Abstract
OBJECTIVE—To provide a simple clinical diabetes risk score and to identify characteristics that predict later diabetes using variables available in the clinic setting as well as biological variables and polymorphisms.
RESEARCH DESIGN AND METHODS—Incident diabetes was studied in 1,863 men and 1,954 women, 30–65 years of age at baseline, with diabetes defined by treatment or by fasting plasma glucose ≥7.0 mmol/l at 3-yearly examinations over 9 years. Sex-specific logistic regression equations were used to select variables for prediction.
RESULTS—A total of 140 men and 63 women developed diabetes. The predictive clinical variables were waist circumference and hypertension in both sexes, smoking in men, and diabetes in the family in women. Discrimination, as measured by the area under the receiver operating curves (AROCs), were 0.713 for men and 0.827 for women, a little higher than for the Finish Diabetes Risk (FINDRISC) score, with fewer variables in the score. Combining clinical and biological variables, the predictive equation included fasting glucose, waist circumference, smoking, and γ-glutamyltransferase for men and fasting glucose, BMI, triglycerides, and diabetes in family for women. The number of TCF7L2 and IL6 deleterious alleles was predictive in both sexes, but after including the above clinical and biological variables, this variable was only predictive in women (P < 0.03) and the AROC statistics increased only marginally.
CONCLUSIONS—The best clinical predictor of diabetes is adiposity, and baseline glucose is the best biological predictor. Clinical and biological predictors differed marginally between men and women. The genetic polymorphisms added little to the prediction of diabetes.
A number of diabetes risk scores have been developed to detect those who should be screened for diabetes (1). In the Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort, we have previously studied the anthropometric variables associated with diabetic levels of fasting glucose and found that BMI, waist circumference, and waist-to-hip ratio were equally useful in the identification of individuals with undiagnosed diabetes (2).
The first score to identify lifestyle and clinical parameters predictive of later diabetes was developed by Lindström and Tuomilehto (3), from a population-based sample of people who responded to questionnaires in 1987; 10-year incident diabetes was identified from a registry of diabetes treatment. A similar Finnish Diabetes Risk Score (FINDRISC) was used in a cross-sectional study (4). In the American Atherosclerosis Risk in Communities (ARIC) study and in a Thai population, predictive risk factors were also identified, wherein diabetes was defined by treatment or diabetic levels of fasting and 2-h glucose from an oral glucose tolerance test (5,6). More recently, Simmons et al. (7) published a score from the European Prospective Investigation into Cancer Study (EPIC)-Norfolk study, wherein incident diabetes was defined by clinical identification of diabetes or A1C >7% and dietary factors and physical activity were included. Finally, dietary and other noninvasive factors associated with 5-year incident, self-reported cases of diabetes were identified in the large EPIC-Potsdam study (8).
In the San Antonio Study, Stern et al. (9) published a score based on prospective clinical and biological data. In the Framingham cohort, four scores were proposed: a clinical score and three scores with both clinical and biological factors with incident diabetes identified at follow-up by diabetic treatment and/or fasting glucose levels (10). Other studies on diabetes risk factors include one in French men with impaired fasting glucose (6.1–6.9 mmol/l), which identified lifestyle and clinical and biological factors predictive of diabetes (11).
As risk scores cannot always be generalized from one country to another (12,13), the aim of this study was to describe sex-specific lifestyle and clinical diabetes risk factors in a French population followed over 9 years in order to aid in identifying those at risk for incident diabetes. Additional aims were to study the impact of biological factors and genetic polymorphisms in predicting diabetes.
RESEARCH DESIGN AND METHODS
The study population consisted of men and women aged 30–64 years who participated in the 9-year follow-up study, DESIR. Participants were recruited from volunteers who were offered periodic health examinations free of charge by the French Social Security at 10 health examination centers in western France. All subjects provided informed written consent, and the protocol was approved by an ethics committee.
Incident cases of diabetes were identified by treatment for diabetes or a fasting plasma glucose ≥7.0 mmol/l at one of the 3-yearly examinations; after exclusion of individuals with diabetes at baseline and those with unknown diabetes status at the 9-year examination, 1,863 men and 1,954 women with glucose, BMI, and waist circumference measures available at baseline were included in the study.
Two measures of blood pressure, using a mercury sphygmomanometer, and heart rate were taken in a supine position after 5 min of rest, and mean values were used for analyses. Weight and height were measured in lightly clad participants, and BMI was calculated. Waist circumference, the smallest circumference between the lower ribs and the iliac crest, was also measured.
The examining physician noted the family history of diabetes and menopausal status in a clinical questionnaire; treatment for diabetes and hypertension and lipids were recorded. Hypertension was defined by systolic/diastolic blood pressure of at least 140/90 mmHg or being on antihypertensive medication. Smoking habits, alcohol consumption (glasses of wine, beer, cider, and spirits per day), and degree of physical activity (at home, at work, and sport) were assessed using a self-administered questionnaire.
All biochemical measurements were from one of four health center laboratories located in France at Blois, Chartres, LA Riche, or Orléans. Fasting plasma glucose, measured by the glucose oxidase method, was applied to fluoro-oxalated plasma using a Technicon RA100 (Bayer Diagnostics, Puteaux, France) or a Specific or a Delta device (Konelab, Evry, France). Total cholesterol, HDL cholesterol, triglycerides, alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), and creatinine were assayed by DAX 24 (Bayer Diagnostics) or KONE (Konelab). Insulin was quantified by microparticle enzyme immunoassay with an automated analyzer (IMX; Abbott, Rungis, France). White cell counts were determined by a Technicon H* or Technicon H3RTX (Bayer Diagnostics), a JT2 (Beckman/Coulter, Roissy, France), or an Argos (ABX, Montpellier, France). Interlaboratory variability was assessed monthly on normal and pathological values for each biologic variable.
Single nucleotide polymorphism (SNP) genotyping was performed with SNPlex Technology (Applied Biosystems, Foster City, CA) based on oligonucleotide ligation assay combined with multiplex PCR target amplification (http://www.appliedbiosystems.com) (14).
Statistical methods
Statistical analysis was performed using SAS version 9.1 (SAS Institute, Cary, NC). Alcohol intake, BMI, fasting glucose, insulin, ALT, GGT, triglycerides, and white blood cell count were log-transformed because of their skewed distributions.
Characteristics of men and women with and without incident diabetes are shown as means ± SD or n (%) and compared by t or χ2 tests or by linear regression for the polymorphisms with additive models. The logistic model was used to test for interactions with sex, and P are reported; significant interactions (P < 0.01) provided the rational for sex-specific models.
The linearity of continuous parameters in logistic analyses was studied by adding a squared term and comparing nested models by likelihood ratio tests; all variables were linearly related with the logit of diabetes incidence except for fasting glucose (log transformed); in the models, glucose(log) was centered by subtracting its mean, and its square was systematically included.
Parsimonious logistic regression models were selected using forwards and backwards as well best model selection criteria using all parameters; the Hosmer-Lemeshow goodness-of-fit test was the principal criteria for selection of a model. Interactions with sex were tested. The area under the receiver operating characteristic curve (AROC) for sensitivity-specificity quantified the discrimination between diabetic and nondiabetic participants. Bootstrap sampling was used to validate the choice of variables in the models, with 1,000 samples of the same sizes as the study populations. The choice of variables was also validated in the Cox model.
To derive a simple clinical score from the clinical equations, we used the β-coefficients from the logistic regression analysis; for waist circumference, four groups were defined, linearly, from the approximate sex-specific quartiles. The score was validated in two French cohorts: E3N and SU.VI.MAX (15,16) (online appendix Fig. 1 [available at http://dx.doi.org/10.2337/dc08-0368]). The first study identified incident diabetes by self-questionnaire or treatment reimbursement, the second by fasting glucose or treatment.
Four polymorphisms were chosen for study (glucokinase: GCK-30 G/A rs1799884, interleukine 6: IL6-174 G/C rs1800795, and Kir6.2: KCNJ11 E23K rs5219 and TCF7L2 rs7903146) following previous analyses in this population (14). Additive models discriminated best between diabetic and nondiabetic people. For the two polymorphisms found to be the most related with incident diabetes (IL6 and TCF7L2), the number of deleterious alleles (as a continuous variable) was calculated and added as a variable to the (clinical + biological) equations chosen above. As this analysis aimed to determine those who should be screened for diabetes, we have analyzed all individuals and have not excluded those born outside of mainland France. This analysis was on a smaller population (1,655 men and 1,740 women), where these two polymorphisms were available.
We compared our clinical risk score with the FINDRISC score (3) using the AROC statistic; FINDRISC includes age, BMI, waist circumference, antihypertensive medication, physical activity, previously known high glucose, and daily consumption of vegetables, and fruits or berries; we were not able to include the latter two items. Our (clinical + biological) equation was compared with the Stern equation, including age, sex, fasting glucose, systolic blood pressure, HDL cholesterol, BMI, and diabetes in the family; we did not include the factor for coding Mexican Americans (9).
RESULTS
In the DESIR population, 140 men and 63 women had incident diabetes.
Clinical predictors of incident diabetes
All of the clinical variables showed similar relations with incident diabetes in both men and women with the exception of diabetes in the family: noted for 43% of women with incident diabetes and 19% without diabetes and for 20 and 18% of men, respectively (P for sex interaction = 0.003) (Table 1).
Table 1.
Clinical and biological characteristics at baseline of men and women with and without incident diabetes during the 9 years of the DESIR study
| Men |
Women |
P for variable | P for interaction with sex | |||||
|---|---|---|---|---|---|---|---|---|
| Diabetes | No diabetes | P* | Diabetes | No diabetes | P* | |||
| n | 140 | 1,723 | 63 | 1,891 | ||||
| Age (years) | 50 ± 9 | 47 ± 10 | 0.0001 | 52 ± 8 | 47 ± 10 | 0.0005 | 0.0001† | 0.6 |
| Diabetes in the family | 28 (20) | 312 (18) | 0.6 | 27 (43) | 368 (19) | 0.0001 | ‡ | 0.003 |
| Current smoker | 52 (37) | 418 (24) | 0.0009 | 10 (16) | 249 (13) | 0.5 | 0.001† | 0.3 |
| Alcohol intake (g/day)§ | 34 ± 32 | 23 ± 22 | 0.005 | 8 ± 11 | 7 ± 11 | 0.5 | 0.006† | 0.2 |
| Physical activity | ||||||||
| Little | 43 (31) | 422 (24) | 22 (35) | 465 (25) | ||||
| Moderate | 72 (51) | 911 (53) | 0.07 | 33 (52) | 1036 (55) | 0.04 | 0.03† | 0.7 |
| Intensive | 25 (18) | 388 (23) | 8 (13) | 386 (20) | ||||
| Waist circumference (cm) | 96 ± 10 | 89 ± 9 | 0.0001 | 90 ± 12 | 76 ± 10 | 0.0001 | 0.0001† | 0.3 |
| BMI (kg/m2)§ | 27.5 ± 4.0 | 25.1 ± 3.0 | 0.0001 | 29.2 ± 5.1 | 23.7 ± 3.8 | 0.0001 | 0.0001† | 0.3 |
| Menopause | 30 (48) | 718 (38) | 0.1 | |||||
| Large baby, birth weight ≥4 kg | 16 (27) | 284 (15) | 0.02 | |||||
| Hypertension‖ | 87 (62) | 678 (39) | 0.0001 | 39 (62) | 527 (28) | 0.0001 | 0.0001 | 0.1 |
| Heart rate (min) | 68 ± 10 | 66 ± 10 | 0.007 | 71 ± 11 | 68 ± 9 | 0.02 | 0.0005† | 0.7 |
| Treatment for lipids | 20 (14) | 126 (7) | 0.004 | 9 (14) | 129 (7) | 0.03 | 0.0003† | 0.9 |
| Fasting glucose (mmol/l)§ | 6.05 ± 0.55 | 5.39 ± 0.49 | 0.0001 | 5.96 ± 0.58 | 5.11 ± 0.46 | 0.0001 | 0.0001† | 0.1 |
| GGT (IU/l)§ | 64.3 ± 67.2 | 39.5 ± 38.3 | 0.0001 | 36.4 ± 33.6 | 21.7 ± 21.2 | 0.0001 | 0.0001† | 0.4 |
| ALT (UI/l)§ | 41.7 ± 28.3 | 30.3 ± 18.1 | 0.0001 | 28.9 ± 22.2 | 20.1 ± 13.8 | 0.0001 | 0.0001† | 0.4 |
| Triglycerides (mmol/l)§ | 1.79 ± 1.45 | 1.26 ± 0.80 | 0.0001 | 1.50 ± 0.78 | 0.93 ± 0.50 | 0.0001 | ‡ | 0.006 |
| HDL cholesterol (mmol/l) | 1.41 ± 0.37 | 1.50 ± 0.38 | 0.01 | 1.53 ± 0.34 | 1.80 ± 0.42 | 0.0001 | ‡ | 0.008 |
| Total cholesterol (mmol/l) | 6.06 ± 1.05 | 5.82 ± 0.97 | 0.009 | 5.94 ± 1.04 | 5.61 ± 0.96 | 0.02 | 0.0001† | 0.6 |
| Creatinine (μmol/l) | 91.0 ± 13.9 | 89.1 ± 11.1 | 0.06 | 77.1 ± 10.7 | 74.1 ± 10.0 | 0.02 | 0.006† | 0.4 |
| White blood cell count (109/l)§ | 6.9 ± 2.1 | 6.4 ± 1.7 | 0.002 | 7.3 ± 4.0 | 6.2 ± 1.6 | 0.0002 | 0.0001† | 0.2 |
| n | 135 | 1,617 | 61 | 1,782 | ||||
| Number of TCF7L2 and IL6 deleterious alleles | 2.0 | 1.8 | 0.008 | 2.2 | 1.8 | 0.03 | 0.0007 | 0.7 |
Data are means ± SD and n (%) unless otherwise indicated.
P comparing means and percentages by t and χ2 tests.
P for variable in logistic model with only variable and sex, as interaction not significant.
P for variable not given, as the interaction is significant.
Log transformation because of a nonsymmetric distribution.
Hypertension: systolic/diastolic blood pressure ≥140/90 mmHg or medication for hypertension.
The first most predictive variable was waist circumference, closely followed by BMI, in both sexes. The selected model in men included waist circumference, smoking, and hypertension and in women included waist circumference, diabetes in the family, and hypertension (Table 2). These models showed a good fit (Hosmer-Lemeshow P = 0.7 and 0.6 in men and women, respectively) and well discriminated the diabetic and nondiabetic populations (AROC 0.733 and 0.839, respectively). In the bootstrap samples, these were the most frequently chosen models. These variables were also chosen by the Cox modeling.
Table 2.
β-Coefficients for the clinical, clinical + biological, and clinical + biological + genetic equations: the DESIR study
| Clinical equation |
Clinical + biological equation |
Clinical + biological + genetic equation |
||||
|---|---|---|---|---|---|---|
|
n = 1,860 men (140 with diabetes) and n = 1,954 women (63 with diabetes) |
n = 1,860 men (140 with diabetes) and n = 1,954 women (63 with diabetes) |
n = 1,655 men (128 with diabetes) and n = 1,740 women (58 with diabetes) |
||||
| β | P | β | P | β | P | |
| Men | ||||||
| Intercept | −10.45 | −10.53 | 10.91 | |||
| Current smoker | 0.72 | 0.0002 | 0.88 | 0.0001 | 0.94 | 0.0001 |
| Waist circumference (cm) | 0.081 | 0.0001 | 0.060 | 0.0001 | 0.060 | 0.0001 |
| Hypertension* | 0.50 | 0.01 | ||||
| Fasting glucose (mmol/l)† | 10.15 | 0.0001 | 10.17 | 0.0001 | ||
| Fasting glucose2† | 24.16 | 0.002 | 22.42 | 0.007 | ||
| GGT (IU/l)† | 0.39 | 0.01 | 0.42 | 0.007 | ||
| Number of TCF7L2 and IL6 deleterious alleles | 0.14 | 0.2 | ||||
| AROC statistic | 0.733 | 0.850 | 0.851 | |||
| Hosmer-Lemeshow fit test | 0.7 | 0.8 | 0.1 | |||
| Women | ||||||
| Intercept | −11.81 | −18.91 | −20.43 | |||
| Diabetes in the family | 1.09 | 0.0001 | 0.80 | 0.01 | 0.75 | 0.02 |
| Waist circumference (cm) | 0.095 | 0.0001 | ||||
| BMI (kg/m2)† | 4.38 | 0.0001 | 4.69 | 0.0001 | ||
| Hypertension* | 0.64 | 0.03 | ||||
| Fasting glucose (mmol/l)† | 9.66 | 0.001 | 9.35 | 0.001 | ||
| Fasting glucose2† | 23.89 | 0.06 | 22.39 | 0.08 | ||
| Triglycerides (mmol/l)† | 0.95 | 0.003 | 0.86 | 0.01 | ||
| Number of TCF7L2 amd IL6 deleterious alleles | 0.36 | 0.04 | ||||
| AROC statistic | 0.839 | 0.917 | 0.912 | |||
| Hosmer-Lemeshow fit test | 0.6 | 0.9 | 0.8 | |||
Systolic/diastolic blood pressure ≥140/90 mmHg or medication for hypertension.
Fasting glucose, GGT, BMI, and triglycerides were log transformed.
Clinical risk score
Clinical risk scores were derived (Table 3) from the above equations. These scores showed a good fit (Hosmer-Lemeshow P = 0.8 and 0.9 in men and women, respectively), and the discrimination was similar to the more exact equation with continuous values of waist circumference.
Table 3.
A clinical diabetes risk score of 5 confers a >30% chance of diabetes in the following 9 years: the DESIR study
| Scores to sum | |
|---|---|
| Men | |
| Waist circumference (cm) | |
| <80 | 0 |
| 80–89 | 1 |
| 90–99 | 2 |
| ≥100 | 3 |
| Current smoker: yes | 1 |
| Hypertension: yes* | 1 |
| AROC statistic | 0.713 |
| Hosmer-Lemeshow fit test | P = 0.8 |
| Women | |
| Waist circumference (cm) | |
| <70 | 0 |
| 70–79 | 1 |
| 80–89 | 2 |
| ≥90 | 3 |
| Diabetes in the family: yes | 1 |
| Hypertension: yes* | 1 |
| AROC statistic | 0.827 |
| Hosmer-Lemeshow fit test | P = 0.9 |
Systolic/diastolic blood pressure ≥140/90 mmHg or medication for hypertension.
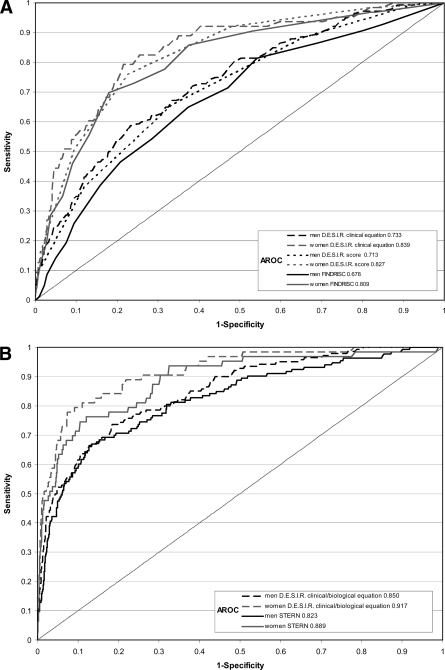
The receiver operating characteristic (ROC) curves for the clinical equation and for the simplified clinical score are shown for men and women (Fig. 1A); the DESIR scores with three variables had AROC values slightly higher than for the five-variable FINDRISC score. The score predicted diabetes in the two French cohorts, with the AROCs similar to those from DESIR (online appendix Fig. 1).
Figure 1.
ROC curves and AROC statistics in men and women for the DESIR French clinical equation, the French clinical risk score, and the FINDRISC clinical score (3) (A) and for DESIR (clinical + biological) (B). French risk equation and Stern risk equation (9).
Biologic predictors of incident diabetes
Fasting glucose was by far the factor most predictive of incident diabetes, with no difference in its effect between men and women (P for interaction = 0.1) (Table 1). Predictive factors differing between sexes were triglycerides and HDL cholesterol—both had a slightly stronger relation in women (P for sex interaction = 0.006 and 0.008, respectively).
Clinical + biological predictors of incident diabetes
Fasting glucose (including its squared term) was the most predictive of all factors. After adjustment for fasting glucose, waist circumference was more predictive than BMI in men, but BMI was more predictive than waist in women (Table 2). In men, the predicting equation included fasting glucose, smoking status, waist circumference, and GGT and in women included fasting glucose, BMI, diabetes in the family, and triglycerides. The same variables were chosen by Cox modeling with five predictive variables. Our (clinical + biological) equation was simpler than the Stern equation with only four variables and discriminated incident diabetic individuals similarly (Fig. 1B).
Genetic polymorphisms as predictors of incident diabetes
None of the four polymorphisms was significantly related to incident diabetes in either men or women, using either the three genotypes or recessive, dominant, or additive models of inheritance (online appendix Table). There was no interaction with sex, and combining men and women, TCF7L2 and IL6 were significantly related with incident diabetes using additive models (P < 0.01 and 0.03, respectively). In comparison with individuals with no deleterious alleles, those with four deleterious alleles had an OR of incident diabetes of 3.60 (95% CI 1.09–11.9) in men and 3.22 (0.62–16.5) in women. The number of deleterious alleles was associated with incident diabetes in both men and women (P < 0.008 and 0.03, respectively) (Table 1). Including the total number of deleterious alleles in the above-determined (clinical + biological) equations, models showed an adequate fit but little changed the AROC (Table 2).
CONCLUSIONS
Both the clinical and the (clinical + biological) equations are able to predict diabetes incidence over a 9-year follow-up, with different variables in the equations for men and women. Age was not selected in any of the equations, but age is highly correlated with adiposity, hypertension, and glucose levels, all of which appear in the equations. Age was included in the equations of many (3,5,8–10) but not all (7) of the other published studies of risk equations. Polymorphisms added little to these scores. As expected, the equations derived on our population performed slightly better than those derived in other populations. The clinical score performed well on two other French cohorts.
Our diabetes risk score based on clinical data has the advantage that it is simple and requires only three parameters. Given a larger population and a higher incidence of diabetes, other parameters might have been included in the equations, but the discrimination and model fit may not be greatly improved. BMI and waist circumference had similarly predictive values in both men and women; once one was included, the other no longer entered the model. Similar comments can be made for GGT and ALT.
In contrast to other scores, we have studied men and women separately and found that the predictive equations differ. In both sexes, waist circumference was the clinical factor most related to incident diabetes; the next most predictive factor in men was smoking, which was more common in men than women; and in women only, diabetes in the family was a predictive factor. Hypertension was predictive of diabetes in both sexes, a factor often present before diabetes (17). Smoking is recognized as a risk factor for diabetes, with a higher risk for the heavy smokers in comparison with the lighter and former smokers (18). Our observation that more diabetic women than men have diabetes in the family is probably due to women being more aware of their family history of diabetes. In the multivariate equations, physical activity was not predictive—this could be because of its negative correlation with waist circumference and hypertension and perhaps because our questions on self-reported physical activity were not sufficiently precise, in comparison with other data such as waist circumference and hypertension. Other studies have indeed included physical activity in their multivariate equations (3,7,8).
The overriding biological factor predictive of diabetes was the baseline glucose level. In the (clinical + biological) equation in men, the GGT also entered the equation and in women the triglycerides concentration entered. We have already shown that GGT is predictive of incident diabetes in this cohort for both sexes (19), and others have shown that triglycerides are predictive (5,10).
The polymorphisms studied provided little toward predicting diabetes: for the 1,655 men and 1,740 women with these data available, the Hosmer-Lemeshow tests showed a poorer fit for men when the genetic data were included but identical AROCs. Of note, in women, the coefficient for the parameter of diabetes in the family was only reduced from 0.80 to 0.75 when genetic parameters were included, indicating that other possible genetic factors are involved. A large panel of SNPs may be needed to outperform even simple clinical parameters.
One of the limitations is that we have not been able to include the 2-h glucose concentration in our definition of diabetes—in France, screening of diabetes with fasting glucose is common; our score is therefore appropriate in the local situation. A further limitation is that the score is only for people between 30 and 65 years of age.
The simplest clinical parameter for identifying those at risk of diabetes is adiposity, and taken alone, either waist circumference or BMI did equally well in predicting later diabetes during the 9-year follow-up. The addition of hypertension, smoking in men, and triglycerides in women provides a clinical score that discriminates well.
The DESIR study group
INSERM U780: B. Balkau, P. Ducimetière, and E. Eschwège; INSERM U367: F. Alhenc-Gelas; CHU D'Angers: Y. Gallois, and A. Girault; Bichat Hospital: F. Fumeron, and M. Marre; CNRS UMR8090, Lille: P. Froguel; Centres d'Examens de Santé: Alençon, Angers, Caen, Chateauroux, Cholet, Le Mans, Tours; Institute de Recherche Médecine Générale: J. Cogneau; general practitioners of the region; Institute inter-Regional pour la Santé: C. Born, E. Caces, M. Cailleau, J.G. Moreau, F. Rakotozafy, J. Tichet, and S. Vol.
Supplementary Material
Acknowledgments
DESIR has been supported by INSERM contracts with CNAMTS (Caisse Nationale d'Assurance Maladie des Travailleurs Salariés [French National Health Insurance Agency for Wage Earners]), Lilly, Novartis Pharma, and sanofi-aventis; by INSERM (Réseaux en Santé Publique, Interactions entre les déterminants de la santé), the Association Diabète Risque Vasculaire, the Fédération Française de Cardiologie, La Fondation de France, ALFEDIAM, ONIVINS, Ardix Medical, Bayer Diagnostics, Becton Dickinson, Cardionics, Merck Santé, Novo Nordisk, Pierre Fabre, Roche, and Topcon.
We thank Francoise Clavel-Chapelon and Serge Hercberg for making their data available from the E3N and SU.VI.MAX studies, respectively, for the validation of our clinical risk score, and Sylviane Vol and Gaëlle Gusto for their careful reading and criticisms of the manuscript.
Published ahead of print at http://care.diabetesjournals.org on 8 August 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, Williams R, John A: Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 11:1–125, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Balkau B, Sapinho D, Petrella A, Mhamdi L, Cailleau M, Arondel D, Charles MA; D.E.S.I.R. Study Group: Prescreening tools for diabetes and obesity-BMI, waist and waist hip ratio: the D.E.S.I.R. Study Eur J Clin Nutr 60:295–304, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindström J, Tuomilehto J: The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 26:725–731, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Saaristo T, Peltonen M, Lindström J, Saarikoski L, Sundvall J, Eriksson JG, Tuomilehto J: Cross-sectional evaluation of the Finnish Diabetes Risk Score: a tool to identify undetected type 2 diabetes, abnormal glucose tolerance and metabolic syndrome. Diab Vasc Dis Res 2:67–72, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, Folsom AR, Chambless LE; The Atherosclerosis Risk in Communities Investigators: Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 28:2013–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Aekplakorn W, Bunnag P, Woodward M, Sritara P, Cheepudomwit S, Yamwong S, Yipintsoi T, Rajatanavin R: A risk score for predicting incident diabetes in the Thai population. Diabetes Care 29:1872–1877, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Simmons RK, Harding AH, Wareham NJ, Griffin SJ; EPIC-Norfolk Project Team: Do simple questions about diet and physical activity help to identify those at risk of type 2 diabetes? Diabet Med 24:830–835, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Mohlig M, Pfeiffer AF, Spranger J, Thamer C, Häring HU, Fritsche A, Joost HG: An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 30:510–515, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Stern MP, Williams K, Haffner SM: Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 136:575–581, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB Sr: Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 167:1068–1074, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Lecomte P, Vol S, Caces E, Born C, Chabrolle C, Lasfargues G, Halimi JM, Tichet J: Five-year predictive factors of type 2 diabetes in men with impaired fasting glucose. Diabete Metab 33:140–147, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Rathmann W, Martin S, Haastert B, Icks A, Holle R, Löwel H, Giani G; KORA Study Group: Performance of screening questionnaires and risk scores for undiagnosed diabetes: the KORA Survey 2000. Arch Intern Med 165:436–441, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Glümer C, Vistisen D, Borch-Johnsen K, Colagiuri S; DETECT-2 Collaboration: Risk scores for type 2 diabetes can be applied in some populations but not all. Diabetes Care 29:410–414, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Clavel-Chapelon F, van Liere MJ, Giubout C, Niravong MY, Goulard H, Le Corre C, Hoang LA, Amoyel J, Auquier A, Duauesnel E: E3N, a French cohort study on cancer risk factors: E3N Group: Etude Epidemiologique aupres de femmes de l'Education Nationale. Eur J Cancer Prev 6:473–478, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, Hercberg S: Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr 200684:395–399, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Vaxillaire M, Veslot J, Dina C, Proença C, Cauchi S, Charpentier G, Tichet J, Fumeron F, Marre M, Meyre D, Balkau B, Froguel P; for the DESIR Study Group: Impact of common type 2 diabetes risk polymorphisms in the DESIR prospective study. Diabetes 57:244–254, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ: Blood pressure and risk of developing type 2 diabetes mellitus: the Women's Health Study. Eur Heart J 28:2937–2943, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J: Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 298:2654–2664, 2007 [DOI] [PubMed] [Google Scholar]
- 19.André P, Balkau B, Born C, Royer B, Wilpart E, Charles MA, Eschwège E: Hepatic markers and development of type 2 diabetes in middle aged men and women: a three-year follow-up study: the D.E.S.I.R. study (Data from an Epidemiological Study on the Insulin Resistance syndrome). Diabet Metab 31:542–550, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.