Abstract
It is often stated that type 1 diabetes results from a complex interplay between varying degrees of genetic susceptibility and environmental factors. While agreeing with this principal, our desire is that this Perspectives article will highlight another complex interplay potentially associated with this disease involving facets related to the gut, one where individual factors that, upon their interaction with each another, form a “perfect storm” critical to the development of type 1 diabetes. This trio of factors includes an aberrant intestinal microbiota, a “leaky” intestinal mucosal barrier, and altered intestinal immune responsiveness. Studies examining the microecology of the gastrointestinal tract have identified specific microorganisms whose presence appears related (either quantitatively or qualitatively) to disease; in type 1 diabetes, a role for microflora in the pathogenesis of disease has recently been suggested. Increased intestinal permeability has also been observed in animal models of type 1 diabetes as well as in humans with or at increased-risk for the disease. Finally, an altered mucosal immune system has been associated with the disease and is likely a major contributor to the failure to form tolerance, resulting in the autoimmunity that underlies type 1 diabetes. Herein, we discuss the complex interplay between these factors and raise testable hypotheses that form a fertile area for future investigations as to the role of the gut in the pathogenesis and prevention of type 1 diabetes.
WHY WRITE A PERSPECTIVES THAT EMPHASIZES THE GUT?
In addition to comprising the largest surface area of the body, the intestinal mucosa is constantly exposed to a vast array of microbes, food antigens, and toxins. The intestinal epithelium must discriminate between pathogenic and nonpathogenic organisms as well as food antigens. It must “tolerate” the commensal flora that maintain mucosal homeostasis by controlling inflammatory responses as well as sensing danger signals of potentially harmful pathogens. It is becoming increasingly clear that the nexus of intestinal microbiota composition, the intestinal barrier, and the mucosal immune system plays pivotal roles in the development of a variety of allergic and autoimmune diseases.
In the case of type 1 diabetes, evidence for a synergism between aberrant intestinal microbiota, a “leaky” intestinal mucosal barrier, and altered mucosal immunity contributing to the disorder's pathogenesis has begun to evolve (Table 1). If these facets do indeed form important components for the pathogenesis of type 1 diabetes, they also offer potential targets for intervention that would include maintenance of a nondiabetogenic microbiota, tightening of interepithelial junctions, as well as prevention of propagation of inflammation and autoimmunity by nutritional or pharmacologic means. In the following sections, we will review each aspect for its physiological function and provide evidence as to how it may form pathogenic significance in type 1 diabetes.
TABLE 1.
Intestinal alterations reported in patients with type 1 diabetes or in individuals at risk of type 1 diabetes
| Finding | Interpretation | Reference |
|---|---|---|
| Increased absorbed ratio of ingested lactulose and mannitol in patients with type 1 diabetes | Altered gut permeability in type 1 diabetes | Kuitunen et al. (24), Sapone et al. (25), Secondulfo et al. (26) |
| Increased absorption of ingested lactulose and mannitol in individuals with β-cell autoimmunity | Altered gut permeability in pre-diabetes | Bosi et al. (23) |
| Alterations in height and thickness of microvilli, space between microvilli and thickness of tight junctions | Mucosal injury in type 1 diabetes | Secondulfo et al. (26) |
| High levels of serum zonulin in patients with type 1 diabetes | Changes in tight junctions in type 1 diabetes | Sapone et al. (25) |
| Enhanced expression of HLA-DR and –DP, ICAM-1, α4β7-integrin, IL-4, IL-1α, IFN-γ in small intestinal biopsy samples from children with type 1 diabetes | Intestinal inflammation in children with type 1 diabetes | Savilahti et al. (28), Westerholm-Ormio et al. (29) |
| Higher density of intraepithelial CD3 and γ/δ-cells and lamina propria CD25 cells in type 1 diabetes | Activation of intestinal immunity in type 1 diabetes | Auricchio et al. (30) |
| Increase in CD3 and CD25 cells and enhanced ICAM-1 and HLA-DR expression in small intestinal biopsy samples cultured with gliadin | Activation of intestinal T-cells by gliadin in type 1 diabetes | Auricchio et al. (30) |
| Increased expression of matrix metalloproteinases and apoptotic cells in small intestinal biopsy samples from children with type 1 diabetes and anti-transglutaminase antibodies | Prominent mucosal inflammation related response in the diabetic children positive for transglutaminase antibodies | Bister et al. (32) |
| Enterovirus detected in small intestinal biopsies from patients with type 1 diabetes | Chronic or recurrent intestinal enterovirus infections in type 1 diabetes | Oikarinen et al. (42) |
| Low numbers of Foxp3-positive cells or Foxp3 transcripts despite increased IL-18 transcription in small intestinal biopsy samples from children with type 1 diabetes | No activation of intestinal regulatory T-cells despite activation of innate immunity in children with type 1 diabetes | Tiittanen et al. (31) |
ALTERED INTESTINAL MICROBIOTA
The presence of a commensal intestinal microbiota in infancy is critical for numerous physiologic processes including growth, angiogenesis, optimization of nutrition, and stimulation of various arms of the innate and adaptive immune systems (1–4). With this, it is surprising that the effects of intestinal microbiota on the development of type 1 diabetes remain an area subject to somewhat limited investigation.
What does seem clear is that rodent models of type 1 diabetes, including NOD mice and related substrains, are more likely to develop disease under specific pathogen-free conditions (5,6). Furthermore, diabetes-prone BB rats (BBDP) subject to Cesarean derivation have been noted to develop accelerated disease (7). In terms of using such information to proactively modulate diabetes formation, the provision of antibiotics, such as fucidic acid, Colistin, and Bactrim, in BB rats after weaning (8,9) lead to diabetes prevention, whereas in our own efforts using the NOD mouse, a decreased frequency of type 1 diabetes was observed with the administration of doxycycline (10). The specific mechanisms of how such therapies modulate disease are unclear, but it is clear that changes in the microbiota affect the development of autoimmune diabetes in both animal models. Supporting this view, oral probiotic administration to NOD mice was noted to induce interleukin (IL)-10 (i.e., an anti-inflammatory cytokine) production and prevented the development of type 1 diabetes (11).
Interest exists on intestinal microbiota composition, a metric that has previously relied almost exclusively on the quantitative cultures from feces. At this time, we are not aware of any published studies that have demonstrated differences in intestinal microbiota of animals or humans with or at high-risk for the development of type 1 diabetes. Furthermore, most microbial species in the intestinal microbiota are not amenable to culture. With the knowledge that environment influences the development of type 1 diabetes and that the gastrointestinal tract provides the greatest surface area for interaction of the environment, this is an area that begs further investigation.
Over 500 species of microbes are known to reside in the human gastrointestinal tract (1,2). Their interaction with the mucosal immune system, especially in the first years of life, may have life-long effects. As but one example, differences in the composition of intestinal microflora between healthy and allergic infants in countries with a high and low prevalence of allergies have been noted and precede clinical symptoms (12). Furthermore, probiotics have been successfully used as immunomodulators in the prevention and treatment of allergies in children (13,14), findings indicating that commensal bacteria are not innocent bystanders in humans but active players in the shaping of the immunological network of the host. Hence, new approaches for evaluating the interactions between the intestinal microbiota and barrier and innate immunity are needed.
Among the most promising molecular techniques that have recently been developed to fill this void are those that enable detection of uncultivatable species and that are amenable to statistical microecologic analyses. Some of these methods target 16Ss RNA gene sequences, as they contain signatures of phylogenetic groups and sometimes even species (15). Other techniques apply PCR with denaturing or temperature gradient gel electrophoresis, fluorescent in situ hybridization and shotgun sequencing DNA (16), and whole metagenomic approaches (17). These techniques offer promise for future efforts seeking to establish a causal link between the intestinal microbiota and type 1 diabetes, as well as to the identification of aberrant microbiota that could be targeted for disease prevention strategies.
ALTERATIONS OF INTESTINAL PERMEABILITY IN TYPE 1 DIABETES
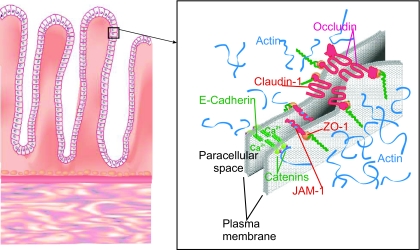
The intestinal surface barrier is one of the most important components of the innate immune system, separating highly immunogenic agents in the intestinal lumen from a highly immunoreactive submucosa. Before aberrant microbes or other antigens can affect the highly immunoreactive submucosa, they must transduce signals mediated by intestinal epithelial toll-like (or similar) receptors, traverse the intestinal epithelial barrier by either the transcellular or interepithelial paracellar route, or influence other cells possessing the capacity to traverse this barrier (e.g., intraepithelial lymphocytes and dendritic cells). Before describing how this system may relate to type 1 diabetes, we will review a few key components of the system (Fig. 1).
FIG. 1.
Constituents of the intestine.
Mucosal barrier cells.
There are numerous cell types in the small intestine that play a role in barrier function, including the following.
Intestinal epithelial cells.
Intestinal epithelial cells (IECs) comprise the lining epithelium of the primitive intestine. These cells are derived from the same undifferentiated stem cell that gives rise to columnar, mucous, goblet, enteroendocrine, and probably M-cells in the intestine. The well-known function of the IEC as the major nutritive absorptive cell should not overshadow its role in transduction of inflammatory signals from luminal microbes via toll-like receptors and other signaling mechanisms. Furthermore, these cells possess intercellular interdigitations and structures that join the intestinal epithelial cells (Fig. 1). The tight junction (i.e., zonula occludens) is the most apical component of the junctional complex and serves as the permeability barrier between the external and internal milieus of the body. The tight junction completely encircles the apical end of absorptive cells as a belt-like band. Several transmembrane proteins and proteins on the cytosolic leaflet are a part of the tight junction; these include the claudins and occludin protein families. Interactions of some of these proteins with the actin cytoskeleton are a major determinant of tight junction structure and play a role in the regulation of paracellular permeability.
M-cells.
M-cells do not have well-developed microvilli and allow macromolecular transport, whereas the absorptive enterocyte has better-developed microvilli. M-cells are specialized for delivering foreign antigens and microorganisms to organized lymphoid tissues within the mucosa of the small and large intestines. They are thought to play a major role in specific immunity to luminal antigens. Little is known about relative contributions of these cell types in the pathogenesis of type 1 diabetes, but as will be discussed later, lines of evidence are pointing toward increased paracellular permeability playing a role.
Goblet cells.
Goblet cells are specialized mucus-secretory cells found throughout the intestine. Intestinal mucus is a complex gel that covers the surface of the villous epithelium and contributes significantly to cytoprotection, offering many ecological advantages for the microbiota.
Paneth cells.
Paneth cells represent one of the four major epithelial cell lineages in the small intestine and are the only lineage that migrates downward into the crypt base after originating in the crypt stem cell region. The location of Paneth cells adjacent to crypt stem cells suggests that they play a critical role in defending epithelial cell renewal. In response to pathogen attack, the Paneth cells secrete a wide spectrum of antimicrobial peptides against gram-negative and gram-positive bacteria, fungi, protozoa, and viruses.
Intraepithelial lymphocytes.
Intraepithelial lymphocytes (IELs) are located at the basolateral side of the epithelial layer. Here they are exposed to a wide range of food and microbial antigens. One well-established function of IELs is their ability to protect the host from invasion by microorganisms that enter through the gastrointestinal tract. One subtype, the γδ intestinal epithelial lymphocytes, are also at the mucosal interface and appear to play a key role in maintaining peripheral tolerance (18).
HOW GUT LEAKINESS MAY INFLUENCE TYPE 1 DIABETES
Multiple tight junction integral proteins have been identified (Fig. 1), including occludin and members of the claudins family; a group of at least 20 tissue-specific proteins are the major sealing proteins that locate between intestinal epithelial cells (19). The claudins, a family of integral tight junction proteins, form ion-selective pores within the tight junction strands, whereas occludin and junctional adhesion molecule may have an adhesive and/or signal transducing function as they interact with various cytosolic complexes (19). In the intestinal epithelium, claudin-1 may directly associate with occludin laterally in the membrane within the same cell but not intercellularly. The combination of these two proteins functioning together performs the major “gatekeeper” or barrier function of the tight junction. These sealing proteins, both transmembrane proteins, interact with cytoplasmic proteins that function as adaptors between the tight junction proteins along with actin and myosin contractile elements within the cell. Acting together, they open and close the paracellular junctions.
Our previous studies using the permeability markers lactulose and mannitol verified that BBDP rats, before the onset of type 1 diabetes, exhibit a highly permeable intestine associated with low levels of intestinal claudin, a major intercellular tight junction protein (20). Intestinal myeloperoxidase activities and goblet cell density are also higher in the diabetes-prone rats than in controls, supporting the notion of an early intestinal inflammatory response. Our studies (20), as well as those of Meddings et al. (21) and Graham et al. (22), show that not only is enteropathy a consistent feature in the BBDP rat, but it precedes the onset of insulitis and appears to be due to mechanisms distinct from those that cause type 1 diabetes. More importantly, this is not a phenomenon that occurs only in rodent models of diabetes, as very recent studies have noted that humans with a propensity to develop type 1 diabetes as well as other autoimmune diseases possess an abnormal intestinal barrier; the so called “leaky gut” (20,21). Namely, intestinal samples from individuals at risk for (23) or already diagnosed with type 1 diabetes demonstrated abnormalities identified with sugar permeability tests (24–26) and associated with interepithelial junctions on electron microscopy (26). These findings are entirely consistent with the concept that a leaky intestine in the setting of type 1 diabetes would allow for greater exposure of the intestinal immune system to antigens (Fig. 2).
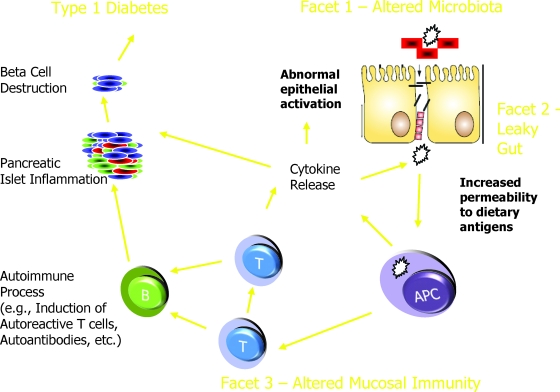
FIG. 2.
Hypothetical model of the contribution of various gut components to the pathogenesis of type 1 diabetes.
Although the mechanisms leading to a “leaky gut” before the development of type 1 diabetes remain largely understood, studies aimed at altering intestinal tight junctions have been initiated. Fasano and colleagues (27) found high levels of the protein zonulin, which has been observed to increase intestinal permeability in rats prone to develop diabetes, and have initiated studies designed to counteract the effects of zonulin. High serum levels of zonulin that correlated with increased ratios in sugar permeability testing have also been noted for patients with type 1 diabetes (25). Other modulators of tight junction proteins (e.g., certain probiotics and short-chain fatty acids such as butyrate) may also play a role in modulation of “intestinal leakiness.”
ALTERED INTESTINAL IMMUNITY
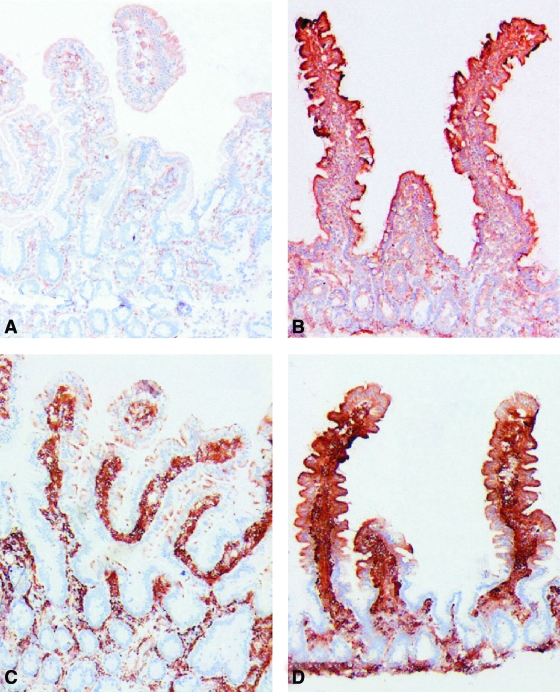
The third facet related to the gut that may be key to type 1 diabetes development relates to intestinal immunity. Of the three, this is the area perhaps subject to the most investigation in settings of type 1 diabetes. To provide a unique glimpse into this area, we and others have investigated markers of immune activation in small intestinal biopsies taken from children with type 1 diabetes that failed to show signs of celiac disease. Specifically, these subjects did not demonstrate signs of celiac disease, they were negative for transglutaminase antibodies, and their intestinal morphology in terms of celiac disease–associated inflammation was normal. These studies noted enhanced expression of antigen-presenting HLA class II molecules (Fig. 3), increased expression of intracellular adhesion molecule (ICAM)-1 on epithelium, and α4β7-integrin on cells of the lamina propria (28,29). In line with these findings, activation of the cytokine network was also demonstrated in the lamina propria. Specifically, increased densities of cells positive for IL-4 and IL-1α (protein and mRNA), as well as increased numbers of interferon-γ mRNA–expressing cells, were observed in patients with type 1 diabetes. The second effort to address this question, involving small intestine biopsies from type 1 diabetic patients, demonstrated high numbers of CD25-positive cells, as well as increased numbers of intraepithelial CD3- and γ/δ-positive cells, in lamina propria (30). Interestingly, the findings of intestinal immune activation were not restricted to individuals with the HLA DQ2 allele (i.e., the HLA risk allele found in 90% of patients with celiac disease). Thus, the altered intestinal immunity in type 1 diabetes appears to represent a separate entity from celiac disease.
FIG. 3.
Immunoperoxidase stainings for HLA-DR (A and B) and HLA-DP (C and D) in jejunal biopsy specimens from a healthy control (A and C) and from a type 1 diabetic patient with normal jejunal mucosa (B and D). Intensive, positive HLA-DR staining is seen throughout the epithelial cells of the villi and also in many crypt cells in the type 2 diabetic specimen (B), whereas the control specimen shows only faint HLA-DR staining in the apical parts of the epithelial cells at the tip of the villi (A). The biopsy specimen from a control patient treated with HLA-DP antibody shows scattered positive granules in the apical parts of the epithelial cells at the tip of the villi (C), whereas strong positive staining is seen throughout the villous epithelial cells in the specimen from a type 1 diabetic patient (D). No positive staining is seen with either HLA-DR or HLA-DP in crypt cells in the control specimen (A and C). AEC-hematoxylin stain, original magnification ×50. (Please see http://dx.doi.org/10.2337/db08-0331 for a high-quality digital representation of this figure.)
Very recent efforts have addressed questions related to regulatory T-cells, which are thought to be vital to the control of autoimmunity by instilling proper immune regulation and are marked by the transcription factor Foxp3. Specifically, these efforts included examination of Foxp3-positive cells in small intestinal biopsies from children with type 1 diabetes as well as from those with celiac disease (31). In children with type 1 diabetes, the densities of Foxp3-positive cells were low and did not show activation of Foxp3 transcripts. This was in contrast to celiac disease, where related inflammation involving Foxp3 expression was noted. The absence of intestinal Foxp3 activation in type 1 diabetes provides a strong rationale for further investigation regarding the whether impaired regulatory mechanisms could explain the subclinical intestinal immune activation and altered responses to dietary antigens.
In terms of how this altered immunity may find its genesis, another potential candidate is matrix metalloproteinases (MMPs), enzymes produced by several different cell types and released in response to inflammation. MMPs participate in the remodeling of extracellular matrix, and when their function goes uncontrolled, they have been noted for the ability to induce tissue injury. When MMP profiles and apoptotic characteristics were studied in a group of children positive for transglutaminase antibodies, the subgroup of children with type 1 diabetes showed higher expression of MMPs and increased proportion of apoptotic mucosal cells than children without type 1 diabetes (32). Taken collectively, these findings performed in small intestinal biopsy samples from type 1 diabetic patients directly implicate altered activation of both innate and adaptive immune cells in the intestinal mucosa and provide a new focus on the potential role of intestinal immunity in the pathogenesis of human type 1 diabetes. With all of this in mind, the question is, why would this occur?
Triggers of intestinal immunity.
In the association of increased intestinal permeability and inflammation, altered responses to food and microflora-derived antigens often develop. It is however indistinguishable whether the aberrant response to food or microbial antigen is the trigger of intestinal inflammation and increased permeability or vice versa.
Regarding type 1 diabetes and aberrant responses to food, enhanced immunity to wheat and cow milk have been reported in type 1 diabetic patients as well as in their relatives (33,34). Interestingly, wheat gliadin stimulation of small intestinal biopsies from children with type 1 diabetes noted an increase in intraepithelial CD3 cell numbers in lamina propria CD25-positive cells and enhanced expression of ICAM-1 and HLA-DR. In contrast, no such activation of T-cells was seen in biopsy samples taken from healthy children (30). This suggests that altered response to wheat gliadin in children with type 1 diabetes may contribute to the intestinal inflammation. It is possible that the HLA-DQ2 and -DQ3 alleles often present in type 1 diabetic and celiac disease patients are responsible for the presentation of wheat gliadin–derived antigens and for the induction of inflammation, which however does not lead to full-blown celiac disease in these cases. Some studies suggest that early exposure to wheat-containing food in infancy increases the risk of β-cell autoimmunity (35,36), which could be explained by induction of intestinal inflammation, as suggested by both human (30) and experimental (37) studies. In individuals at risk of type 1 diabetes who had β-cell autoantibodies, insulin response to glucose improved after 6 months of gluten-free diet, but no changes in the autoimmune response were seen (38). Thus, there is no evidence that elimination of wheat would decrease the intestinal immune activation in type 1 diabetes. Indeed, one could argue that the aberrant immune responses to several dietary antigens noted for subjects with type 1 diabetes, including cow milk–derived proteins, supports the notion that the altered responsiveness is not limited to wheat but may rather be non–antigen-specific phenomenon in the gut of those with type 1 diabetes.
As far as microbial stimulators of intestinal inflammation, enterovirus and rotavirus infections have been linked to development of β-cell autoimmunity (39,40), with some studies demonstrating enterovirus antigens in pancreatic β-cells of patients with type 1 diabetes (41). The role of enteroviruses as candidate triggers of intestinal immunity in type 1 diabetes has been suggested by a recent study in which enteroviral-derived protein VP1 and enterovirus-specific RNA was demonstrated more frequently in small intestinal biopsy samples from type 1 diabetic patients compared with those from nondiabetic patients (42). These findings should be considered preliminary because the number of patients was small and all patients were studied years or decades after diagnosis of type 1 diabetes. Given that activation of immunity as a response to enterovirus CVB4 is decreased in peripheral blood mononuclear cells from children with type 1 diabetes (43), this impaired immunity and poor support of cytotoxic mechanisms important for virus clearance could explain the findings of enterovirus in the intestine even years after diagnosis of type 1 diabetes. The observation, if confirmed, suggests that either chronic enterovirus infection in type 1 diabetes or predisposition to recurrent enterovirus infections, rather than accidental role of enterovirus infections, is responsible for the induction of β-cell autoimmunity. Indeed, enteral infections are known to change the cytokine pattern (44) and increase the permeability of the gut (45), which in some cases may lead to enhanced immunity to food proteins. On the other hand, the demonstration of enterovirus in the intestine of patients with type 1 diabetes could be secondary to the altered mucosal immunity associated with the disease itself.
Mucosal immunity and tolerance.
While the definition of immunological “tolerance” is often subject to debate, at one level it denotes the absence of a detectable functional immune response. With rare exception, most individuals develop immune systems that are tolerant to self, as well as toward substances they ingest. However, in the setting of type 1 diabetes, it is this loss of immunological tolerance that leads to the autoimmunity ascribed with the disease (46).
At this time, however, our understanding of the relationship between intestinal microbiota, the “leaky intestine” in diabetes-prone animals and humans, and tolerance is poorly understood. One pathway (Fig. 2) could involve the association of a highly permeable intestine that permits the inappropriate passage of intestinal contents that, in the presence of a certain microbiota, diminishes tolerance to self via altered regulatory T-cells. In support of this notion, recent work from our laboratories using BB rats demonstrate long-term differences in pro- and anti-inflammatory cytokine/chemokine patterns between animals fed using their own mother's milk versus formula (47). Whether there is any relationship between this finding and differences in intestinal microbiota under the different feeding conditions remains speculative.
Recently, it has been proposed that maintenance of a commensal microbiota closely corresponds to the regulatory and cytotoxic T-cell systems (48,49). This is the basis of the “old friends” hypothesis, which states that the presence of normal microbes (i.e., old friends) stimulates a low-grade upregulation of regulatory T-cells that produce IL-10 and transforming growth factor-β, which in turn diminishes the effects of proinflammatory processes (48). In addition to decreasing a response that would eliminate the “old friends,” this regulatory response would also result in maintenance of tolerance to self. This is likely to be one of the critical mechanisms underlying the benefits of maintaining gastrointestinal commensal microorganisms as well as supplementation with probiotics. In infants with a family history of allergy, probiotics that protected from atopic eczema induced low-grade inflammation characterized by IL-10 upregulation (13). Accordingly, the administration of certain antibiotics and or probiotics may affect the development of type 1 diabetes by altering the balance of gut microbiota toward either a tolerogenic or nontolerogenic state, depending on the intestinal microbes present. Dietary factors, especially those during infancy when new foreign proteins are introduced into the diet, modulate the intestinal microbiota (50). In NOD mice, diabetes-preventive gluten free–diet has been shown to modulate microbiota (51). It is notable that dietary interventions may thus not only modulate the immune response to dietary exposure but also immunity in general by changes in microbiota and permeability. The interaction of tolerance, microflora, and permeability was demonstrated when probiotics given to children with atopic dermatitis resulted in a decrease of the intestinal permeability and alleviated the symptoms of dermatitis (52).
As noted earlier, autoimmune insulitis may result from dysregulated oral tolerance to dietary antigens combined with increased intestinal inflammation and permeability in genetically predisposed individuals. For example, bovine insulin, a cow milk protein, has been hypothesized as a trigger of type 1 diabetes autoimmunity (53). Bovine insulin may sensitize intestinal T-cells early in life to later participation in the autoimmune destruction of insulin-secreting β-cells (53). The activation of luminal antigen-specific T-cells in pancreatic lymph nodes has been demonstrated in experimental animal models (54). In NOD mice, the lymphocytes infiltrating the islets express the gut-homing receptor α-4β-7-integrin, and the endothelium in the insulitis display the ligand MadCam-1 (55,56). The lymphocytes from intestine may therefore circulate between the pancreas and gut, or dendritic cells may transport luminal antigens for presentation in the pancreatic lymph nodes. These findings provide an immunological link between dietary antigens and insulitis, which has been earlier observed as modulation of diabetes outcome by dietary manipulation in animal models. The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study is currently testing the elimination of cow milk proteins and the use of hydrolyzed formula during the first months of life in children at genetic risk of type 1 diabetes for the purpose of preventing β-cell autoimmunity. The trial is based on encouraging results from a pilot study in which a 50% reduction of the β-cell autoimmunity was observed in children who received hydrolyzed formula versus those who received ordinary cow milk–based formula (57). This intervention may have multiple actions that modulate the development of β-cell autoantibodies. The intervention may alter the composition of intestinal microbiota, promote tolerance by diminishing intestinal permeability and inflammation, or modulate immune response to bovine insulin by delaying exposure until the time of normal intestinal maturation.
FUTURE DIRECTIONS
Clearly, much work remains in terms of understanding the role of the gut in type 1 diabetes development. Despite numerous studies showing health benefits of probiotics, the enthusiasm for their application as well as other means to alter the intestinal microbial ecosystem in the prevention of type 1 diabetes needs to be tempered due to the current lack of knowledge of the normal developing intestinal microbiota, in addition to questions as to how it affects the developing immune system. Future studies using newly developed techniques to evaluate intestinal microbiota, coupled with the rapidly emerging fields of proteomics and metabolomics, are certainly needed. Indeed, when used to evaluate the intestinal barrier, we believe the end result will provide not only important insights toward the pathogenic cascade that leads to type 1 diabetes but also strategies for prevention of type 1 diabetes. Finally, the role of gut as modulator of β-cell autoimmunity, and/or as the place for initiation of β-cell autoimmunity, should provide new understanding of the changing incidence of type 1 diabetes at different times and in different populations.
Acknowledgments
The studies of the authors that formed the scientific information for this Perspectives article were supported by the National Institutes of Health, the Juvenile Diabetes Research Foundation, and the Jeffrey Keene Professorship.
REFERENCES
- 1.Hooper LV: Bacterial contributions to mammalian gut development. Trends Microbiol 12:129–134, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Gordon JI: Commensal host-bacterial relationships in the gut. Science 292:1115–1118, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Stappenbeck TS, Hooper LV, Gordon JI: Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A 99:15451–15455, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL: An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL: Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J 45:278–291, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Greiner DL, Rossin IAA, Mordes JP: Translating data from animal models into methods for preventing human autoimmune diabetes mellitus: caveat emptor and primum non nocere. Clin Immunol 100:134–143, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Like AA, Guberski DL, Butler L: Influence of environmental viral agents on frequency and tempo of diabetes mellitus in BB/Wor rats. Diabetes 40:259–262, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Buschard K, Pedersen C, Hansen SV, Hageman I, Aaen K, Bendtzen K: Anti-diabetogenic effect of fusidic acid in diabetes prone BB rats. Autoimmunity 14:101–104, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, Bos NA: Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat: is the gut flora involved in the development of type 1 diabetes? Diabetologia 49:2105–2108, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz RF, Neu J, Schatz D, Atkinson MA, Wasserfall C: Comment on: Brugman S et al: (2006) Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat: is the gut flora involved in the development of type 1 diabetes? Diabetologia 50:220–221, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Calcinaro FDS, Marinaro M, Candeloro P, Bonato V, Marzotti S, Corneli RB, Ferretti E, Gulino A, Grasso F, De Simone C, Di Mario U, Falorni A, Boirivant M, Dotta F: Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia 48:1565–1575, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Björkstén B: Effects of intestinal microflora and the environment on the development of asthma and allergy. Semin Immunopathol 25:257–270, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Pohjavuori E, Viljanen M, Korpela R, Kuitunen M, Tiittanen M, Vaarala O, Savilahti E: Lactobacillus GG effect in increasing IFN-gamma production in infants with cow's milk allergy. J Allergy Clin Immunol 114:131–136, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Marschan E, Kuitunen M, Kukkonen K, Poussa T, Sarnesto A, Haahtela T, Korpela R, Savilahti E, Vaarala O: Probiotics in infancy induce protective immune profiles that are characteristic for chronic low-grade inflammation. Clin Exp Allergy 38:611–618, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Tannock GW: What immunologists should know about bacterial communities of the human bowel. Semin Immunol 19:94–105, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Tringe SG, von Mering C, Kobayashi A, Salamov AA, Chen K, Chang HW, Podar M, Short JM, Mathur EJ, Detter JC, Bork P, Hugenholtz P, Rubin EM: Comparative metagenomics of microbial communities. Science 308:554–557, 2005 [DOI] [PubMed] [Google Scholar]
- 17.EF: A molecular revolution in the study of intestinal microflora. Gut 55:141–143, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke NR, Stankovic S, Funda DP, Harrison LC: TCR gamma delta intraepithelial lymphocytes are required for self-tolerance. J Immunol 176:6533–6539, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Tsukita S, Furuse M: Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol 9:268–273, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek-Tenace LM, Hatch M, Li N, Caicedo RA, Schatz DA, Atkinson M: Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr 40:589–595, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG: Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol 276:G951–G957, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Graham S, Courtois P, Malaisse WJ, Rozing J, Scott FW, Mowat AMI: Enteropathy precedes type 1 diabetes in the BB rat 10.1136/gut. Gut 53:1437–1444, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, Piemonti L, Pastore MR, Paroni R: Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 49:2824–2827, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E: Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity 35:365–368, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Count SD, Fasano A: Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55:1443–1449, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Secondulfo M, Iafusco D, Carratù R, deMagistris L, Sapone A, Generoso M, Mezzogiomo A, Sasso FC, Cartenì M, De Rosa R, Prisco F, Esposito V: Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type 1 diabetic patients. Dig Liver Dis 36:35–45, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A: Role of the intestinal tight junction modulator zonulin in the pathogenesis of type 1 diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A 102:2916–2921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savilahti E, Ormälä T, Saukkonen T, Sandini-Pohjavuori U, Kantele JM, Arato A, Ilonen J, Akerblom HK: Jejuna of patients with insulin-dependent diabetes mellitus (IDDM) show signs of immune activation. Clin Exp Immunol 116:70–77, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E: Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes 52:2287–2295, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Auricchio R, Paparo F, Maglio M, Franzese A, Lombardi F, Valerio G, Nardone G, Percopo S, Greco L, Troncone R: In vitro–deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes 53:1680–1683, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Tiittanen M, Westerholm-Ormio M, Verkasalo M, Savilahti E, Vaarala O: Infiltration of Foxp3 expressing cells in jejunal mucosa in celiac disease but not in type 1 diabetes. Clin Exp Immunol 152:498–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bister V, Kolho KL, Karikoski R, Westerholm-Ormio M, Savilahti E, Saarialho-Kere U: Metalloelastase (MMP-12) is upregulated in the gut of pediatric patients with potential celiac disease and in type 1 diabetes. Scand J Gastroenterol 40:1413–1422, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Vaarala O, Klemetti P, Savilahti E, Reijonen H, Ilonen J, Åkerblom HK: Cellular immune response to cow's milk beta-lactoglobulin in patients with newly diagnosed IDDM. Diabetes 45:178–182, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Klemetti P, Savilahti E, Ilonen J, Åkerblom HK, Vaarala O: T-cell reactivity to wheat gluten in patients with insulin-dependent diabetes mellitus. Scand J Immunol 47:48–53, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E: Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 290:1721–1728, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, Rewers M: Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 290:1713–1720, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Scott FW, Rowsell P, Wang GS, Burghardt K, Kolb H, Flohé S: Oral exposure to diabetes-promoting food or immunomodulators in neonates alters gut cytokines and diabetes. Diabetes 51:73–78, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Pastore MR, Bazzigaluppi E, Belloni C, Arcovio C, Bonifacio E, Bosi E: Six months of gluten-free diet do not influence autoantibody titers, but improve insulin secretion in subjects at high risk for type 1 diabetes. J Clin Endocrinol Metab 88:162–165, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Lönnrot M, Korpela K, Knip M, Ilonen J, Simell O, Korhonen S, Savola K, Muona P, Simell T, Koskela P, Hyöty H: Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes 49:1314–1318, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Honeyman MC, Coulson BS, Stone NL, Gellert SA, Goldwater PN, Steele CE, Couper JJ, Tait BD, Colman PG, Harrison LC: Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 49:1319–1324, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M: Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47:225–239, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Oikarinen M, Tauriainen S, Honkanen T, Oikarinen S, Vuori K, Kaukinen K, Rantala I, Mäki M, Hyöty H: Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin Exp Immunol 151:71–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skarsvik S, Puranen J, Honkanen J, Roivainen M, Ilonen J, Holmberg H, Ludvigsson J, Vaarala O: Decreased in vitro type 1 immune response against coxsackie virus B4 in children with type 1 diabetes. Diabetes 55:996–1003, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Azevedo MS, Yuan L, Pouly S, Gonzales AM, Jeong KI, Nguyen TV, Saif LJ: Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol 80:372–382, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jalonen T, Isolauri E, Heyman M, Crain-Denoyelle AM, Sillanaukee P, Koivula T: Increased beta-lactoglobulin absorption during rotavirus enteritis in infants: relationship to sugar permeability. Pediatr Res 30:290–293, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Kamradt T, Mitchison NA: Tolerance and autoimmunity. N Engl J Med 344:655–664, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Caicedo RA, Li N, DES Robert C, Scumpia PO, Hubsher CP, Wasserfall CH, Schatz DA, Atkinson MA, Neu J: Neonatal formula feeding leads to immunological alterations in an animal model of type 1 diabetes. Pediatr Res 63:1–5, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Rook GA, Brunet LR: Microbes, immunoregulation, and the gut. Gut 54:317–320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan F, Polk DB: Commensal bacteria in the gut: learning who our friends are. Curr Opin Gastroenterol 20:565–571, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE: Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118:511–512, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Hansen AK, Ling F, Kaas A, Funda DP, Farlov H, Buschard K: Diabetes preventive gluten-free diet decreases the number of caecal bacteria in non-obese diabetic mice. Diabete Metab Res Rev 22:220–225, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Rosenfeldt V, Benfeldt E, Valerius NH, Paerregaard A, Michaelsen KF: Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr 145:612–616, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Vaarala O, Knip M, Paronen J, Hämäläinen AM, Muona P, Väätäinen M, Ilonen J, Simell O, Akerblom HK: Cow's milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes 48:1389–1394, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C: Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci U S A 102:17729–17733, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang XD, Sytwu HK, McDevitt HO, Michie SA: Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes 46:1542–1547, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Hanninen A, Salmi M, Simell O, Jalkanen S: Mucosa-associated (beta7-integrinhigh) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes 45:1173–1180, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Akerblom HK, Virtanen SM, Ilonen J, Savilahti E, Vaarala O, Reunanen A, Teramo K, Hämäläinen AM, Paronen J, Riikjärv MA, Ormisson A, Ludvigsson J, Dosch HM, Hakulinen T, Knip M; National TRIGR Study Group: Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia 48:829–837, 2005 [DOI] [PubMed] [Google Scholar]