Abstract
OBJECTIVE—That type 2 diabetes is associated with the metabolic syndrome is known. However, information is lacking regarding the long-term and adverse changes of metabolic syndrome variables in the development of type 2 diabetes from childhood to adulthood.
RESEARCH DESIGN AND METHODS—Observations were examined, retrospectively, in a community-based cohort of normoglycemic (n = 1,838), pre-diabetic (n = 90), and type 2 diabetic (n = 60) subjects followed serially for cardiovascular risk factors during childhood (4–11 years), adolescence (12–18 years), and adulthood (19–44 years).
RESULTS—Diabetic subjects versus normoglycemic subjects had significantly higher levels of subscapular skinfold, BMI, triglycerides, glucose, insulin, and homeostasis model assessment of insulin resistance and lower levels of HDL cholesterol beginning in childhood and higher levels of mean arterial pressure (MAP) in adolescence and adulthood. In a multivariate model including BMI, MAP, HDL cholesterol, LDL cholesterol, triglycerides, glucose, and insulin, adjusted for age, age2, race, sex, and race × sex interaction, adverse changes in glucose and LDL cholesterol were independently associated with pre-diabetic subjects, whereas adverse changes in BMI, glucose, and HDL cholesterol were associated with diabetic subjects. As young adults, pre-diabetic and diabetic groups displayed a significantly higher prevalence of obesity, hypertension, dyslipidemia, hyperinsulinemia, and metabolic syndrome.
CONCLUSIONS—These findings indicate that adverse levels of risk variables of metabolic syndrome, adiposity, and measures of glucose homeostasis accelerating since childhood characterize the early natural history of type 2 diabetes and underscore the importance of early prevention and intervention on risk factors beginning in childhood.
More than 19 million people have type 2 diabetes and another 54 million individuals show impaired fasting glucose as adults, which may represent a pre-diabetic state (1). This carbohydrate-insulin imbalance becomes one of the most common causes of death in the U.S. (2). It is also widely recognized that type 2 diabetes increases the risk for cardiovascular morbidity and premature death (3). On the basis of the progressive global epidemic of obesity, it is expected that the worldwide prevalence of type 2 diabetes will rise by 50% to >360 million people over the next 30 years (4). Because a much larger population can be classified as pre-diabetic, the additional risk of cardiovascular mortality becomes enormous (5).
As for type 2 diabetes, the pathogenesis of pre-diabetes is linked to a relative insulin deficiency and/or tissue insulin resistance associated with elevated blood glucose levels, despite secondary hyperinsulinemia (6). According to the classification of the American Diabetes Association (7), pre-diabetes and type 2 diabetes represent the two categories of impaired glucose regulation and are associated with a constellation of disorders characteristic of the metabolic syndrome (8,9). However, most studies have been performed on single, baseline measurements at middle and older ages (10,11). Information is lacking on long-term, longitudinal, and progressive changes of the risk variables of the metabolic syndrome from childhood to younger adulthood. This study observed changes over a 21-year period beginning in childhood.
RESEARCH DESIGN AND METHODS
The Bogalusa Heart Study is being conducted in the semirural, biracial (65% white and 35% black) community of Bogalusa, Louisiana. Between 1976 and 1994, six cross-sectional studies of school-aged children were conducted. In addition, eight cross-sectional surveys were conducted between 1978 and 2002 using young adults who had been examined previously as children. A detailed description of the study design, participation, and protocols was published elsewhere (12). This panel design, based on repeated cross-sectional examinations conducted approximately every 3–4 years, resulted in serial observations from childhood to young adulthood, allowing longitudinal analyses. The participant rate was ≥80% for the children and ∼60% for the adult cohort.
Subjects from six cross-sectional studies of children who participated in at least one of the 14 cross-sectional surveys of children and adults were eligible for this retrospective cohort study. Of these, a total of 1,988 fasting subjects (68% white and 43% male) were selected from the last three surveys of adults from 1995–2002. At the baseline examination, the children with a history of treatment of diabetes or those who had a fasting glucose level ≥7 mmol/l were excluded. At the initial screening, the mean ± SD age was 10.9 ± 4.0 years (range 4–18). At the most recent screening, the age was 32.0 ± 6.5 years (19–44). The mean follow-up interval was 21 years. The number of screenings and observations between childhood and adulthood ranged from 2 to 9. In all, 91% of subjects were screened ≥3 times and 63% were screened 4–6 times, with a total of 9,232 sets of observations.
On the basis of the data at the last survey, adult subjects were classified as normoglycemic, pre-diabetic, or diabetic according to the American Diabetes Association criteria (7). Individuals were considered normoglycemic (n = 1,838) if they had a fasting glucose level <5.6 mmol/l, pre-diabetic (n = 90) if their fasting glucose level was between 5.6 and 6.9 mmol/l, or diabetic (n = 60) if 1) their fasting glucose level was ≥7 mmol/l or 2) they had a history of treatment of diabetes. The institutional review board approved consent forms used for these surveys, and informed consent was obtained from adult study participants and from parents or guardians of children.
General examination
Identical protocols were used by trained examiners, nurses, and technicians across all surveys; procedures for general examination were reported previously (13). In brief, subjects were instructed to fast for 12 h before the screening, with compliance ascertained by an interview on the day of examination. Serum samples were obtained from antecubital venous blood and kept at 4°C until analysis the following day. Each screening day, on a random 10% subsample, a second blood sample was collected, labeled, and analyzed in a blind fashion to estimate the measurement error. Information on personal health and medication history was obtained by questionnaires. All measurements were made in replicate, and mean values were used. In the longitudinal analysis, BMI (weight in kilograms divided by the square of height in meters) was used as a measure of overall adiposity and subscapular skinfold measured with Lange skinfold calipers was used for truncal fatness. Young adults were considered obese if their BMI was ≥30 kg/m2. Blood pressure measurements were obtained on the right arm with the subjects in a relaxed, sitting position. Systolic and diastolic blood pressures were recorded at the first and fifth Korotkoff phases using a mercury sphygmomanometer. Blood pressure levels were reported as the mean of six replicate readings, with three taken by each of two randomly assigned and trained observers. Mean arterial pressure (MAP), calculated as diastolic blood pressure plus one-third pulse pressure, was used in the analysis.
Laboratory analyses
From 1973 to 1986, cholesterol and triglycerides levels were measured using chemical procedures on a Autoanalyzer II (Technicon) according to the Laboratory Manual of the Lipid Research Clinics Program. These variables were determined by enzymatic procedures on a VP instrument (Abbott) between 1987 and 1996 and on the Hitachi 902 Automatic Analyzer (Roche) afterward. Both chemical and enzymatic procedures met the performance requirements of the Lipid Standardization Program of the Centers for Disease Control and Prevention, which routinely monitored the precision and accuracy of cholesterol, triglyceride, and HDL cholesterol measurements since the beginning of this study. Measurements on the quality-control samples assigned by the agency showed no consistent bias over time within or between surveys. Serum lipoprotein cholesterol levels were analyzed by using a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures (14). The intraclass correlation coefficients between the blind duplicate (10% random sample) values ranged from 0.86 to 0.98 for HDL cholesterol, from 0.86 to 0.98 for LDL cholesterol, and from 0.88 to 0.99 for triglycerides. On the basis of the National Cholesterol Education Program Adult Treatment Panel III guidelines (15), adult subjects were classified as having dyslipidemia if they had high LDL cholesterol (≥4.14 mmol/l), high triglycerides (≥2.26 mmol/l), or low HDL cholesterol (<1.03 mmol/l) or if they were taking medication for dyslipidemia and as having metabolic syndrome if they had at least three risk factors consistent with that diagnosis.
From 1976 to 1991, plasma glucose was measured by a glucose oxidase method using a glucose analyzer (Beckman). Since then, it has been measured enzymatically as part of a multichemistry (SMA20) profile. Plasma immunoreactive insulin levels were measured by a commercial radioimmunoassay kit (Phadebas; Pharmacia). The intraclass correlation coefficients between the blind duplicate values ranged from 0.94 to 0.98 for insulin and from 0.86 to 0.98 for glucose. Hyperinsulinemia was arbitrarily defined as a fasting value >108 pmol/l, a value considered indicative of insulin resistance in normoglycemic subjects (16). In addition, an index of insulin resistance was calculated according to the homeostasis model assessment for insulin resistance formula: HOMA-IR = [insulin (microunits per milliliter) × glucose (millimoles per liter)/22.5]. This model was considered useful for assessing insulin resistance in epidemiological studies.
Statistical analysis
All of the statistical analyses were performed with SAS (version 9.1; SAS Institute, Cary, NC). In the analyses, the race-sex groups were combined to increase statistical power and to simplify the presentation. Continuous variables were tested for normality using a Kolmogorov-Smirnov test. Values for triglycerides, glucose, and insulin and HOMA-IR variables used in the analyses were log transformed to improve normality as necessary. Mean levels of risk variables in 4- to 11-, 12- to 18-, and ≥19-year-old age-groups corresponding with the preadolescence, adolescence, and adulthood period were compared by their diabetes status in adulthood (normoglycemic versus pre-diabetic, normoglycemic versus diabetic, and pre-diabetic versus diabetic). A single measurement per subject was used to calculate mean levels of risk variable within age-groups. General linear models were used to examine impaired glucose regulation differences in risk factor variables, adjusted for age, race, and sex. By using data from the last survey in adulthood, significant differences in the prevalence of pre-diabetes and diabetes by race and sex and metabolic syndrome and its related cardiovascular risk factors by diabetes status were tested by Pearson's χ2 test.
Multivariate analyses (generalized estimation equation method) were used to determine which longitudinal changes in risk variables since childhood predicted adult diabetes status. The model included BMI, MAP, HDL cholesterol, LDL cholesterol, triglycerides, glucose, and insulin, adjusted for age, age2, race, sex, and sex- by-race interaction, as applicable. Nonsignificant terms (P > 0.05) were removed from the model by backward stepwise procedure.
RESULTS
Mean levels of anthropometric, hemodynamic, and metabolic variables at baseline are presented in Table 1 by diabetes status. The pre-diabetic group had significantly higher age, LDL cholesterol, and glucose than the normoglycemic group. With the exceptions of diastolic blood pressure and LDL cholesterol, the diabetic group versus normoglycemic group showed significantly higher age, subscapular skinfold, BMI, systolic blood pressure, MAP, triglycerides, glucose, insulin, and HOMA-IR and lower HDL cholesterol. Compared with the pre-diabetic group, diabetic subjects had significantly higher subscapular skinfold, BMI, and triglycerides and lower HDL cholesterol.
Table 1.
Levels at baseline in childhood and prevalence at last survey in adulthood of risk variables related to metabolic syndrome by adult diabetes status: the Bogalusa Heart Study
| Variable | Normoglycemia | Pre-diabetes | Diabetes |
|---|---|---|---|
| n | 1,838 | 90 | 60 |
| At baseline | |||
| Age (years)* | 10.8 ± 0.1 | 12.4 ± 0.4** | 12.3 ± 0.5¶ |
| Subscapular skinfold (mm)† | 12.6 ± 0.2 | 14.2 ± 0.7 | 17.0 ± 1.1††‡‡ |
| BMI (kg/m2)† | 18.3 ± 0.1 | 19.5 ± 0.4 | 22.2 ± 0.8††‖‖ |
| Systolic blood pressure (mmHg)† | 100.8 ± 0.3 | 105.0 ± 1.2 | 107.5 ± 1.7** |
| Diastolic blood pressure (mmHg)† | 61.8 ± 0.2 | 64.5 ± 1.2 | 65.0 ± 1.3 |
| MAP (mmHg)† | 74.8 ± 0.2 | 78.0 ± 1.1 | 79.2 ± 1.3‖ |
| LDL cholesterol (mmol/l)† | 2.27 ± 0.02 | 2.36 ± 0.07‖ | 2.32 ± 0.10 |
| HDL cholesterol (mmol/l)† | 1.60 ± 0.01 | 1.62 ± 0.06 | 1.38 ± 0.06**§§ |
| Triglycerides (mmol/l)† | 0.73 ± 0.01 | 0.78 ± 0.04 | 0.90 ± 0.05††‡‡ |
| Glucose (mmol/l)† | 4.7 ± 0.01 | 5.0 ± 0.1** | 5.1 ± 0.1†† |
| Insulin (pmol/l)† | 53.6 ± 1.6 | 76.7 ± 15.9 | 98.1 ± 17.4¶ |
| HOMA-IR† | 1.8 ± 0.1 | 2.8 ± 0.6 | 3.8 ± 0.8** |
| At last survey | |||
| Obesity: BMI ≥30 kg/m2 | 32.9‡ | 60.0†† | 75.0†† |
| Hypertension: ≥140/90 or treatment | 10.6 | 23.3** | 38.3†† |
| Dyslipidemia | |||
| HDL cholesterol <1.03 mmol/l | 24.8 | 31.1 | 47.5††‡‡ |
| LDL cholesterol ≥4.14 mmol/l | 11.9 | 24.4** | 18.6 |
| Triglycerides ≥2.26 mmol/l | 10.1 | 25.6†† | 32.2†† |
| Hyperinsulinemia: >108 pmol/l | 13.6 | 55.1†† | 51.7†† |
| Metabolic syndrome§ | 14.1 | 68.9†† | 78.0†† |
Data are means ± SD or %.
P values were adjusted for race and sex.
P values were adjusted for age, race, and sex.
Prevalence (%) at last survey.
Defined by the National Cholesterol Education Program Adult Treatment Panel III. Different from normoglycemia:
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001. Different from pre-diabetes:
P < 0.05;
P < 0.01;
P < 0.0001.
The prevalence of metabolic syndrome and its related cardiovascular risk factors in adulthood at the last survey is shown in Table 1 by diabetes status. Compared with the normoglycemic group, obesity in terms of excess generalized adiposity (BMI), hypertension, dyslipidemia, and hyperinsulinemia (indicative of insulin resistance), and metabolic syndrome were significantly more prevalent among the pre-diabetic (except HDL cholesterol) and diabetic (except LDL cholesterol) groups. Compared with the pre-diabetic group, high risk levels of HDL cholesterol were more prevalent among the diabetic group. The overall prevalence of the metabolic syndrome was 18.4% in the total sample (data not shown).
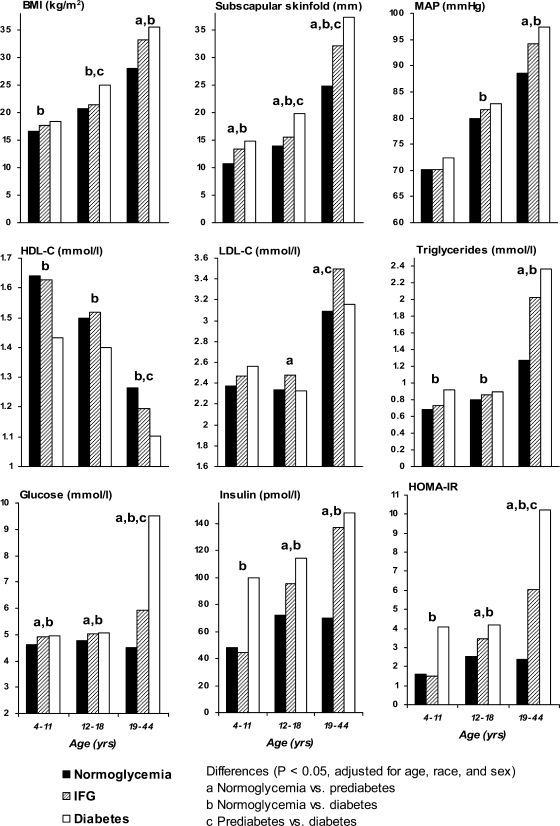
Mean levels of variables of metabolic syndrome in childhood (4–11 years), adolescence (12–18 years), and adulthood (19–44 years) are shown in Fig. 1 by diabetes status in adulthood. A single measurement per subject was used in each age-group. Comparisons were made after adjustment for age, race, and sex. The pre-diabetic group versus normoglycemic individuals showed higher levels of glucose and subscapular skinfold from childhood through adulthood; higher levels of LDL cholesterol, insulin, and HOMA-IR in adolescence and adulthood; and higher levels of BMI, MAP, and triglycerides in adulthood. On the other hand, the diabetic group versus the normoglycemic group had higher levels of subscapular skinfold, BMI, triglycerides, glucose, insulin, and HOMA-IR and lower levels of HDL cholesterol from childhood through adulthood and higher levels of MAP in adolescence and adulthood. In addition, the diabetic group versus the pre-diabetic group had higher levels of subscapular skinfold in adolescence and adulthood, higher BMI in adolescence, and lower levels of HDL cholesterol and LDL cholesterol and higher levels of glucose and HOMA-IR in adulthood.
Figure 1.
Mean levels of BMI, subscapular skinfold, MAP, HDL cholesterol, LDL cholesterol, triglycerides, fasting glucose, insulin, and HOMA-IR from childhood to adulthood by adult diabetes status: the Bogalusa Heart Study.
As shown in Supplementary Fig. 1 of the online appendix (available at http://dx.doi.org/10.2337/dc08-0898), white male subjects had a significantly higher prevalence of pre-diabetes than white female subjects. With respect to diabetes, white male versus white female subjects and black female versus white female subjects had a significantly greater prevalence. Male versus female subjects with pre-diabetes and black versus white subjects with diabetes had a significantly higher prevalence; overall prevalences of pre-diabetes and diabetes were 4.5 and 3.0% in the total sample, respectively (data not shown).
Longitudinal rates of change in risk variables of metabolic syndrome by diabetes status were observed (Supplementary Table 1 of the online appendix). Compared with the normoglycemic group, the rates of increase in subscapular skinfold, BMI, triglycerides, glucose, insulin, and HOMA-IR were significantly higher among the pre-diabetic and diabetic groups and the rates of increase in diastolic blood pressure and MAP were significantly higher among the diabetic group.
Independent relationships between adverse longitudinal changes in risk variables since childhood and adult pre-diabetes and diabetes conditions were determined in the multivariate models, presented in Table 2. Adverse changes in LDL cholesterol and glucose were independently associated with pre-diabetes status and adverse changes in BMI, HDL cholesterol, and glucose with diabetes status. When HOMA-IR index, instead of glucose and insulin, was included in the models, HOMA-IR index, LDL cholesterol, and glucose were significant independent predictors in the pre-diabetic group and HOMA-IR, BMI, and HDL cholesterol were predictors in the diabetic group; further, alternate multivariate analyses using subscapular skinfold, instead of BMI, gave essentially identical results (data not shown).
Table 2.
Adverse longitudinal changes in risk variables since childhood as independent correlates of adult diabetes status in the study cohort: the Bogalusa Heart Study
| Independent variable | Pre-diabetes vs. normoglycemia |
Diabetes vs. normoglycemia |
||||
|---|---|---|---|---|---|---|
| β* | 95% CI | P | β* | 95% CI | P | |
| BMI | — | — | — | 0.09 | 0.06–0.12 | <0.0001 |
| HDL cholesterol | — | — | — | −0.60 | −1.11 to −0.10 | <0.05 |
| LDL cholesterol | 0.37 | 0.18–0.55 | <0.0001 | — | — | — |
| Glucose | 1.87 | 1.64–2.11 | <0.0001 | 1.76 | 1.49–2.03 | <0.0001 |
Generalized equation estimation method regression coefficient adjusted for age, age2, race, sex, and the race × sex interaction, as applicable. Model includes BMI, MAP, LDL cholesterol, HDL cholesterol, triglycerides, glucose, and insulin.
CONCLUSIONS
These observations explore the natural history of impaired glucose regulation and diabetes status in a community-based population of children free from a selection bias monitored longitudinally over a period of 21 years. Data linked the conditions of pre-diabetes and type 2 diabetes in young adults with concurrent longitudinal changes in some metabolic syndrome risk variables from childhood to young adulthood. The present population study shows that among the metabolic syndrome risk variables, in a comparison with normoglycemic subjects, glucose was consistently higher from childhood through adulthood in both pre-diabetic and diabetic subjects; LDL cholesterol, insulin, and HOMA-IR were higher in pre-diabetic subjects since adolescence; and obesity, triglycerides, insulin, and HOMA-IR were higher and HDL cholesterol was lower in diabetic subjects beginning in childhood. In terms of adverse longitudinal changes from childhood to adulthood, LDL cholesterol and glucose were independently related with pre-diabetic status, whereas obesity, HDL cholesterol, and glucose were related to diabetic status.
In the current study, the prevalence of both pre-diabetes, which may represent an impaired fasting glucose state, and diabetes was lower than that found previously (1,8,10). This difference can be explained by the lower average age of our cohort. The observed differences of sex (male > female) in the pre-diabetic group (1,8,10) and race (black > white) in the diabetic group (1) in the study cohort are in agreement with the earlier reports. Further, compared with other race-sex groups, pre-diabetes (10) and diabetes (1) were less prevalent among white females.
It is of interest that levels of obesity were higher beginning in childhood, changed adversely through adulthood, and related independently with diabetes in adulthood. This observation is consistent with the known tracking of risk factor variables over time and especially of childhood obesity in predicting adulthood obesity (17). The persistent elevation of obesity has influenced the onset of type 2 diabetes occurring at a younger age (17,18). With respect to the longitudinal changes in this present study cohort, obesity was the most consistent predictor of adverse changes leading to diabetes, regardless of age, race, or sex. A number of studies have shown baseline obesity as an independent and modifiable risk factor for type 2 diabetes (18,19). Although of different populations, studies showed obesity in young children and adolescents as a strong predictor of subsequent diabetes (17,18). Such observations suggest the molecular mechanisms by which obesity plays a part in glucose intolerance are complex and include a combination of genetic factors and mechanisms in which skeletal myocytes and central adipocytes play a role (19).
This study demonstrates that both pre-diabetic and diabetic groups displayed excess basal glucose, insulin, and HOMA-IR index values at least by adolescence. Of note, the diabetic group displayed a persistent elevation of glucose from childhood through adulthood. Further, glucose, but not insulin, along with lipid and obesity (in the diabetic group) variables were the independent predictors of adverse longitudinal changes of impaired glucose regulation. Of interest, blood pressure was not independently associated in the models, which is consistent with an earlier study in childhood and adolescence (18). Although blood pressure and insulin were individually predictors of diabetes, they were not independently correlated once obesity, glucose, and lipid variables were introduced in the multivariate analyses (18).
The deterioration in glucose levels to pre-diabetes or diabetes that, after a relatively stable period, occurs as a rapid, incremental increase accompanied by a decline in insulin sensitivity has been noted (20). In the current cohort study, both showed progressively increased glucose levels beginning in early life, before the onset of impaired glucose regulation status, suggesting that even small changes in glucose levels may be a marker of altered carbohydrate-insulin imbalance.
In the present study, adverse changes in LDL cholesterol and HDL cholesterol were independently correlated among the pre-diabetic and diabetic groups. Of relevance to this present finding, HDL cholesterol was demonstrated as an independent modifiable predictor of diabetes in childhood and adolescence in Pima Indians (18). Individuals with diabetes usually have increased triglycerides and decreased HDL cholesterol resulting from the release of fatty acids from adipose tissue, the elevation of delivery of free fatty acids to the liver, and the hepatic synthesis of VLDLs (21). This abnormal lipid profile is characterized by modestly elevated LDL cholesterol and high triglycerides levels with a markedly increased cardiovascular risk among diabetic patients (22).
Perhaps most important is the observation that young adults with pre-diabetes and diabetes showed an increased prevalence of metabolic syndrome and its multiple risk factors. The observed overall prevalence of metabolic syndrome is in agreement with an earlier report (23). Also, metabolic syndrome status was more prevalent in the pre-diabetic and diabetic groups, as might be expected and is consistent with previous findings (11). A pre-diabetic status reflects an atherogenic profile of metabolic syndrome risk variables long before an overt clinical macrovascular event (24). Further, these multiple risk factors related to autopsy findings of coronary atherosclerosis that we studied in our young individuals, evidence of “silent,” subclinical disease that evolves from childhood.
As limitations, this study lacks postchallenge glucose and in vivo insulin action and secretion. Instead, an established simple surrogate measure HOMA-IR index applicable to population studies was used. Also, because of the lack of dietary intake data, this study did not address the role of diet in the regulation of glucose homeostasis and related risk of obesity, cardiovascular disease, and type 2 diabetes (25). The fasting status was based on self-report.
In summary, these findings indicate that adverse levels of risk variables of metabolic syndrome, adiposity, and measures of glucose homeostasis in particular, and their accelerated rates of change since childhood, characterizes the early natural history of carbohydrate-insulin imbalance. The current findings reinforce a primary role for early prevention of and intervention for these risk factors, especially obesity, beginning in childhood.
Supplementary Material
Acknowledgments
This work was supported by grants AG16592 from the National Institute on Aging and 0555168B from the American Heart Association.
The Bogalusa Heart Study is a joint effort of many investigators and staff members, whose contributions are gratefully acknowledged. We especially thank the study participants.
Published ahead of print at http://care.diabetesjournals.org on 15 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW: Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 29:1263–1268, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Hoyert DL, Heron MP, Murphy SL, Kung H: Deaths: final data for 2003. Natl Vital Stat Rep 54(13):1–120 [PubMed]
- 3.Fox CS, Sullivan L, D'Agostino RB Sr, Wilson PW: The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care 27:704–708, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Saydah SH, Loria CM, Eberhardt MS, Brancati FL: Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care 24:447–453, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Ghani MA, Tripathy D, DeFronzo RA: Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29:1130–1139, 2006 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 27(Suppl. 1):S5–S10, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Twigg SM, Kamp MC, Davis TM, Neylon EK, Flack JR, Australian Diabetes Society, Australian Diabetes Educators Association: Prediabetes: a position statement from the Australian Diabetes Society and Australian Diabetes Educators Association. Med J Aust 186:461–465, 2007 [DOI] [PubMed] [Google Scholar]
- 9.McClain MR, Srinivasan SR, Chen W, Steinmann WC, Berenson GS: Risk of type 2 diabetes mellitus in young adults from a biracial community: the Bogalusa Heart Study. Prev Med 31:1–7, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Pankow JS, Kwan DK, Duncan BB, Schmidt MI, Couper DJ, Golden S, Ballantyne CM: Cardiometabolic risk in impaired fasting glucose and impaired glucose tolerance: the Atherosclerosis Risk in Communities Study. Diabetes Care 30:325–331, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Nóvoa FJ, Boronat M, Saavedra P, Díaz-Cremades JM, Varillas VF, La Roche F, Alberiche MP, Carrillo A: Differences in cardiovascular risk factors, insulin resistance, and insulin secretion in individuals with normal glucose tolerance and in subjects with impaired glucose regulation: the Telde Study. Diabetes Care 28:2388–2393, 2005 [DOI] [PubMed] [Google Scholar]
- 12.The Bogalusa Heart Study 20th anniversary symposium. Am J Med Sci 310(Suppl. 1):S1–S138, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC, Foster TA, Blonde CV: Cardiovascular Risk Factors in Children: The Early Natural History of Atherosclerosis and Essential Hypertension. New York, Oxford University Press, 1980
- 14.Srinivasan SR, Berenson GS: Serum lipoproteins in children and methods for study. In Handbook of Electrophoresis. Lewis LA, Ed. Boca Raton, FL: CRC Press, 1983, p. 185–204
- 15.Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Laakso M: How good a marker is insulin level for insulin resistance? Am J Epidemiol 137:959–965, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Cheung YB, Machin D, Karlberg J, Khoo KS: A longitudinal study of pediatric body mass index values predicted health in middle age. J Clin Epidemiol 57:1316–1322, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, Infante AM, Krakoff J, Looker HC: Childhood predictors of young-onset type 2 diabetes. Diabetes 56:2964–2972, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn BB, Flier JS: Obesity and insulin resistance. J Clin Invest 106:473–481, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laspa E, Christen A, Efstathiadou Z, Johnston DG, Godsland IF: Long-term changes and variability in diabetes risk factors prior to the development of impaired glucose homeostasis. Diabet Med 24:1269–1278, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Haffner S: Rationale for new American Diabetes Association Guidelines: are National Cholesterol Education Program goals adequate for the patient with diabetes mellitus? Am J Cardiol 96:33E–36E, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Brown AS: Lipid management in patients with diabetes mellitus. Am J Cardiol 96:26E–32E, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 287:356–359, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK: Cardiovascular risk factors in confirmed prediabetic individuals: does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 263:2893–2898, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Ludwig DS: The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287:2414–2423, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.