT stimulus produced significantly higher concentrations of C-peptide than the GST. Whereas both tests were highly reproducible, the MMTT was significantly more so (R2 = 0.96 for peak C-peptide response). Overall, the majority of subjects preferred the MMTT, and there were few adverse events. Some older subjects preferred the shorter duration of the GST. Nausea was reported in the majority of GST studies, particularly in the young age-group.
CONCLUSIONS—The MMTT is preferred for the assessment of β-cell function in therapeutic trials in type 1 diabetes.
The measurement of C-peptide in response to a stimulus provides a direct measure of β-cell function. Whereas type 1 diabetes results from an immune-mediated loss of pancreatic β-cells, higher residual β-cell function early in the disease has strong long-term beneficial effects (1). The international diabetes research community has highlighted the need to establish the validity of C-peptide and other assessments used in clinical trials in recent-onset type 1 diabetes (2), weighing the scientific properties of a test against the burden imposed on a subject.
A recently issued draft guidance recognized preservation of β-cell function, as measured by C-peptide, as an appropriate outcome for therapeutic trials in early type 1 diabetes (3). Results from trials using therapies such as immunosuppression, T-cell modulation, B-cell modulation, costimulation blockade, antigen-specific therapy, and metabolic control are likely to change the standard of care for individuals with type 1 diabetes.
Generally, one of two methods for stimulating C-peptide response is used in clinical trials. In the mixed-meal tolerance test (MMTT), commonly used in the U.S., a liquid meal (Sustacal/Boost) is ingested in the fasting state with timed measurements of C-peptide over the subsequent 2–4 h. In the glucagon stimulation test (GST), glucagon is injected intravenously with timed measurements of C-peptide over the subsequent 10 min. Two parallel studies were conducted simultaneously by the Type 1 Diabetes TrialNet (TrialNet) Research Group and the European C-peptide Trial (ECPT) Study Group to evaluate the properties and tolerability of these tests.
RESEARCH DESIGN AND METHODS
Details about study design and laboratory and statistical methods are provided in an online appendix (available at http://dx.doi.org/10.2337/dc07-2451).
TrialNet enrolled 148 subjects between September 2004 and December 2005 at 14 clinical centers in North America, 1 in Europe, and 1 in Australia. The ECPT enrolled 118 subjects between April 2004 and December 2004 in 12 European centers. Institutional or ethics board approval was obtained at each site, and each subject or parent, as appropriate, provided informed consent and/or assent. Type 1 diabetes was diagnosed by American Diabetes Association criteria within the past 1 month to 3 years (TrialNet) or by the World Health Organization 1999 criteria within 4 years (ECPT). Patients being treated with drugs that influence β-cell function or influence insulin sensitivity were excluded.
TrialNet randomly allocated subjects 8–35 years of age to receive either two MMTTs followed by two GSTs or the opposite sequence 3–10 days apart. ECPT randomly allocated subjects to receive two MMTTs or GSTs followed by one GST or MMTT. Subjects were stratified by age (8–17 vs. 18–35 years) and duration of diabetes (1 year ± 3 months, 2 years ± 6 months, or 4 years ± 12 months) with approximately equal numbers within each stratum. The latter two duration subgroups were randomly allocated together.
The MMTTs and GSTs were initiated before 10 a.m. Subjects receiving a continuous subcutaneous insulin infusion (insulin pump) could continue to use the usual basal rate. Subjects were instructed to withhold long-acting insulin on the morning of the test. TrialNet allowed use of rapid-acting insulin by injection or continuous subcutaneous insulin infusion up to 2 h before the test and short-acting insulin up to 6 h before the test. ECPT subjects withheld rapid or short-acting insulin for 6 h before the test. Tests were rescheduled if the subject had a capillary glucose value >200 mg/dl or <70 mg/dl. C-peptide concentrations are presented as picomoles per milliliter.
Statistical methods
The ECPT GST only obtained a 6-min value, which was treated as the peak. The poststimulus area under the curve (AUC) was calculated using the trapezoidal rule. The mean AUC (in picomoles per milliliter) is the AUC divided by the time of the test. Distributions were positively skewed, and a log transformation was used.
The covariate-adjusted mean C-peptide value for MMTT and GST was estimated, and the difference was tested using normal error regression models for repeated measures (4). The reliability of repeated MMTT or GST tests in the same subject was assessed by the intraclass correlation coefficient (5). The difference in reliability of the MMTT versus the GST in TrialNet was tested (6) using subjects who completed all four visits. All analyses were performed using the Statistical Analysis System (SAS Institute, Cary, NC).
RESULTS
TrialNet randomly allocated 77 subjects to receive two MMTTs followed by two GSTs and 71 subjects to receive the opposite sequence. Of these, 123 completed all four tests, 15 completed three of the four tests, and 10 completed one or two of the tests. ECPT randomly allocated 59 subjects to receive two GSTs followed by a single MMTT and 59 subjects to receive the opposite sequence. Of these, 115 completed three tests and 3 completed only one test. The baseline characteristics were similar within each sequence group within each study and were comparable across the two studies with the exception of differences by design in duration of type 1 diabetes (Table 1). (See also supplemental Tables A1–A6 and Figs. A1 and A2 in the online appendix.)
Table 1.
Baseline characteristics of the TrialNet and ECPT participants
| TrialNet | ECPT | Combined data* | |
|---|---|---|---|
| n | 148 | 118 | 230 |
| Male sex (%) | 91 (61) | 70 (59) | 138 (60) |
| Age (years) | |||
| Median (range) | 15 (8–35) | 19 (8–40) | 16 (8–35) |
| Means ± SD | 16.2 (6.19) | 20.3 (7.54) | 17.2 (6.53) |
| Categories | |||
| 8–12 years (%) | 51 (34) | 26 (22) | 73 (32) |
| 13–17 years (%) | 49 (33) | 26 (22) | 66 (29) |
| ≥18 years (%) | 48 (32) | 66 (56) | 91 (40) |
| Race | |||
| White | 128 (86) | 116 (98) | 208 (90) |
| African American | 6 (4) | 0 (0) | 6 (3) |
| American Indian | 3 (1) | 0 (0) | 3 (1) |
| Asian | 4 (3) | 1 (0.85) | 5 (2) |
| Other | 7 (5) | 1 (0.85) | 8 (3) |
| Ethnicity | |||
| % Hispanic | 10 (7) | N/A | N/A |
| Duration of type 1 diabetes (years) | |||
| Median (range) | 1.36 (0.08–2.98) | 2.32 (0.75–4.97) | 1.49 (0.08–2.98) |
| Mean ± SD | 1.4 ± 0.88 | 2.5 ± 1.27 | 1.54 ± 0.84 |
| Categories | |||
| 0 to <1 year | 60 (41) | 15 (13) | 75 (33) |
| 1 to <2 years | 39 (26) | 34 (29) | 72 (31) |
| 2 to <3 years | 49 (33) | 34 (29) | 83 (36) |
| 3 to <4 years | N/A | 14 (12) | N/A |
| 4 to <5 years | N/A | 21 (18) | N/A |
| A1C (%) | 7.3 ± 1.4 |
Data are n (%), median (range), and means ± SD.
Restricted to TrialNet and ECPT study subjects with diabetes duration of 3 months to 3 years and 8–35 years of age. N/A, not applicable.
Measurable values
The C-peptide assay cannot measure a value below the lower limit of quantification (LOQ). In TrialNet, the LOQ was lowered during the study. Among assays completed with the new limit, C-peptide was measurable in 88% of the basal samples, 87–95% of stimulated MMTT samples, and 92–94% of stimulated GST samples. Results in the ECPT were similar. There were no significant differences either between tests (MMTT versus GST) or between studies (TrialNet versus ECPT). Specimens with nonmeasurable C-peptide were assigned the LOQ value at the time of testing.
C-peptide values
In TrialNet, the MMTT values increased steadily over the first hour and then increased more slowly during the second hour, nearly doubling over the fasting concentration. The GST values reached a peak at 6 min and then declined. A similar pattern was observed in the ECPT among the 82 subjects in the same range of age (8–35 years) and duration (3 months–3 years) as in TrialNet. The mean ± SE time to the peak value was 88.4 ± 2.5 min for the TrialNet MMTT, 80.3 ± 10.7 min for the ECPT MMTT, and 5.8 ± 0.3 min for the TrialNet GST. Summary measures from each test were highly correlated. The correlations between MMTT fasting and stimulated peak and between MMTT fasting and AUC were at least 0.92; those for the GST measures were slightly lower.
Table 2 presents fasting and stimulated C-peptide values obtained from the two tests. In each study, the covariate-adjusted stimulated MMTT value was significantly higher than the GST value. The MMTT-stimulated values in TrialNet tended to be higher than those from the ECPT. However, in a further analysis (data not shown) using the subset of ECPT subjects in the same range of age and duration of diabetes as in the TrialNet, the MMTT stimulated values were equivalent in the TrialNet and ECPT, although the TrialNet basal value remained significantly higher than that in the ECPT.
Table 2.
Fasting and stimulated C-peptide concentrations
| TrialNet |
ECPT |
|||
|---|---|---|---|---|
| MMTT | GST | MMTT | GST | |
| n | 143 | 135 | 116 | 117 |
| Tests | 278 | 271 | 174 | 174 |
| Fasting (pmol/ml) | 0.17 (0.14–0.19) | 0.17 (0.15–0.20) | 0.07 (0.05–0.08) | 0.07 (0.05–0.08) |
| Peak stimulated (pmol/ml) | 0.40 (0.38–0.42) | 0.30 (0.28–0.32)* | 0.13 (0.12–0.15) | N/A |
| 90-min MMTT/6-min GST (pmol/ml) | 0.36 (0.34–0.38) | 0.27 (0.25–0.28)* | 0.12 (0.11–0.13) | 0.10 (0.09–0.10)* |
| AUC mean (pmol/ml) | 0.31 (0.29–0.33) | 0.25 (0.24–0.26)* | 0.11 (0.10–0.11) | N/A |
Data are geometric means (95% CI). Geometric means, calculated as exp[mean log(x)], are from a mixed-model analysis of the log(C-peptide) adjusted for fasting glucose concentration, age, sex, continuous diabetes duration, test order, and sequence group. Analyses of stimulated values were also adjusted for basal log(C-peptide). Analyses were conducted separately for TrialNet and EPCT.
P < 0.0001 for the comparison between the TrialNet GST and MMTT. N/A, not applicable.
Covariate effects on C-peptide
Peak stimulated MMTT and GST values were positively associated with fasting C-peptide. Fasting glucose was inversely associated with peak MMTT but not peak GST values. Age and duration of diabetes were associated with peak C-peptide only in the TrialNet studies. Similar associations were observed after exclusion of the small numbers of subjects with a fasting glucose measurement outside of 70–200 mg/dl on the day of the test.
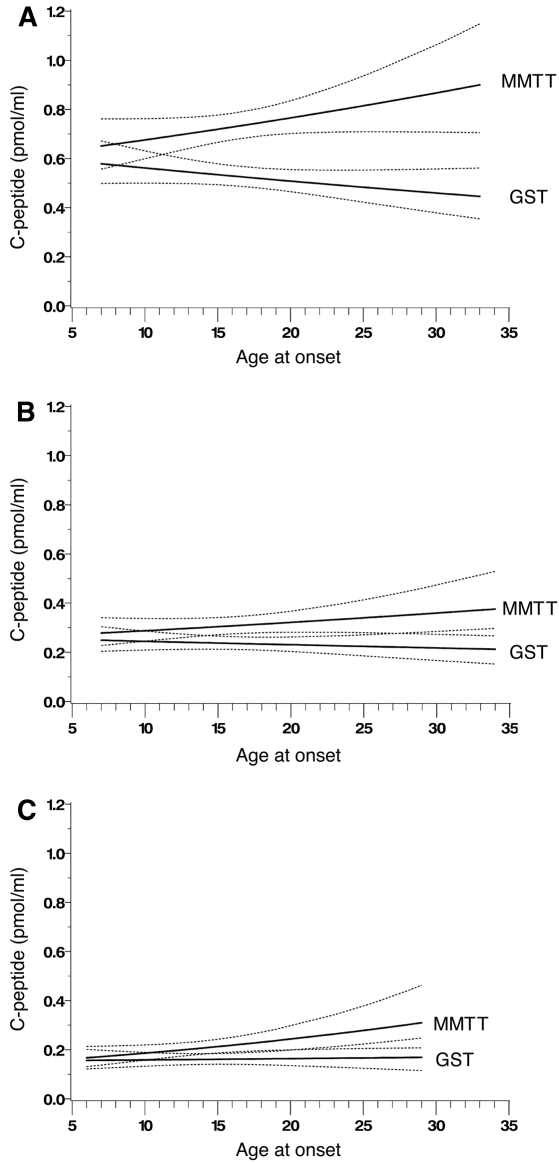
In the TrialNet but not in the ECPT, there was a significant interaction between age and test type (MMTT versus GST) for both the peak and the AUC mean values (P < 0.01 for each), adjusted for other covariates. Figure 1 shows that the mean peak values with MMTT tended to increase with increasing age, especially among those with duration of diabetes of <1 year (Fig. 1A), whereas those with GST decrease with increasing age. Thus, the difference between the MMTT and GST peak values also increased with age.
Figure 1.
TrialNet mean peak C-peptide (solid lines) and 95% CI bands (dotted lines), from an MMTT (upper solid lines) and a GST (lower solid lines) with respect to the age of onset in subjects with duration of type 1 diabetes <1 year (A), 1–2 years (B), and 2–3 years (C) obtained from separate models within each duration category with an interaction between test (MMTT versus GST) and age at onset of type 1 diabetes, adjusted for fasting C-peptide, fasting glucose, weight, visit number, and randomization group.
Reproducibility and reliability
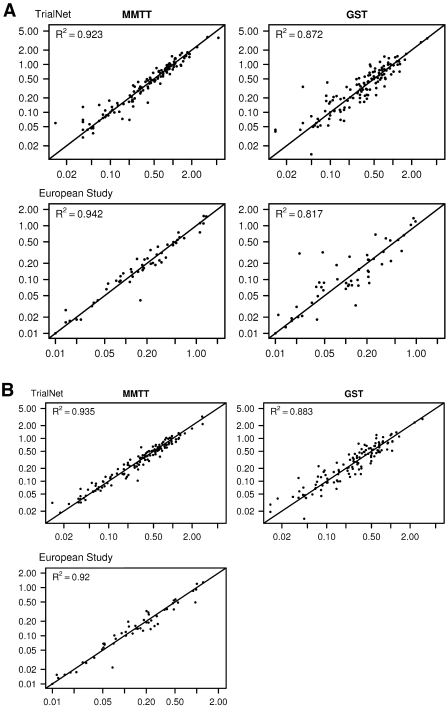
Figure 2 shows the relationship between peak and AUC values from the repeat tests for each subject. For each test, the relationship is strongly linear, with a high R2. The correlation coefficients (reliability) between the duplicate values were high in both studies, although those among the duplicate fasting values were lower (about r = 0.90) than those among the stimulated values. Further, the correlations among stimulated MMTT values were significantly higher than those among the GST values. The correlations were similar in the TrialNet and ECPT and among subgroup categories of sex, age, and duration of diabetes.
Figure 2.
Relationship between the duplicate measurements of the peak (A) and AUC mean C-peptide values (B) (pmol/ml) from the MMTT and the GST. The 6-min European GST values are presented in lieu of the peak values.
Adverse effects
The incidence of adverse effects was substantially higher with the GST than with the MMTT. With the GST, the incidence of nausea was ∼95% in 8–12 year olds, falling to ∼66% in those aged >18 years.
Preference
In TrialNet, 53% of subjects preferred the MMTT to the GST. The preference depended on age: 86% of those aged <13 years preferred the MMTT versus 45% of those aged 13–17 years and 30% of those aged ≥18 years (P < 0.01). Male and female subjects did not differ significantly: 61% of female versus 48% of male subjects preferred the MMTT (P = 0.17).
Among subjects who preferred the MMTT, 85% cited nausea experienced during the GST as a reason. Among those who preferred the GST, 94% cited the shorter duration of the test as a reason.
CONCLUSIONS
Retention of β-cell function in type 1 diabetes, as measured by stimulated C-peptide response, results in improved glycemic control and reduced incidence of hypoglycemia, retinopathy, and nephropathy (3,7). Thus, intervention studies aimed to preserve β-cell function have usually measured plasma C-peptide after either a liquid mixed meal (MMTT) or an intravenous injection of glucagon (GST). However, the scientific validity of the MMTT versus the GST and the relative tolerability and practicality have not been definitively studied.
Two parallel studies have provided clear, concordant results. On repeat testing 3–10 days apart, both tests provided highly reliable (reproducible) measures of stimulated C-peptide responses (more so with the MMTT), over a wide range of age and diabetes duration. Thus, changes in these measures over time in clinical trials (and in individual subjects) can be attributed to progression of disease and/or influence of therapy. These data also confirm the fact that almost all individuals with type 1 diabetes with a duration of up to 4 years have measurable stimulated C-peptide, even with an undetectable fasting value, and that most maintain clinically meaningful amounts (>0.2 pmol/ml) as defined by the Diabetes Control and Complications Trial (DCCT) (7).
Both studies also show that the MMTT is a more sensitive test of residual β-cell function, with the peak C-peptide response being significantly greater than that in the GST. In the MMTT, the peak response occurred at about 90 min compared with 6 min for the GST, thus confirming the selection of this time point for the GST in the ECPT. In multivariate models, longer diabetes duration and younger age were associated with a lower C-peptide response to the MMTT in the TrialNet but not the ECPT, perhaps owing to the smaller number of tests and/or the longer allowed duration of diabetes. Further, the difference in peak response with MMTT versus GST increased with age.
Use of a fixed glucagon dose, rather than dosing based on weight, as was done with the mixed meal, may have resulted in lower peak C-peptide responses in the GST. However, this is unlikely because identical results are obtained after adjustment for weight; the greater sensitivity of the MMTT is more likely due to an incretin effect in which an oral glucose stimulus elicits greater insulin secretion because of the actions of gastric inhibitory polypeptide and glucagon-like peptide 1 compared with a similar intravenous stimulus. Although there is a known impairment in the incretin effect early in type 1 diabetes (8), these data suggest that this impairment may be influenced by age. Alternatively, the insulin response to glucagon may be inherently less than the response to the combined stimuli of fat, protein, and carbohydrate, a hypothesis that could be tested in individuals without type 1 diabetes.
Because glucose affects the C-peptide response (9), the MMTT and GST were performed only if the fasting blood glucose by meter was within 70–200 mg/dl. Within this range, however, the fasting glucose significantly affected the MMTT C-peptide response. Thus, for intervention trials, the distributions of fasting glucose values on the day of the test should be comparable across groups or controlled for in the statistical analysis.
Nausea occurred in 75–81% of the GSTs and was more prevalent among younger subjects, perhaps owing to the fixed glucagon dose regardless of body weight. The relatively high frequency is possibly due to directed questioning. Most episodes of nausea were mild, although 5–11% involved vomiting.
Most subjects preferred the MMTT. However, older individuals, who were more concerned with time and who experienced less nausea, preferred the GST. Nevertheless, compliance with the sequence of tests was high; 83% of TrialNet subjects completed all four tests and 93% completed three or four tests, and 97% of ECPT subjects completed all three tests, all within a 1 month period. Thus, it is unlikely that the choice of MMTT versus GST will affect compliance with testing in a clinical trial.
Recently, C-peptide response during a two-phase glucose-clamp procedure was used to measure β-cell function (10), which poses a substantial additional burden on the subject and investigator. However, responses to this procedure have not been compared with those for the GST or MMTT in subjects with type 1 diabetes. An arginine stimulation test (AST) has also been used, particularly in post-transplant studies. The AST, like the GST, is short, but unlike the GST it does not cause nausea and thus might be the test with the lightest subject burden. One small study did not show any significant differences in sensitivity between the AST and MMTT (11); however, it did not assess reproducibility or overall comparability.
Any stimulated test is expensive and time consuming and thus may not provide benefits in clinical practice beyond a fasting or a random C-peptide measurement. As shown herein, a fasting (basal) C-peptide value has a high correlation with the preferred MMTT mean AUC response (r = 0.95 and 0.93 in the two studies; see supplemental material in the online appendix) and a random C-peptide value has been shown to differentiate subjects with type 1 versus type 2 diabetes (12). However, the emphasis on stimulated C-peptide levels in type 1 diabetes stems from the DCCT demonstration that stimulated C-peptide response at baseline in the intensive therapy group was directly associated with improved glycemic control, with lower risks of microvascular complications and less hypoglycemia (1,7). The DCCT has not published an analysis of the like associations with the fasting C-peptide levels. Without a formal study using a fasting or random C-peptide value in addition to a stimulated test, it is not possible to determine which is more clinically meaningful in an individual subject.
In summary, two parallel studies definitively show that the MMTT is superior to the GST when performed under standardized conditions. The MMTT is more sensitive, providing higher poststimulus C-peptide responses; is more reproducible; and is better tolerated and thus is the preferred method to measure residual β-cell function in clinical trials in type 1 diabetes. Clinicians interpreting results from clinical trials to arrest the type 1 diabetes disease process should be aware that these two commonly used outcome measures are not directly comparable.
Supplementary Material
Acknowledgments
TrialNet was performed under the auspices of Type 1 Diabetes TrialNet, a collaborative clinical research project sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Child Health and Human Development, the National Center for Research Resources, the Juvenile Diabetes Research Foundation International, and the American Diabetes Association. ECPT was supported by the European Association for the Study of Diabetes and the Instituto Carlos III, Spain (FIS PI061104 to D.M.).
APPENDIX
Complete affiliation information for the authors
Type 1 Diabetes TrialNet.
Carla J. Greenbaum, MD, Benaroya Research Institute, Seattle, WA; Paula F. McGee, MS, TrialNet Coordinating Center, The Biostatistics Center, The George Washington University, Rockville MD; and John M. Lachin, ScD; TrialNet Coordinating Center, The Biostatistics Center, The George Washington University, Rockville MD.
European C-peptide Trial.
Thomas Mandrup-Poulsen, MD, PhD, Steno Diabetes Center, Gentofte (Central Laboratory), and Department of Biomedical Sciences, University of Copenhagen, Denmark; Tadej Battelino, MD, PhD, Coordinating Centre, Department of Pediatric Endocrinology, University Childreny's Hospital, Ljubljana, Slovenia; Burkhard Haastert, PhD, German Diabetes Center, Düsseldorf, Germany; Johnny Ludvigsson, MD, PhD, Division of Pediatrics, Faculty of Health Sciences, Linköping University, Linköping, Sweden; Paolo Pozzilli, MD, PhD, University Campus BioMedico, Rome, Italy; and Hubert Kolb, MD, PhD, German Diabetes Center, Düsseldorf, Germany.
Published ahead of print at http://care.diabetesjournals.org on 15 July 2008.
Clinical trial reg. no. NCT00105352, clinicaltrials.gov.
C.J.G., T.M.-P., J.M.L., and H.K. contributed equally to this study.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW: C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 53:250–264, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Greenbaum CJ, Harrison LC: Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes 52:1059–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 3.FDA Guidance for Industry: Diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention [article online, 2008]. Available from http://www.fda.gov/cder/guidance/7630dft.pdf. Accessed 14 April 2008
- 4.Demidenko E: Mixed Models: Theory and Applications. New York, John Wiley and Sons, 2004
- 5.Lachin JM: The role of measurement reliability in clinical trials. Clin Trials 1:553–566, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Dunn OJ, Clark V: Correlation coefficients measured on the same individuals. J Am Stat Assoc 64:366–377, 1969 [Google Scholar]
- 7.The DCCT Research Group: Effect of intensive therapy on residual β-cell function in patients with type I diabetes in the Diabetes Control and Complications Trial. Ann Intern Med 128:517–523, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Greenbaum CJ, Prigeon RL, D’Alessio DA: Impaired β-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes 51:951–957, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gjessing HJ, Reinholdt B, Faber OK, Pedersen O: The effect of acute hyperglycemia on the plasma C-peptide response to intravenous glucagon or to a mixed meal in insulin-dependent diabetes mellitus. Acta Endocrinol (Copenh) 124:556–562, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352:2598–2608, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Greenbaum C, Seidel K, Pihoker C: The case for intravenous arginine stimulation in lieu of mixed-meal tolerance tests as outcome measure for intervention studies in recent-onset type 1 diabetes. Diabetes Care 27:1202–1204, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Berger B, Stenstro G, Sundkvist G: Random C-peptide in the classification of diabetes. Scand J Clin Lab Invest 60:687–694, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.