Abstract
OBJECTIVE—We have previously shown that hypogonadotrophic hypogonadism is common in middle-aged patients with type 2, but not with type 1, diabetes. We have now investigated the total and free testosterone concentrations in young (aged 18–35 years) type 1 and type 2 diabetic patients.
RESEARCH DESIGN AND METHODS—In this study carried out in a tertiary referral center, serum concentrations of total and free testosterone were measured in 38 type 1 diabetic (mean age 26.45 ± 0.89 years) and 24 type 2 diabetic (mean age 27.87 ± 0.97 years) subjects. The mean BMI of type 1 and type 2 diabetic patients was 27.41 ± 1.18 and 38.55 ± 2.04 kg/m2, respectively (P < 0.001).
RESULTS—The mean total testosterone concentration of type 1 and type 2 diabetic patients was 22.89 ± 1.23 and 11.14 ± 0.99 nmol/l, respectively (P < 0.001). The mean free testosterone concentration of type 1 and type 2 diabetic patients was 0.489 ± 0.030 and 0.296 ± 0.022 nmol/l, respectively (P < 0.001). Eight of 24 (33%) type 2 diabetic patients had subnormal free testosterone concentrations (<0.225 nmol/l). Using an age-based reference range, 14 of 24 (58%) type 2 diabetic patients had low free testosterone concentrations (<0.278 nmol/l). Three of 38 (8%) type 1 diabetic patients had free testosterone concentrations below the lower limit of normal (P = 0.02 when compared with type 2 diabetes). Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) concentrations in type 2 diabetic patients with low free testosterone concentrations were in the normal range and were similar to those in type 1 diabetic patients.
CONCLUSIONS—Young type 2 diabetic patients have significantly lower plasma concentrations of total and free testosterone and inappropriately low LH and FSH concentrations with a very high prevalence of hypogonadotrophic hypogonadism, when compared with type 1 diabetic patients of a comparable age. The potential implications for their sexual and reproductive function during prime reproductive years are profound.
We have previously shown that patients with type 2 diabetes have a frequent occurrence of hypogonadotrophic hypogonadism, as reflected in low plasma concentrations of testosterone and inappropriately low luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (1). These studies were carried out in patients with a mean age of 55 years. Other studies (2,3), also carried out in middle-aged populations, have confirmed the frequent occurrence of this defect in type 2 diabetes. We have also shown that the prevalence of this defect is very low in type 1 diabetes (4). In view of the increasing incidence of obesity and type 2 diabetes in younger populations and in children, the occurrence of this defect in younger populations needs to be investigated, since such an abnormality would affect their sexual function, reproductive capacity, and the quality of life during their peak reproductive years. In addition, the lack of testosterone would also potentially promote further weight gain and loss of skeletal muscle and would thus promote insulin resistance (5–7). In view of the above observations, we have now hypothesized that younger patients with type 2 diabetes have significantly lower testosterone concentrations and a higher prevalence of low testosterone concentrations (hypogonadism) when compared with patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
The study was conducted in the Diabetes Endocrinology Center of Western New York, a tertiary referral center affiliated with the State University of New York and Kaleida Health in Buffalo, New York. This is a cross-sectional analysis of total and free testosterone concentrations in 24 type 2 diabetic (mean age 27.87 ± 0.97 years) and 38 type 1 diabetic (mean age 26.45 ± 0.89 years) male patients aged between 18 and 35 years. These patients were referred to our center for management and control of diabetes. Data were collected from January 2007 to January 2008. Patients with a known history of hypogonadism, panhypopituitarism, or chronic debilitating disease, such as renal failure, cirrhosis, or HIV, were excluded from the study. Demographic parameters were collected. Fasting blood samples were then obtained to measure serum total testosterone, free testosterone, sex hormone–binding globulin (SHBG), LH, FSH, prolactin, and A1C. We evaluate testosterone concentrations routinely in diabetic patients in view of the high frequency (one in three) of low testosterone concentrations. Informed consent was therefore not obtained. Health Insurance Portability and Accountability Act forms were signed by all patients. The collected data were kept confidential by the investigators, and patient identifiers were eliminated from the database once the master database had been created.
Total testosterone was measured by solid-phase radioimmunoassay (Coat-A-Count; Diagnostic Products, Los Angeles, CA) (normal range 10.4–34.7 nmol/l). SHBG was tested at Specialty Laboratories (Santa Monica, CA) by immunochemiluminometric assay. LH and FSH were measured by chemiluminescent immunometric assays. Free testosterone was calculated from SHBG and testosterone using the method of Vermeulen and colleagues (8,9). Hypogonadism was defined as low free testosterone (0.225 nmol/l [6.5 ng/dl] was taken as lower limit of normal for free testosterone) (10).
Statistical analysis
Data are presented as means ± SE. Mann-Whitney rank-sum test was used to compare nonparametric data, and t test was used to compare parametric data. Fisher's exact test or χ2 test was also used to compare the groups whenever appropriate. Spearman correlation (for nonparametric data) and Pearson correlation (for parametric data) was used to establish statistical relationships. P < 0.05 was considered significant. Sigma Stat software (SPSS, Chicago, IL) was used for analysis.
RESULTS
Total and free testosterone concentrations (11.14 ± 0.99 and 0.296 ± 0.022 nmol/l, respectively) in type 2 diabetic patients were significantly lower (P < 0.001 for both) than the total and free testosterone concentrations (22.89 ± 1.23 and 0.489 ± 0.030 nmol/l, respectively) in type 1 diabetic patients (Table 1) (Fig. 1). We defined hypogonadism as a low free testosterone concentration. On the basis of usual normal ranges, 8 of 24 type 2 diabetic patients were hypogonadal, whereas 3 of 38 type 1 diabetic patients were hypogonadal (P < 0.001). Two of three hypogonadal type 1 diabetic patients were morbidly obese (BMI 57 and 41 kg/m2, respectively), while one was grossly underweight (BMI 15.8 kg/m2) and was under investigation for malabsorption and anorexia nervosa. Five other patients with obesity and type 1 diabetes had total and free testosterone concentrations in the middle- to high-normal range. Since testosterone concentrations decline with age, we searched the literature for information on the concentrations of calculated free testosterone in younger patients. One article provided information on the concentrations of total and free testosterone in men aged 20–29 years (11). Using the range of free testosterone cited for the decade 20–29 (0.278–0.777 nmol/l), 14 of 24 type 2 diabetic patients would have subnormal concentrations and thus would be hypogonadal. All patients except three with type 2 diabetes had a BMI ≥30 kg/m2 and were, therefore, obese. All three had normal total and free testosterone concentrations. Thus, the prevalence of hypogonadism in obese, young subjects with type 2 diabetes was even higher (8 of 21 using the usual range and 14 of 21 using the range for young male subjects).
Table 1.
Comparison of demographics and hormonal concentrations of type 1 and type 2 diabetic patients
| Type 2 diabetes | Type 1 diabetes | P | |
|---|---|---|---|
| n | 24 | 38 | |
| Age (years) | 27.87 ± 0.97 | 26.45 ± 0.89 | 0.37 |
| BMI (kg/m2) | 38.55 ± 2.04 | 27.41 ± 1.18 | <0.001 |
| Total testosterone (nmol/l) | 11.14 ± 0.99 | 22.89 ± 1.23 | <0.001 |
| Free testosterone (nmol/l) | 0.296 ± 0.022 | 0.489 ± 0.030 | <0.001 |
| % hypogonadal | 33% | 8% | 0.02 |
| SHBG (nmol/l) | 19.63 ± 2.41 | 36.43 ± 2.28 | <0.001 |
| LH (IU/l) | 3.5 ± 0.4 | 3.79 ± 0.44 | 0.58 |
| FSH (IU/l) | 3.46 ± 0.43 | 4.54 ± 0.87 | 0.61 |
| Prolactin (mcg/l) | 6.97 ± 0.65 | 7.44 ± 0.59 | 0.57 |
| A1C (%) | 7.09 ± 0.42 | 7.73 ± 0.22 | 0.17 |
| Caucasian (%) | 50 | 92 | <0.001 |
| African American (%) | 25 | 5 | <0.001 |
| Hispanic (%) | 21 | 0 | <0.001 |
| Asian (%) | 4 | 3 | 0.61 |
| Insulin use | 57 | 100 | <0.001 |
| Mean insulin dose (units) | 72 ± 18 | 60 ± 4 | 0.96 |
| Exenatide use [% (dose)] | 21% (6.7 ± 1.7 μg) | 3% (5 ± 0 μg) | <0.001 |
| Metformin use [% (dose)] | 86% (1.6 ± 0.2 g) | 7% (2 ± 0 g) | <0.001 |
| Sulfonylurea use [% (dose)] | 64% (9 ± 1.4 mg) | 0 | <0.001 |
| Thiazolidinedione use (%) | 21 | 0 | <0.001 |
| C-peptide (ng/ml) | 2.13 ± 0.45 | 0.36 ± 0.13 | 0.001 |
Data are means ± SE or percent, unless otherwise indicated.
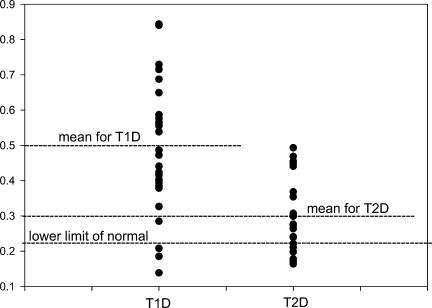
Figure 1.
Comparison of free testosterone concentrations and their distribution in type 1 and type 2 diabetic patients. Note that the highest free testosterone concentration in type 2 diabetes is similar to the mean concentration of type 1 diabetes.
As previously reported, SHBG concentrations were significantly lower in patients with type 2 diabetes than in those with type 1 diabetes (4). LH and FSH concentrations in patients with type 2 diabetes were in the normal range and were therefore inappropriately low. LH, FSH, and prolactin concentrations in these patients were similar to those in type 1 diabetic patients.
Relationship of testosterone with age and BMI
Data for both type 1 and type 2 diabetes were combined to calculate correlation coefficients. Free and total testosterone were related to each other (r = 0.89, P < 0.001). Free testosterone was negatively related to age (r = −0.39, P < 0.01) and BMI (r = −0.50, P < 0.001) (Fig. 2). The relatively small number of subjects in each group did not allow enough power to calculate correlations separately in type 2 and type 1 diabetic patients for each index measured. However, total testosterone concentrations were related to LH (r = 0.46, P = 0.036) and FSH (r = 0.67, P < 0.001) in type 1, but not in type 2, diabetic patients. Free testosterone concentrations were related to FSH (r = 0.62, P < 0.01) and had a trend toward a relationship to LH in type 1 (r = 0.39, P = 0.1) but not in type 2 diabetic patients. LH and FSH concentrations were related to each other in type 1 (r = 0.58, P < 0.01), but not in type 2 (r = 0.06, P = 0.79), diabetic patients.
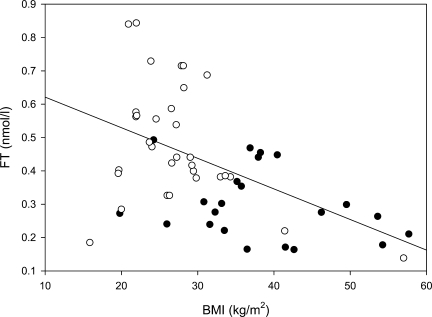
Figure 2.
Inverse relation of BMI with free testosterone in type 1 (○) and type 2 (•) diabetes. r = −0.50; P < 0.001.
The ethnicity of patients is mentioned in Table 1. Caucasians were the majority group in both type 1 and type 2 diabetes. A larger percentage of type 2 diabetic subjects (25%) were African American compared with type 1 diabetic men (5%). Demographic and hormonal concentrations of type 2 diabetic subjects according to their ethnicity are described in Table 2. The lower free testosterone concentrations of Hispanic men compared with African American men can be explained by the higher age of Hispanic men. The higher free testosterone concentration of African Americans compared with Caucasians could be explained by the lower BMI of African American men (although the difference not significant [P = 0.32], probably due to the small sample size). It is, however, interesting that none of the seven African American men in our study had a low free testosterone concentration. These findings need to be confirmed in a larger number of age- and BMI-matched type 2 diabetic subjects. A recent epidemiological survey (12) did not find any relation of ethnicity to testosterone concentrations.
Table 2.
Relationship of ethnicity to age, BMI, and hormone concentrations in type 2 diabetic men
| Caucasian | Hispanic | African American | |
|---|---|---|---|
| n | 12 | 5 | 7 |
| Age (years) | 27.17 ± 1.32 | 31.78 ± 0.73* | 26.28 ± 2† |
| BMI (kg/m2) | 41.08 ± 3.39 | 35.38 ± 2.72 | 36 ± 2.84 |
| Total testosterone (nmol/l) | 9.89 ± 1.63 | 11.36 ± 1.23 | 13.13 ± 1.65 |
| SHBG (nmol/l) | 16.99 ± 3.25† | 30.2 ± 6.12 | 16.21 ± 2.68† |
| Free testosterone (nmol/l) | 0.275 ± 0.028 | 0.215 ± 0.027 | 0.388 ± 0.032*† |
| % hypogonadal | 36 | 60 | 0 |
Data are means ± SD or percent, unless otherwise indicated.
P < 0.05 vs. Caucasian;
P < 0.05 vs. Hispanic.
CONCLUSIONS
Our data show clearly that the mean total and free testosterone concentrations in young patients with type 2 diabetes are significantly lower than those in patients with type 1 diabetes. Eight of 24 type 2 diabetic patients had low free testosterone concentrations. According to previously published data, normal free testosterone concentrations in young men (age 21–29 years) are 0.278–0.777 nmol/l (11). The mean free testosterone concentration of type 2 diabetic patients in our study was 0.296 nmol/l. This is clearly low for a group in its peak reproductive years. Fourteen of 24 patients had free testosterone concentrations <0.278nmol/l. Thus, this small sample of younger patients with type 2 diabetes had a prevalence of hypogonadism of 33% on the basis of the standard normal ranges. However, if the normal range from a study on younger male subjects aged 20–29 years is used, 14 of 24 (58%) type 2 diabetic subjects were subnormal. Consistent with our previous data, hypogonadal patients had inappropriately low LH and FSH concentrations and thus had hypogonadotrophic hypogonadism (1). It is also noteworthy that all the eugonadal type 2 diabetic patients were not obese, and, therefore, the prevalence of hypogonadotrophic hypogonadism in obese type 2 diabetic patients was even higher.
Patients with type 1 diabetes, on the other hand, had much higher total and free testosterone concentrations that were comparable with those observed in younger normal subjects. Three of 38 patients had subnormal free testosterone concentrations and therefore were hypogonadal. Two of them were morbidly obese (BMI 57 and 41.4 kg/m2, respectively). These patients’ hypogonadal state could, in part, be attributed to gross obesity. One other patient who was markedly underweight (BMI 15.8 kg/m2) had subnormal total and free testosterone concentrations. He was being investigated for malabsorption and anorexia nervosa. The rest had middle- to high-normal total and free testosterone concentrations similar to those observed in normal subjects, consistent with their age. The group with normal total and free testosterone included five other patients with nonmorbid obesity and type 1 diabetes.
Since the association of obesity with type 2 diabetes led to a greater chance of developing hypogonadotrophic hypogonadism, and its existence in type 1 diabetes occurred only in association with morbid obesity, BMI is a major determinant of hypogonadotrophic hypogonadism. It is well known that obesity is inversely associated with free and total testosterone concentrations in middle-aged and elderly men. A recent report from the Netherlands found a 35.6% prevalence of hypogonadotrophic hypogonadism in obese men (mean age 58 years). The study population included type 2 diabetic men. Comparison of prevalence of hypogonadotrophic hypogonadism in type 2 diabetic and nondiabetic obese men was not available in this publication. We (1) and others (2) have described that the negative association of BMI with free testosterone is also present in middle-aged (mean age 53–58 years) type 2 diabetic men. These men have a high prevalence (33–50%) of hypogonadotrophic hypogonadism.
Nielsen et al. (11) examined testosterone concentrations of 615 nonobese and 70 obese young Danish men (aged 20–29 years) in association with subcutaneous and visceral fat mass, measured by dual-energy X-ray absorptiometry and magnetic resonance imaging. The purpose of this study was to investigate the impact of obesity on reference intervals for testosterone in young men. The reference interval of plasma free testosterone in nonobese men was 0.29–0.78 nmol/l and in obese men 0.23–0.67 nmol/l. Total and bioavailable testosterone concentrations were negatively related to all measures of fat mass. A total of 23% of obese young men had subnormal total testosterone concentrations, while 10% had subnormal total and bioavailable testosterone concentrations. Thus, obesity without diabetes may be related to hypogonadotrophic hypogonadism.
The strength of our current report is that it describes the prevalence of hypogonadotrophic hypogonadism in young type 2 diabetic men (aged 18–35 years; mean age 28 years). In addition, BMI was inversely related to free testosterone. Since testosterone falls with age, it is important to highlight that BMI is negatively associated with hypogonadotrophic hypogonadism, even at an early age in men, and that there is a very high prevalence of hypogonadotrophic hypogonadism (58% on the basis of age-matched control subjects) in young type 2 diabetic men. Using the reference range for obese young men (11), the prevalence of hypogonadotrophic hypogonadism in young type 2 diabetic men is 33%. This prevalence is higher than the rate of 10% found in young obese men (11). More focused investigations on the relationship of obesity, especially visceral adiposity, in type 2 diabetic men (using imaging techniques or surrogate clinical measures such as waist circumference) to total and free testosterone concentrations are required.
While obesity contributes to the association of type 2 diabetes with hypogonadotrophic hypogonadism, the association is not entirely dependent on obesity. In our first study on type 2 diabetic men, we found that 31% of lean type 2 diabetic men also had hypogonadotrophic hypogonadism (1). It is likely that factors other than obesity also contribute to hypogonadotrophic hypogonadism (13). It is possible that the association is mediated via insulin resistance. Type 2 diabetic patients generally have higher insulin resistance, while all obese men are not insulin resistant. A recent analysis (14) from the Third National Health and Nutrition Examination Survey showed that low androgens were a risk factor for type 2 diabetes in men. In multivariable models adjusted for age, race/ethnicity, and adiposity, men in the lowest tertile of free testosterone concentrations were four times more likely to have type 2 diabetes compared with men in the third tertile (odds ratio 4.12 [95% CI 1.25–13.55]). Whether obesity or insulin resistance is the major determinant of hypogonadotrophic hypogonadism has to be addressed in future studies, and the pathogenesis of hypogonadotrophic hypogonadism needs to be defined. It has been suggested that increased aromatase activity in the adipose tissue may lead to a greater degree of conversion from testosterone to estradiol and that the excess of estradiol suppresses the hypothalamus hypophyseal axis. On the other hand, it has also been shown that the deletion of the insulin receptor gene in neurons of mice leads to hypogonadotrophic hypogonadism. Thus, the insulin-resistant state in the hypothalamus may lead to hypogonadotrophic hypogonadism.
As previously reported, the concentrations of SHBG tended to be low in type 2 diabetic subjects and to be middle to high normal in type 1 diabetic subjects (4). Despite corrections made to total testosterone concentrations on the basis of SHBG, the concentrations of calculated free testosterone remained markedly lower in type 2 diabetes and relatively high in type 1 diabetes.
These observations have several clinical implications, since low testosterone concentrations may contribute to the impairment of sexual function, diminished libido, and erectile dysfunction. Furthermore, the lack of testosterone during these crucial years may lead to diminished peak bone mass and the lack of development or loss of skeletal muscle (7). In addition, these patients may develop increased adiposity and, therefore, may become more insulin resistant (2,15). Clearly, patients with type 2 diabetes, especially if they are obese, need further focused attention and systematic investigation. This is even more relevant to younger type 2 diabetic patients. Furthermore, patients with hypogonadotrophic hypogonadism and type 2 diabetes have been shown to have very high C-reactive protein concentrations (16). Thus, they may be at a heightened risk of atherosclerosis and cardiovascular disease. Indeed, low testosterone concentrations in male subjects are known to be associated with enhanced cardiovascular risk in observational studies (17,18). A Japanese study (19) has shown that free testosterone (but not total testosterone) concentrations are inversely related to carotid intimal medial thickness in type 2 diabetic patients. Since free testosterone was measure by radioimmunoassay in that study, these findings need to be confirmed by using a more reliable assay.
In addition, these patients are also candidates for infertility, since subnormal FSH concentrations are likely to adversely affect the development of seminiferous tubules and spermatogenesis. Indeed, in two patients with hypogonadotrophic hypogonadism and type 1 diabetes, we have found marked oligospermia following semen analysis (A. Chandel, S.D., S.T., and P.D., unpublished observations). The treatment plans for such patients should include not only testosterone therapy but also the consideration of gonadotropin therapy in order to restore fertility.
In conclusion, young patients with type 2 diabetes have significantly lower plasma concentrations of total and free testosterone, with inappropriately low LH and FSH concentrations, when compared with type 1 diabetic patients of a comparable age. The prevalence of absolute or relative hypogonadism is also markedly higher in this group. There is an inverse relationship between BMI and total and free testosterone concentrations. Type 2 diabetic patients with obesity appear to be at a greater risk of hypogonadotrophic hypogonadism than those without. The implications for their sexual and reproductive function, as well as the underlying defect of insulin resistance, are profound. This area requires careful further assessment and investigation.
Acknowledgments
P.D. is supported by National Institutes of Health Grant 5R01DK069805-04.
Published ahead of print at http://care.diabetesjournals.org on 23 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P: Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 89:5462–5468, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH: Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 30:911–917, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C: Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 60:762–769, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomar R, Dhindsa S, Chaudhuri A, Mohanty P, Garg R, Dandona P: Contrasting testosterone concentrations in type 1 and type 2 diabetes. Diabetes Care 29:1120–1122, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Woodhouse LJ, Gupta N, Bhasin M, Singh AB, Ross R, Phillips J, Bhasin S: Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab 89:718–726, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, Tripathy D, Yialamas M, Groop L, Elahi D, Hayes FJ: Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 28:1636–1642, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Dhindsa S, Bhatia V, Dhindsa G, Chaudhuri A, Gollapudi GM, Dandona P: The effects of hypogonadism on body composition and bone mineral density in type 2 diabetic patients. Diabetes Care 30:1860–1861, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen A, Verdonck L, Kaufman JM: A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Morley JE, Patrick P, Perry HM 3rd: Evaluation of assays available to measure free testosterone. Metabolism 51:554–559, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen A, Kaufman JM: Diagnosis of hypogonadism in the aging male. Aging Male 5:170–176, 2002 [PubMed] [Google Scholar]
- 11.Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, Haug E, Larsen R, Andersen M: Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab 92:2696–2705, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB: Serum androgen levels in black, Hispanic, and white men. J Clin Endocrinol Metab 91:4326–4334, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Dandona P, Dhindsa S, Chaudhuri A, Bhatia V, Topiwala S: Hypogonadotrophic hypogonadism in type 2 diabetes, obesity and the metabolic syndrome. Current Mol Med, 2008, In press [DOI] [PubMed]
- 14.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA: Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 30:234–238, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM, Karhapaa P, Mykkanen L, Laakso M: Insulin resistance, body fat distribution, and sex hormones in men. Diabetes 43:212–219, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P: Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care 29:2289–2294, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Laughlin GA, Barrett-Connor E, Bergstrom J: Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93:68–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P: Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation 112:332–340, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Fukui M, Kitagawa Y, Nakamura N, Kadono M, Mogami S, Hirata C, Ichio N, Wada K, Hasegawa G, Yoshikawa T: Association between serum testosterone concentration and carotid atherosclerosis in men with type 2 diabetes. Diabetes Care 26:1869–1873, 2003 [DOI] [PubMed] [Google Scholar]