Abstract
OBJECTIVE—The association between changes in triglyceride concentrations over time and diabetes is unknown. We assessed whether two triglyceride determinations obtained 5 years apart can predict incident type 2 diabetes.
RESEARCH DESIGN AND METHODS—Triglyceride levels at baseline (time 1) and 5 years later (time 2), followed by subsequent follow-up of 5.5 years, were measured in 13,953 apparently healthy men (age 26–45 years) with triglycerides <300 mg/dl (<3.39 mmol/l).
RESULTS—During 76,742 person-years, 322 cases of diabetes occurred. A multivariate model adjusted for age, BMI, total cholesterol–to–HDL cholesterol ratio, family history of diabetes, fasting glucose, blood pressure, physical activity, and smoking status revealed a continuous independent rise in incident diabetes with increasing time 1 triglyceride levels (Ptrend < 0.001). Men in the lowest tertile of time 1 triglyceride levels who progressed to the highest tertile over follow-up (low-high) exhibited a hazard ratio (HR) of 12.62 (95% CI 3.52–31.34) compared with those remaining in the lowest tertile at both time points (reference group: low-low). Whereas men who were at the top triglyceride level tertile throughout follow-up (high-high) had a HR for diabetes of 7.08 (2.52–14.45), those whose triglyceride level decreased to the lowest tertile (high-low) exhibited a HR of 1.97 (0.67–6.13). Alterations in triglyceride levels during follow-up were associated with changes in BMI, physical activity, and eating breakfast habit (P < 0.05), but remained an independent modifier of diabetes risk even after adjustment for such changes.
CONCLUSIONS—Two measurements of fasting triglyceride levels obtained 5 years apart can assist in identifying apparently healthy young men at increased risk for diabetes, independent of traditional risk factors and of associated changes in BMI and lifestyle parameters.
Elevated triglyceride levels are a common dyslipidemic feature accompanying type 2 diabetes and pre-diabetic states (1). A fasting triglyceride level of ≥150 mg/dl (≥1.70 mmol/l) is one of five accepted criteria for defining individuals at high risk for cardiovascular disease and type 2 diabetes, arguably termed the “metabolic syndrome” (2–4). Some evidence suggests that fasting triglyceride levels can aid in predicting future type 2 diabetes (5,6). However, this was shown mainly when triglyceride levels were combined with additional clinical parameters, such as BMI, blood pressure (7), and other classic risk factors for cardiovascular disease (8,9), or with “high-normal” fasting plasma glucose levels (10).
The level of circulating triglycerides is highly influenced by the fed-fasted state, insulin sensitivity, and lifestyle factors such as diet and physical activity (1,11,12). These make triglyceride levels a highly sensitive lifestyle biomarker at a given time point but suggest that a triglyceride determination at a single time point may inaccurately reflect long-term triglyceridemia, particularly if lifestyle modification occurred during follow-up. Whether assessment of triglyceride levels at more than one time point could improve the association between triglyceride levels and diabetes is largely unknown. Recently, we reported in a large cohort of young (aged 26–45 years) men that significant changes between two fasting triglyceride measurements obtained 5 years apart corresponded with alterations in lifestyle parameters (13). Furthermore, such changes modified the risk for heart disease attributed to elevated triglyceride levels (13).
Most studies assessing the risk factors for type 2 diabetes have overlooked the parameters specifically relevant for the apparently healthy young adult population. Although the incidence rate of diabetes in this group is relatively low, recent studies suggested a surge in type 2 diabetes in young adults (14). Identifying individuals in this group who have a high risk of developing diabetes is therefore challenging but potentially of significant benefit if preventive measures are used. Here, we used the cohort of young, apparently healthy men (10,13) to assess whether baseline triglyceride measurements as well as lifestyle-associated changes in triglyceride levels over time can predict the risk of diabetes.
RESEARCH DESIGN AND METHODS
The MELANY cohort
The Metabolic, Life-Style, and Nutritional Assessment in Young Adults (MELANY) study is based on a computerized database established in 1992 that includes all medical records of Israel Defense Forces (IDF) career service personnel, as detailed previously (10,13). Of this population, >95% are Caucasians. During every visit to the staff periodic examination center, participants complete a detailed questionnaire assessing demographic, nutritional, lifestyle, and medical parameters. Height, weight, and blood pressure are recorded by trained medics, venous blood samples are obtained after a 14-h fast, and a complete physical examination is performed by physicians. Between visits, primary care for IDF personnel is obtained at designated military clinics, and all medical information is recorded in the same central database.
The IDF Medical Corps Institutional Review Board approved this study, which was exempt from the requirement for written informed consent on the basis of maintaining participants’ anonymity. IDF authorities did not censor or limit any aspect of the study design, analyses, and reporting.
Criteria for inclusion, exclusion, and outcome
Women were not included, as the database contains an insufficient number of incident cases of diabetes to facilitate meaningful analyses. Included are apparently healthy men aged 26–45 years, with fasting triglyceride levels <300 mg/dl (<3.40 mmol/l) at their initial visit. Of 15,165 men who had at least two visits, the following were excluded because they were referred for nutritional/pharmacological intervention: 227 men with diabetes and 17 men with coronary heart disease at baseline and 686 men with triglyceride levels of ≥300 mg/dl (≥3.40 mmol/l). An additional 282 men were excluded because they received chronic medications.
Two analyses are presented. Analysis 1 determined the association between triglyceride values at enrollment (time 1) and incident diabetes during 10 years of follow-up. Analysis 2 evaluated the effect of change in fasting triglyceride level between two measurements: time 1′ at enrollment and time 2′ obtained 5 years later. In this analysis, the time 2 value became the baseline measurement (time 1 turning into a historical, prebaseline determination), and incident diabetes was assessed in the subsequent 5.2 years of follow-up. For analysis 1, 13,953 men were included, and the predictive value of time 1 triglyceride measurement for incident diabetes was assessed. For analysis 2, an additional 413 men were excluded: 363 did not have triglyceride results at time 2, and in 38 and 12 men diabetes or coronary heart disease was diagnosed, respectively, between time 1 and time 2. Therefore, analysis 2 included 13,540 men.
Diagnosis of type 2 diabetes was defined as the primary study end point. All cases of diabetes were diagnosed according to the American Diabetes Association Expert Committee criteria (15). The diagnosis of all 322 incident cases of diabetes was based on two fasting plasma glucose levels of ≥126 mg/dl (≥7.00 mmol/l). End point determination was made at each sequential staff periodic examination center visit by measuring fasting glucose levels. Between visits, diabetes was diagnosed by the IDF primary care physician (15), and the diagnosis was reviewed by a military physicians’ committee.
Laboratory methods
Fresh blood samples were analyzed in a core laboratory facility. The laboratory is authorized according to international quality standard ISO-9002 and is subject to periodic quality control assessment (by the British National External Quality Assessment Service). For venous fasting plasma glucose determinations, blood samples were collected in sodium fluoride–containing tubes. All biochemical parameter measurements were performed using a BM/Hitachi 917 automated analyzer (Boehringer Mannheim).
Statistical analysis
A general linear model was used to assess the age-adjusted means and proportions of the population's characteristics across quintiles of triglyceride levels and to fit the median of the quintiles as a continuous variable to estimate the trend of variables across quintiles. For analysis 1, we conducted a Cox proportional hazards analysis during each interval of follow-up to estimate the hazard ratios (HRs) and 95% CIs for the development of diabetes according to triglyceride levels of time 1 (first measurement). In stepwise models, values for BMI, fasting plasma glucose, and family history of diabetes were added separately into the age-adjusted model to evaluate the potential role of each as a confounder. In the final multivariate model, we controlled for age, BMI, total cholesterol–to–HDL cholesterol ratio, family history of diabetes, fasting plasma glucose, mean arterial blood pressure, physical activity, and smoking status.
For analysis 2, triglyceride levels at both time 1 and time 2 were cross-classified by tertiles. In parallel, we determined changes in BMI, smoking status, physical activity, and pattern of eating breakfast between time 2 and time 1. Next, we evaluated the joint risk attributed to triglyceride levels at time 1 and time 2; categorized them according to low, intermediate, and high tertiles; and used the group of men in the low-low triglyceride level group as a reference group (HR = 1). To evaluate the direct association of triglyceride level changes, we further adjusted for the changes between time 2 and time 1 in BMI, physical activity, smoking, and eating breakfast pattern in a final multivariate model. In this analysis, we added the calculated differences (Δs) of BMI, and for categorical variables we created four groups, describing the positive or negative states at time 1/time 2 of smoking, physical activity, and eating breakfast pattern (yes/yes, yes/no, no/yes, and no/no). All statistical analyses were performed using SAS statistical software (version 9.1; SAS Institute, Cary, NC).
RESULTS
We analyzed data from 13,953 apparently healthy men (mean age 32.4 years; range 26–45 years) with triglyceride levels <300 mg/dl (<3.40 mmol/l) at enrollment (time 1). Age-adjusted values for BMI, LDL cholesterol, and fasting plasma glucose levels, as well as the proportion of men with a family history of diabetes and the percent that were current smokers, were more likely to increase across triglyceride quintiles (Table 1). Concomitantly, levels of HDL cholesterol and the proportion of men who were physically active or who reported eating breakfast regularly were more likely to decrease across quintiles of triglyceride levels.
Table 1.
Baseline characteristics of 13,953 young adult men across quintiles of triglycerides (time 1)
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Ptrend | Total (mean) | |
|---|---|---|---|---|---|---|---|
| n | 2,844 | 2,739 | 2,789 | 2,795 | 2,786 | 13,953 | |
| Triglyceride level (mg/dl) | |||||||
| Mean | 52 ± 9.6 | 78.1 ± 6.9 | 104 ± 8.4 | 139 ± 12.6 | 212 ± 35.7 | 116.9 | |
| Median (25th, 75th) | 53 (45, 60) | 78 (72, 84) | 104 (97, 111) | 138 (128, 150) | 204 (182, 236) | ||
| Range | 30–66 | 67–90 | 91–119 | 120–163 | 164–299 | ||
| Age (years) | 31.0 ± 4.8 | 31.7 ± 5.1 | 32.3 ± 5.3 | 33.0 ± 5.4 | 33.9 ± 5.2 | <0.001 | 32.4 |
| Mean follow-up (years) | 10.4 | 10.3 | 10.1 | 10.6 | 10.7 | 0.58 | 10.4 |
| Age adjusted | |||||||
| Family history of type 2 diabetes (%)* | 16.0 | 16.7 | 16.7 | 21.0 | 23.2 | <0.001 | 18.7 |
| BMI (kg/m2) | 24.0 ± 3.1 | 24.7 ± 3.4 | 25.5 ± 3.5 | 26.3 ± 3.5 | 27.3 ± 3.6 | <0.001 | 25.6 |
| Blood pressure (mmHg) | |||||||
| Systolic | 117 ± 11.4 | 119 ± 11.6 | 120 ± 12.2 | 121 ± 12.3 | 122 ± 12.6 | <0.001 | 119.8 |
| Diastolic | 75.0 ± 8.4 | 76.3 ± 8.6 | 77.1 ± 9.1 | 77.8 ± 9 | 78.9 ± 9.7 | <0.001 | 77.0 |
| Mean arterial pressure | 88.4 ± 8.6 | 89.2 ± 8.7 | 90.5 ± 9.4 | 91.2 ± 9.3 | 92.3 ± 9.8 | <0.001 | 90.3 |
| Smoking status (%) | |||||||
| Current | 22.0 | 27.7 | 29.7 | 32.9 | 37.0 | <0.001 | 29.8 |
| Former | 20.0 | 18.5 | 22.0 | 19.8 | 20.5 | 0.81 | 20.2 |
| Physical activity | |||||||
| >60 min/week (%) | 13.0 | 11.8 | 9.0 | 9.3 | 6.6 | <0.001 | 9.9 |
| Minutes per week (mean) | 91 | 89 | 88 | 81 | 83 | 0.057 | 86.4 |
| Eating breakfast (%)† | 22 | 19.3 | 17.7 | 17.9 | 16.4 | 0.002 | 18.7 |
| Biomarkers | |||||||
| HDL cholesterol (mg/dl) | 54.0 ± 11.2 | 48.2 ± 10.5 | 47.1 ± 9.8 | 44.3 ± 9.8 | 40.8 ± 9.6 | <0.001 | 46.9 |
| Total cholesterol–to–HDL cholesterol ratio | 3.70 ± 0.9 | 4.28 ± 1.1 | 4.80 ± 1.3 | 5.34 ± 1.5 | 6.03 ± 1.6 | <0.001 | 4.8 |
| Fasting plasma glucose (mg/dl) | 89.0 ± 9.7 | 90.3 ± 9.6 | 91.4 ± 9.8 | 92.0 ± 10.1 | 93.3 ± 10.3 | 0.005 | 91.2 |
Data are means ± SD unless indicated otherwise. Values represent the first of two repeated measurements of triglycerides within a 5-year interval.
A family history for type 2 diabetes indicates the presence of type 2 diabetes in a first-degree relative.
Eating breakfast denotes the percentage of persons reporting eating breakfast regularly. To convert the values for triglycerides to millimoles per liter, multiply by 0.0113. To convert the values for glucose to millimoles per liter, multiply by 0.0555. To convert the values for cholesterol to millimoles per liter, multiply by 0.0259.
Analysis 1
During nearly 77,000 person-years (mean follow-up 10.5 years), there were 322 documented incident cases of type 2 diabetes. Age-adjusted HRs for diabetes increased across quintiles of triglycerides (Table 2), reaching 4.77 (95% CI 3.22–7.06) for the top quintile compared with the bottom quintile (Ptrend < 0.001). Further adjustment for BMI attenuated the risk values to 2.61 for the top quintile (1.75–3.91). The upper two quintiles of triglyceride (median triglyceride 138 mg/dl [1.57 mmol/l] and 204 mg/dl [2.31 mmol/l]) remained significantly associated with elevated diabetes risk when plasma glucose and family history of diabetes, two independent risk factors for diabetes in this population (10), were added to the model. Furthermore, a multivariate model adjusting for age, BMI, total cholesterol–to–HDL cholesterol ratio, family history of diabetes, glucose levels, mean arterial blood pressure, physical activity, and smoking status revealed a significantly increased risk for diabetes attributable to triglyceride levels in quintiles 4 and 5 (HR 1.72 [95% CI 1.12–2.64)] and 2.11 [38–3.22], respectively), compared with the bottom quintile (median triglyceride level 53 mg/dl [0.60 mmol/l]). To exclude the possibility that elevated triglyceride levels already reflected a clinically unrecognized diabetic state at baseline, the findings above were confirmed in a secondary analysis that excluded individuals whose diagnosis of diabetes was made within the first 2 years of follow-up (data not shown).
Table 2.
HRs for type 2 diabetes among 13,953 young adult men across quintiles of triglyceride levels
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Ptrend | |
|---|---|---|---|---|---|---|
| Triglyceride (mg/dl) | 30–66 | 67–90 | 91–119 | 120–163 | 164–299 | |
| Person-years of follow-up | 15,941 | 15,115 | 15,340 | 15,428 | 15,100 | |
| No. incident cases of type 2 diabetes | 22 | 41 | 43 | 79 | 137 | <0.001 |
| Adjusted risk ratio (95% CI) | ||||||
| Age | 1 | 1.49 (0.94–2.35) | 1.72 (1.10–2.68) | 2.88 (1.90–4.36) | 4.77 (3.22–7.06) | <0.001 |
| Age and BMI | 1 | 1.21 (0.77–1.93) | 1.34 (0.86–2.08) | 1.89 (1.24–2.87) | 2.61 (1.75–3.91) | <0.001 |
| Age, BMI, and FPG | 1 | 1.16 (0.73–1.83) | 1.23 (0.79–1.92) | 1.69 (1.11–2.57) | 2.13 (1.42–3.19) | <0.001 |
| Age, BMI, FPG and family history of diabetes* | 1 | 1.18 (0.75–1.87) | 1.25 (0.80–1.95) | 1.72 (1.13–2.61) | 2.10 (1.40–3.14) | <0.001 |
| Multivariate† | 1 | 1.15 (0.73–1.82) | 1.24 (0.78–1.94) | 1.72 (1.12–2.64) | 2.11 (1.38–3.22) | <0.001 |
Family history of diabetes is a reported first-degree relative with type 2 diabetes
The multivariate Cox regression model was adjusted for age, BMI, total cholesterol–to–HDL cholesterol ratio, fasting plasma glucose, mean arterial blood pressure (continuous variables), family history of coronary heart disease (positive, negative, or missing information), physical activity (yes, no, or missing information), and smoking status (current smoker, noncurrent smoker, or missing information). To convert the values for triglycerides to millimoles per liter, multiply by 0.0113.
Analysis 2
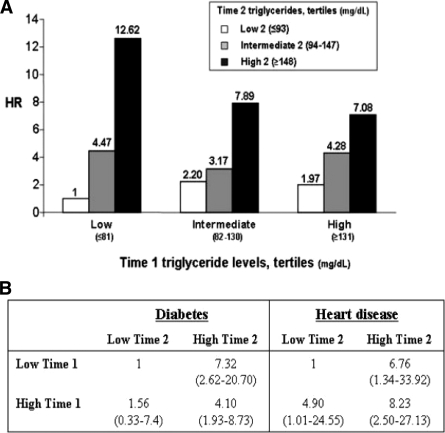
We next evaluated the association between changes in triglyceride levels and diabetes risk. We analyzed data from 13,540 men as described in research design and methods. Triglyceride levels at time 1 and time 2 were divided into tertiles (low, intermediate, and high), and diabetes risk was assessed using the multivariate model described above during the mean 5.2 years after time 2. Consistent with analysis 1 (Table 2), the risk of incident diabetes was 2.01-fold higher (95% CI 1.20–4.38) in the high tertile of time 1 triglyceride levels compared with that for the low tertile (Ptrend < 0.001). Adding time 2 triglyceride levels resulted in nine groups cross-classified according to triglyceride level at both time points, for which the HR for diabetes was calculated and expressed using individuals in the low tertile of triglyceride levels at both time points (low-low) as the reference group (Fig. 1A). In this group the incidence rate of diabetes during follow-up was 52.2 cases per 10,000 individuals (16 cases of 3,066). In the multivariate model, which now also controlled for the time interval between time 1 and time 2, the HRs for diabetes were 3.17 (95% CI 1.09–8.67) and 7.08 (2.52–14.45) in individuals with triglyceride levels at both time points in the intermediate- or high- tertile levels, respectively. By contrast, individuals who had low time 1 triglyceride but whose triglyceride levels increased to intermediate or high tertiles in time 2 were 4.47 (95% CI 1.37–9.48) and 12.62 (3.52–31.34) times, respectively, more likely to develop diabetes compared with those remaining with low triglyceride levels at time 2 (low-low). Importantly, time 2 triglyceride values also modified diabetes risk in those with triglyceride levels in the high tertile at time 1: HR for diabetes was 7.08 in the high-high compared with the low-low group, but decreased to 4.28 (95% CI 1.42–9.69) and 1.97 (0.67–6.13) in individuals whose time 2 triglyceride levels decreased to intermediate or low tertiles, respectively.
Figure 1.
Association between changes in triglyceride levels and future morbidity (analysis 2). A: Multivariate model showing the association between fasting serum triglyceride levels obtained at two measurements 5 years apart* and incidence of type 2 diabetes. The multivariate Cox regression model was adjusted for age, BMI, total cholesterol–to–HDL cholesterol ratio, fasting plasma glucose, time lapse between time 1 and time 2 determinations, and mean arterial blood pressure as continuous variables and physical activity (yes, no, or missing information), family history of diabetes (positive, negative, or missing information), and smoking status (current, noncurrent smoker, or missing information). To convert the values for triglycerides to millimoles per liter, multiply by 0.0113. B: Multivariate model comparing HRs for diabetes or heart disease associated with fasting triglyceride levels in two measurements 5 years apart*. The model was adjusted, as in A, for age, family history of coronary heart disease (positive, negative, or missing information), interval between time 1 and time 2, time 1 levels of fasting plasma HDL cholesterol, glucose, mean arterial blood pressure, and BMI (as continuous variables). In addition, the model was adjusted for the changes between time 1 and time 2 in BMI, physical activity (nonactive/nonactive, nonactive/active, active/nonactive, or active/active), smoking status (current/current, current/noncurrent, noncurrent/current, or noncurrent/noncurrent), and habit of eating breakfast (no/no, no/yes, yes/no, or yes/yes). The results regarding heart disease have been published in Ref. 13. *Time 1 is determination at enrollment; time 2 is determination obtained 5 years after time 1 determination. In this analysis (analysis 2), follow-up begins from time 2, as detailed under research design and methods.
In a recent article (13), we characterized the low-low and high-high (as well as low-high and high-low) groups representing individuals with stable or dynamic triglyceride levels during follow-up, respectively. Men in the low-low triglyceride level group were more likely to retain a lower BMI, maintain high physical activity parameters, and continue a high percentage of reported habit of eating breakfast. Importantly, men in the high-low group, i.e., those whose triglyceride decreased between time 1 and time 2 from the high to the low tertile without lipid-lowering medication, were the only group of the four whose BMI decreased. Furthermore, they were the group who exhibited the highest increase in physical activity and percentage of individuals reporting eating breakfast regularly.
To assess whether changes in triglyceride had a risk-modifying effect beyond changes in BMI, physical activity, smoking, and eating breakfast habit, the HRs for diabetes were further adjusted for the change in these parameters. In this model, dynamic changes in triglyceride levels remained a significant modifying factor of the risk of incident diabetes: individuals whose triglyceride levels progressed from the low to the high tertile had a HR for diabetes of 7.32 (95% CI 2.62–20.70) compared with those remaining at the low triglyceride tertile at both time points. Conversely, in those whose triglyceride levels decreased from the high to the low tertile, the HR for diabetes was 1.56 (0.33–7.40), not significantly different from that for those who retained low triglyceride levels at both time points. The comparison with the association between changes in triglyceride level and heart disease in a model controlling also for the change in BMI and lifestyle factors in the same population (13) is described in Fig. 1B. Stable high triglyceride levels (high-high group) increased the risk of heart disease eightfold and the risk of diabetes fourfold compared with the stable low triglyceride level group. However, young men with an initial high triglyceride level followed by a lifestyle-associated decrease in triglyceride level to the low level at time 2 (high-low group) had a HR of 4.9 for heart disease, representing a lower risk than that observed in the high-high group but still significantly elevated compared with that for the low-low group. In contrast, risk of diabetes among the high-low group was statistically indistinguishable from that for men with stable low tertile triglyceride levels.
CONCLUSIONS
Three major observations emerge from this study on triglyceride level as an independent risk predictor for future type 2 diabetes in young men. First, baseline triglyceride level was found in our large-scale cohort of young men to be an independent risk factor for diabetes during > 10 years of follow-up. Second, two triglyceride measurements 5 years apart revealed that in individuals whose triglyceride level changed, diabetes risk was correspondingly modified: diabetes risk increased >12-fold if the triglyceride level increased between time 1 and time 2 from the lowest to the highest tertile. Conversely, in individuals with triglyceride levels at the high tertile in the first determination, diabetes risk changed from a HR of 7 to <2 if the second triglyceride measurement was in the low tertile range (compared with the low-low group). Importantly, such triglyceride level changes were not related to initiation of lipid-lowering therapy but instead were associated with measurable changes in lifestyle parameters (13). This seemingly tight association between dynamic triglyceride level and diabetes risk not only strengthens the role of triglyceride levels in determining diabetes risk but also signifies that triglyceride level is a sensitive biomarker for lifestyle habits related to the risk of developing diabetes. Third, fasting triglyceride level predicts incident diabetes in a more “acute” manner than it associates with heart disease. This is reflected by the facts that 1) individuals with initial low but subsequent high triglyceride have a higher HR even compared with those with stable high levels at both time points and 2) in the high-low group, which exhibited positive lifestyle changes, a HR statistically indistinguishable from that for those with stable low triglyceride levels was observed. Both phenomena are not present in the association between changes in triglyceride level and heart disease in the same population (Fig. 1B). Thus, although type 2 diabetes and heart disease share multiple common risk factors, a shorter “metabolic memory” of triglyceride levels may underlie the pathogenesis of diabetes versus heart disease, at least in the young, apparently healthy male population.
The strengths and weaknesses of this cohort have been discussed in detail in previous publications (10,13). Worth mentioning here is the fact that baseline measurements are highly similar to those of other published cohorts of young adults (16,17), possibly excluding the “healthy worker bias” in our cohort. Second, although we could not draw conclusions regarding the relation between triglyceride level and diabetes in women, a previous study assessing a cohort of 39- to 65-year-old Swedish women also reported triglyceride level as an independent diabetes risk factor (5). Third, although laboratory parameters do not include advanced measurements such as circulating insulin levels, they constitute a set of routine tests that are typically available to the practicing physician but not routinely screened in young adults. Finally, the large size of the cohort enabled us to define subgroups with a relatively high risk for development of type 2 diabetes in a population otherwise characterized by a low background incidence rate of the disease.
Baseline triglyceride levels were found to be an independent risk factor for diabetes in our cohort of young men, even after adjustment for metabolic parameters, clinical variables, and lifestyle indexes. Many of these factors are known to be tightly correlated with triglyceride (11,12). Consistently, controlling for these parameters in the stepwise multivariate model demonstrated a marked attenuation of the diabetes risk associated with higher quintiles of triglyceride level. Nevertheless, a significantly elevated HR was observed in the multivariate model for individuals with triglyceride levels at the fourth and fifth quintiles (i.e., already at triglyceride levels >120 mg/dl [>1.36 mmol/l]) compared with those at the bottom quintile (triglyceride level of ≤66 mg/dl [≤0.75 mmol/l]). The notion that triglyceride level constitutes an independent diabetes risk factor was proposed by several studies on cohorts of older individuals or of women (5,6,8,9). Here, we also show the relevance of triglyceride measurements for diabetes risk assessment in the young adult, apparently healthy, male population.
The modifying effect of changes in triglyceride levels on diabetes risk provides potential new insights into diabetes prevention strategies and mode of action of lifestyle modification. Compared with pharmacological intervention with metformin, lifestyle modification showed superior efficacy in preventing diabetes (18). The close association observed between changes in triglyceride levels and alterations in BMI, physical activity, and eating habits (13) suggests triglyceride level as a sensitive lifestyle biomarker that is relevant to diabetes risk assessment. Intriguingly, dynamics in triglyceride levels remained a significant determinant of diabetes risk even after adjustment for the accompanying changes in BMI, smoking, physical activity, and eating breakfast (Fig. 1B). This may suggest an effect of triglyceride on diabetes risk that is independent of lifestyle factors altogether and, indeed, conditions such as familial hypertriglyceridemia suggest that genetic factors can determine triglyceride levels. Alternatively, lifestyle factors that were unaccounted for in this study may mediate the change in triglyceride and/or the associated modification of diabetes risk.
Circulating triglyceride levels represent a balance between triglyceride synthesis and utilization (1). These are greatly affected by lifestyle factors (nutritional habits and exercise) and by insulin sensitivity. Consistently, an increasing triglyceride level, particularly when accompanied by low HDL, was shown to be a surrogate marker of insulin resistance (19), a strong predisposing condition for type 2 diabetes. Furthermore, high free fatty acids potentially derived from triglyceride may further deteriorate insulin sensitivity (20), creating a vicious cycle between triglyceride level and insulin resistance. Such a process may have operated to acutely increase diabetes risk when triglyceride levels progressed during follow-up from the lowest to the highest tertile, potentially surpassing the excessive risk associated with persistently elevated triglyceride levels. Finally, improving insulin sensitivity and glucose tolerance by pharmacological means decreased circulating free fatty acids or triglyceride levels (21–23). Thus, although our observational study falls short in unraveling cause-effect relationships, it is tempting to speculate that lowering triglyceride levels, either pharmacologically or through lifestyle modification, may constitute a viable means to attenuate diabetes risk in apparently healthy young men.
Acknowledgments
This work was supported by “Talpiyot Sheba”–Career Development Grant (A.T.), Ben-Gurion University of the Negev, and the Israel Defense Forces National Budget.
Published ahead of print at http://care.diabetesjournals.org on 30 June 2008.
A.T. and I.S. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Ginsberg HN, Zhang YL, Hernandez-Ono A: Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res 36:232–240, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet P, Shaw J: IDF Epidemiology Task Force Consensus Group: the metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kahn R, Buse J, Ferrannini E, Stern M: The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 48:1684–1699, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Dotevall A, Johansson S, Wilhelmsen L, Rosengren A: Increased levels of triglycerides, BMI and blood pressure and low physical activity increase the risk of diabetes in Swedish women: a prospective 18-year follow-up of the BEDA study. Diabet Med 21:615–622, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG: Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ 310:560–564, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, Folsom AR, Chambless LE: Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 28:2013–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 8.McPhillips JB, Barrett-Connor E, Wingard DL: Cardiovascular disease risk factors prior to the diagnosis of impaired glucose tolerance and non-insulin-dependent diabetes mellitus in a community of older adults. Am J Epidemiol 131:443–453, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Mykkanen L, Kuusisto J, Pyorala K, Laakso M: Cardiovascular disease risk factors as predictors of type 2 (non-insulin-dependent) diabetes mellitus in elderly subjects. Diabetologia 36:553–559, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A: Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 353:1454–1462, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg HN: Nonpharmacologic management of low levels of high-density lipoprotein cholesterol. Am J Cardiol 86:41–45L, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Szapary PO, Bloedon LT, Foster GD: Physical activity and its effects on lipids. Curr Cardiol Rep 5:488–492, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Tirosh A, Rudich A, Shochat T, Tekes-Manova D, Israeli E, Henkin Y, Kochba I, Shai I: Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med 147:377–385, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bloomgarden ZT: Type 2 diabetes in the young: the evolving epidemic. Diabetes Care 27:998–1010, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Juonala M, Viikari JS, Hutri-Kahonen N, Pietikainen M, Jokinen E, Taittonen L, Marniemi J, Ronnemaa T, Raitakari OT: The 21-year follow-up of the Cardiovascular Risk in Young Finns Study: risk factor levels, secular trends and east-west difference. J Intern Med 255:457–468, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Urbina EM, Srinivasan SR, Kieltyka RL, Tang R, Bond MG, Chen W, Berenson GS: Correlates of carotid artery stiffness in young adults: the Bogalusa Heart Study. Atherosclerosis 176:157–164, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM: Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol 96:399–404, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Boden G, Shulman GI: Free fatty acids in obesity and type 2 diabetes: defining the role in the development of insulin resistance and β-cell dysfunction. Eur J Clin Invest 32(Suppl. 3):14–23, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Enger SC, Johnsen V, Samuelsen A, Laws EA: The effect of clofibrate on glucose tolerance, insulin secretion, triglycerides and fibrinogen in patients with coronary heart disease. Acta Med Scand 201:563–566, 1977 [DOI] [PubMed] [Google Scholar]
- 22.Panz VR, Wing JR, Raal FJ, Kedda MA, Joffe BI: Improved glucose tolerance after effective lipid-lowering therapy with bezafibrate in a patient with lipoatrophic diabetes mellitus: a putative role for Randle's cycle in its pathogenesis? Clin Endocrinol (Oxf) 46:365–368, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL: Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese, diabetic and non-diabetic subjects. Diabetes 48:1836–1841, 1999 [DOI] [PubMed] [Google Scholar]