Abstract
OBJECTIVE—To describe and discuss a case of superior mesenteric artery syndrome (SMAS) presenting with gastrointestinal signs and symptoms mistakenly attributed to, and treated as, diabetic gastroparesis.
RESEARCH DESIGN AND METHODS—A case report was compiled describing the clinical presentation, including history and physical examination, evaluation, diagnosis, and treatment of a patient with type 1 diabetes presenting with gastrointestinal complications.
RESULTS—Clinical suspicion combined with the appropriate radiological evaluation led to a diagnosis of SMAS, with classic findings of reduced aortomesenteric angle and distance. Surgical intervention resulted in resolution of symptoms and recovery of glycemic control.
CONCLUSIONS—The possibility of SMAS should be considered in patients with type 1 diabetes presenting with gastrointestinal symptomatology, especially when associated with weight loss.
Patients with type 1 diabetes experience greater frequency of gastrointestinal complications, such as diabetic enteropathy (manifested by diarrhea), steatorrhea and/or constipation (1), and gastroparesis, which commonly presents as bloating but may also result in early satiety, epigastric pain or discomfort, nausea, vomiting, postprandial fullness, and anorexia. The clinician must be vigilant because these symptoms may be due to less common etiologies such as superior mesenteric artery syndrome (SMAS). The patient in this study is only the second reported patient with type 1 diabetes complicated by SMAS (2).
RESEARCH DESIGN AND METHODS
An 18-year-old white female with a history of type 1 diabetes since age 6 years presented with a 3-year history of poorly controlled diabetes, increasing insulin requirements (up to 3 units · kg−1 · day−1) and frequent hospitalizations for diabetic ketoacidosis. The patient's diabetes had been under excellent control (A1C 6.9%) until she began a low-carbohydrate diet. The ensuing 50-lb (22.7-kg) weight loss was exacerbated by personal stressors and the development of gastrointestinal symptoms. She reported nausea, which after dinner progressed to retching and vomiting along with the onset of abdominal pain, tachycardia, and the sudden development of severe nighttime hyperglycemia (with capillary blood glucose levels often in the 400–600 mg/dl [22.2–33.3 mmol/l] range) with ketonuria, which was poorly responsive to large doses of insulin (60–80 units of insulin delivered overnight). Associated symptoms included depression, painful sensory peripheral neuropathy, arthralgias, constipation, hair loss, dry skin, and amenorrhea.
On exam, she had orthostatic changes with a baseline heart rate of 116 bpm and a blood pressure of 133/92 mmHg. Weight and BMI were 123 lbs and 17.7 kg/m2, respectively. No findings of neuropathy or retinopathy were evident. A1C was 11.5%. Additional evaluation included a urine analysis for free cortisol, dehydroepiandrosterone, transglutaminase antibodies, serum IGF-1, and glucagon levels; a complete blood count; and a fecal fat measurement: all were within normal levels. Urine albumin levels were slightly elevated on repeated evaluations. A 6-week trial of domperidone and oral dietary supplementation along with an intensive insulin regimen failed to improve the patient's condition, with only a marginal improvement in A1C to 10.5%.
RESULTS
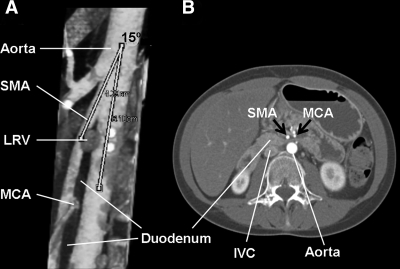
The patient was further assessed for the possibility of anorexia nervosa and insulin bulimia (avoidance of prescribed insulin administration). She had previously undergone extensive gastrointestinal evaluation (upper GI series and endoscopy), which had only revealed delayed transit time on gastric emptying scintigraphy. Because of the suspicious presentation and negative workup, a computed tomography angiography (CTA) was ordered that demonstrated dilatation of the first and second portions of the duodenum with normal gastric volume. Compression of the third portion of the duodenum was evident, and a reduced aortomesenteric angle of 15° (normal >22°) and an aortomesenteric distance of 3.3 mm (normal >10 mm) were identified (Fig. 1). These findings were consistent with SMAS. Upper gastrointestinal series and upper endoscopy were unremarkable.
Figure 1.
A: Sagittal computed tomography angiography demonstrating a narrow (15°) aortomesenteric angle (normal >22°). B: Axial computed tomography angiography demonstrating a reduced aortomesenteric distance of 3.3 mm causing duodenal compression and dilatation of the proximal portion of the duodenum. IVC, inferior vena cava; LRV, left renal vein; MCA, middle colic artery.
An exploratory laparotomy, duodenojejunostomy, and division of the ligament of Treitz were subsequently performed to relieve the mechanical obstruction. The patient's postoperative course was uneventful: her symptoms resolved and her A1C was 8.4% at the 1-month postoperative visit and 7.0% six months later, concomitant with a 10-lb (4.5 kg) weight gain.
CONCLUSIONS
Patients with poorly controlled diabetes may present with symptoms of bloating, early satiety, epigastric pain or discomfort, nausea, vomiting, postprandial fullness, and anorexia, often attributed to gastroparesis. We report a case of SMAS in a patient with type 1 diabetes mistakenly diagnosed as gastroparesis. This case is interesting because the most severe manifestations of SMAS occurred after the evening meal. They were exacerbated by the patient lying supine and were associated with ketogenesis and a significant degree of resistance to insulin therapy.
SMAS is a term used to define the clinical signs and symptoms caused by compression of the third portion of the duodenum between the angle made by the aorta and the superior mesenteric artery (SMA). The compression is caused by a decrease in the aortomesenteric angle and distance, with complete obstruction observed with angles between 6 and 16° and a distance of 2–8 mm (3–6). SMAS is thought to occur because of duodenal compression following the loss of the mesenteric fat pad, which maintains the angle and distance between aorta and SMA. This can be seen in acute catabolic states, dietary disorders such as anorexia nervosa, chronic wasting diseases, and rapid weight loss following bariatric surgery.
The importance of reporting this case lies in heightening the diagnostic index of suspicion for SMAS in patients with type 1 diabetes presenting with suspicious gastrointestinal symptoms and glycemic instability. Patients manifesting intermittent and recurrent postprandial abdominal pain, nausea, vomiting, and anorexia associated with weight loss (all often attributed to diabetic gastroparesis) should be screened for SMAS.
A variety of modalities have been utilized for diagnosis (7,8); most have been largely replaced by the noninvasive and more accurate helical computed tomography with oral and intravenous contrast and three-dimensional reconstruction (9).
In conclusion, patients with type 1 diabetes presenting with gastrointestinal symptoms associated with recent weight loss or low BMI should be evaluated for SMAS. Computed tomography angiography is a quick and reliable diagnostic tool to identify the structural and anatomic abnormalities associated with this syndrome.
Published ahead of print at http://care.diabetesjournals.org on 15 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Feldman M, Schiller LR: Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med 98:378–384, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Azami Y: Diabetes mellitus associated with superior mesenteric artery syndrome: report of two cases. Intern Med 40:736–739, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Welsch T, Buchler MW, Kienle P: Recalling superior mesenteric artery syndrome. Dig Surg 24:149–156, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Hines JR, Gore RM, Ballantyne GH: Superior mesenteric artery syndrome: diagnostic criteria and therapeutic approaches. Am J Surg 148:630–632, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Neri S, Signorelli SS, Mondati E, Pulvirenti D, Campanile E, Di Pino L, Scuderi M, Giustolisi N, Di Prima P, Mauceri B, Abate G, Cilio D, Misseri M, Scuderi R: Ultrasound imaging in diagnosis of superior mesenteric artery syndrome. J Intern Med 257:346–351, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Wayne ER, Burrington JD: Duodenal obstruction by the superior mesenteric artery in children. Surgery 72:762–768, 1972 [PubMed] [Google Scholar]
- 7.Adams JB, Hawkins ML, Ferdinand CH, Medeiros RS: Superior mesenteric artery syndrome in the modern trauma patient. Am Surg 73:803–806, 2007 [PubMed] [Google Scholar]
- 8.Chin LW, Chou MC, Wang HP: Ultrasonography diagnosis of superior mesenteric artery syndrome in the ED. Am J Emerg Med 25:864.e5–864.e6, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Townsend CM: Vascular compression of the duodenum. In Mastery of Surgery. Vol. 1. Fischer JE, Ed. Philadelphia, Lippincott Williams & Wilkins, 2006, p. 955–961