The dogma regarding the pathogenesis of type 2 diabetes has evolved over the last decade to view the islet β-cell as the final determinant of whether glucose tolerance is normal or abnormal (1). While obesity and insulin resistance have reached epidemic proportions around the world, the presence of an appropriate compensation of insulin secretion (“healthy β-cells”) allows daylong glycemia to be indistinguishable from metabolically normal individuals (2). In turn, pre-diabetes and type 2 diabetes result from progressive β-cell dysfunction (3). As such, an army of researchers worldwide is searching for the pathogenic basis of this β-cell dysfunction, along with strategies of when, and how, to intervene. What has resulted is a reasonably good mapping of the natural history of the β-cell dysfunction, plus a lengthy list of potential mechanisms. Most are based on animal studies; therefore, homing in on the operative mechanisms in humans remains a challenge. Still, there is high confidence within the β-cell research arena that we are on the right track to identifying the molecular details of β-cell compensation and failure.
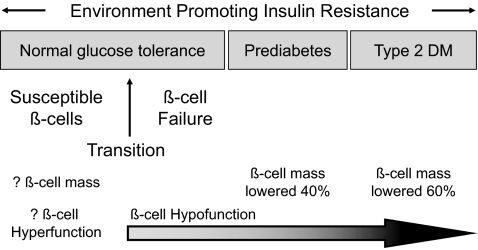
Figure 1 shows the proposed stages of β-cell dysfunction. It begins early, perhaps at birth, with β-cells that are programmed to be at risk to fail (“susceptible β-cells”). Indeed, the recent genome-wide scans have identified many susceptibility genes for type 2 diabetes that likely impact the development and ongoing homeostasis of the mass of β-cells, as well as insulin secretion and synthesis (4). Although direct evidence is lacking, many are betting that a lowered mass of normally functional β-cells, from genetics or environmentally based imprinting during fetal or early life, will end up being a common cause of susceptible β-cells, based on animal (5,6) and human (Kumar et al. [7]) studies posthemipancreatectomy showing a predilection for diabetes. Eventually, β-cell dysfunction (actually failed β-cell compensation) occurs when the subject is still normally glucose tolerant, resulting in a slow rise in glycemia (8). Whether this reflects dysfunctional β-cells, a loss of β-cells, or both is unknown. By the onset of pre-diabetes, defective β-cell function (glucose unresponsivesness and impaired insulin pulsatility) and a lowered β-cell mass are both found and worsen with time (1). Many mechanisms for the β-cell dysfunction and death are being studied, such as glucotoxicity, glucolipotoxicity, oxidative or endoplasmic reticulum stress, amyloid infiltration, inflammation, and so on.
FIG. 1.
Proposed schema for the pathogenesis of type 2 diabetes that incorporates the available literature on β-cell mass and function through the evolution from normal glucose tolerance to overt type 2 diabetes.
However, there is one stage of this sequence called “transition” (i.e., the time when insulin secretion switches from successful to failed compensation) that has gone relatively unstudied. In this issue of Diabetes, the article by Chakravarthy et al. (9) may provide important insight into this event, although that was not the original intent. They intensively studied a mouse model of intrauterine growth retardation (IUGR) due to haploinsufficiency for fatty acid synthase (FAS), noting modest hyperglycemia on a standard diet at 12 months of age and even worse hyperglycemia after fat feeding compared with the wild-type mice. The reason was defective insulin secretion and a falling β-cell mass. Importantly, this propensity to diabetes came without the postnatal catch-up in body weight and insulin resistance that characterize most other models of IUGR, which allowed the authors to conclude that β-cells were programmed for failure by events related to the IUGR. The story became even more interesting when they looked earlier (at 3 months) and found an opposite phenotype. The FAS heterozygous mice were hypoglycemic/hyperinsulinemic on a standard diet, with a larger β-cell mass and insulin secretion that was hyperresponsive to glucose, when they were more insulin sensitive than age-matched wild-type mice. Furthermore, the FAS haploinsufficient mice compensated better to fat feeding than did wild-type mice in terms of a greater increase in β-cell mass and insulin secretion; plus, there were lower-than-normal glucose values during a glucose challenge. So, early on there was super β-cell compensation, and later there was failed compensation.
The authors concluded that they had discovered a body weight–sensing mechanism during fetal development that regulates β-cell mass. As such, growth retardation feeds back to enhance β-cell mass and insulin secretion to augment whole-body fat and protein deposition. They supported this conclusion with a second mouse model of IUGR, also with enhanced insulin sensitivity (muscle-specific uncoupling protein-1 transgenic mice), that showed the same early increases in β-cell mass and function. Finally, they concluded that it was the initial β-cell hyperfunction that programmed the β-cells for later failure (i.e., creating susceptible β-cells).
It is impossible to know, from this study, about the plausibility of the proposed fetal size–sensing regulatory system for β-cell mass and function. No biological details for such an effect are known in mammals, and one can envision fairly profound metabolic changes in fatty acid homeostasis and cellular metabolism in the studied mice. Instead, the concept of β-cell hyperfunction programming β-cells for later failure (termed “β-cell overwork” or “β-cell exhaustion”) is supported by studies over several decades from many investigators (10), although the relevant literature is often forgotten. Hansen and Bodkin (11) performed seminal studies that mapped the stages to type 2 diabetes in rhesus monkeys, finding that β-cell hyperresponsiveness for insulin was the first identifiable event preceding obesity and insulin resistance. We studied Zucker fatty rats that are obese and normoglycemic and noted that the compensatory increase in β-cell mass started well before the time reported by others for the onset of insulin resistance (12). Studies in humans have shown that insulin hypersecretion precedes insulin resistance in nondiabetic obese juveniles (13), and a high fasting insulin level independent of insulin resistance was shown to be an important risk factor for type 2 diabetes in Pima Indians (14). Moreover, intervention studies (15–18) using inhibitors of insulin secretion (diazoxide, novel ATP-sensitive K+ channel openers, and somatostatin), so-called “β-cell rest strategies” that have shown paradoxical improvements in β-cell function in animals and humans with type 2 diabetes, have been highly influential for the β-cell overwork concept. Indeed, one wonders if the improved β-cell function after aggressive blood glucose control using insulin that originally led to the glucose toxicity concept (19,20) might more correctly stem from β-cell rest.
A key question is how to distinguish healthy β-cell compensation versus unhealthy increases in β-cell function. Again, the study by Chakravarthy et al. (9) may provide an important clue, as the 3-month-old FAS haploinsufficient mice were mildly hypoglycemic basally and during the glucose challenge after fat feeding. In other words, the enhanced β-cell mass and function, at this early time point, were neither appropriate nor normally regulated but instead resulted in a condition that is reminiscent of the honeymoon period in type 1 diabetes, another state of failing β-cells. Whether there is a comparable phase in human type 2 diabetes is unknown, although it was commonly believed by clinicians years ago that ∼20% of individuals presenting with type 2 diabetes had gone through a prior period of reactive postmeal hypoglycemia.
Thus, extensive literature supports that enhanced β-cell function (and likely mass) is an early stage, and likely causative event, in the progression to β-cell failure and type 2 diabetes. What is needed now is a better understanding of the biology that links β-cell hyperfunction to eventual failure, followed by the development of targeted pharmaceuticals in order to test the concept of β-cell rest for the prevention and treatment of early type 2 diabetes.
See accompanying original article, p. 2698.
REFERENCES
- 1.Leahy JL: β-Cell dysfunction in type 2 diabetes mellitus. In Joslin's Diabetes Mellitus. 14th ed. Kahn CR, Weir GC, King GL, Jacobson AM, Moses AL, Smith RJ, Eds. Philadelphia, Lippincott, Williams & Wilkins, 2005, p. 449–461
- 2.Polonsky KS, Given BD, Van Cauter E: Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest 81:442–448, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florez JC: Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia 51:1100–1110, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Leahy JL, Bonner-Weir S, Weir GC: Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest 81:1407–1414, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matveyenko AV, Veldhuis JD, Butler PC: Mechanisms of impaired fasting glucose and glucose intolerance induced by an approximate 50% pancreatectomy. Diabetes 55:2347–2356, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kumar AF, Gruessner RWG, Seaquist ER: Risk of glucose intolerance and diabetes in hemipancreatectomized donors selected for normal preoperative glucose metabolism. Diabetes Care 31:1639–1643, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA: San Antonio metabolism study. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 47:31–39, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Chakravarthy MV, Zhu Y, Wice WB, Coleman T, Kappan KL, Marshall CA, McDaniel ML, Semenkovich CF: Decreased fetal size is associated with β-cell hyperfunction in early life and failure with age. Diabetes 57:2698–2707, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leahy JL: β-cell dysfunction with chronic hyperglycemia: the “overworked β-cell” hypothesis. Diabetes Revs 4:298–319, 1996 [Google Scholar]
- 11.Hansen BC, Bodkin NL: Beta-cell hyperresponsiveness: earliest event in development of diabetes in monkeys. Am J Physiol 259:R612–R617, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL: Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 54:2294–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Le Stunff C, Bougnères P: Early changes in postprandial insulin secretion, not insulin sensitivity, characterize juvenile obesity. Diabetes 43:696–702, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE: A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 49:2094–2101, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Leahy JL, Bumbalo LM, Chen C: Diazoxide causes recovery of beta-cell glucose responsiveness in 90% pancreatectomized diabetic rats. Diabetes 43:173–179, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Greenwood RH, Mahler RF, Hales CN: Improvement in insulin secretion in diabetes after diazoxide. Lancet 1:444–447, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Laedtke T, Kjems L, Porksen N, Schmitz O, Veldhuis J, Kao PC, Butler PC: Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab 279:E520–E528, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Song SH, Rhodes CJ, Veldhuis JD, Butler PC: Diazoxide attenuates glucose-induced defects in first-phase insulin release and pulsatile insulin secretion in human islets. Endocrinology 144:3399–3405, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Turner RC, McCarthy ST, Holman RR, Harris E: Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J 1:1252–1254, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG: The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes 34:222–2234, 1985 [DOI] [PubMed] [Google Scholar]