Abstract
OBJECTIVE— Cerebral edema (CE) is a potentially life-threatening complication of diabetic ketoacidosis (DKA) in children. Osmotic fluctuations during DKA treatment have been considered responsible, but recent data instead suggest that cerebral hypoperfusion may be involved and that activation of cerebral ion transporters may occur. Diminished cerebral blood flow (CBF) during DKA, however, has not been previously demonstrated. We investigated CBF and edema formation in a rat model of DKA and determined the effects of bumetanide, an inhibitor of Na-K-Cl cotransport.
RESEARCH DESIGN AND METHODS— Juvenile rats with streptozotocin-induced DKA were treated with intravenous saline and insulin, similar to human treatment protocols. CBF was determined by magnetic resonance (MR) perfusion–weighted imaging before and during treatment, and CE was assessed by determining apparent diffusion coefficients (ADCs) using MR diffusion–weighted imaging.
RESULTS— CBF was significantly reduced in DKA and was responsive to alterations in pCO2. ADC values were reduced, consistent with cell swelling. The reduction in ADCs correlated with dehydration, as reflected in blood urea nitrogen concentrations. Bumetanide caused a rapid rise in ADCs of DKA rats without significantly changing CBF, while saline/insulin caused a rapid rise in CBF and a gradual rise in ADCs. DKA rats treated with bumetanide plus saline/insulin showed a trend toward more rapid rise in cortical ADCs and a larger rise in striatal CBF than those observed with saline/insulin alone.
CONCLUSIONS— These data demonstrate that CE in DKA is accompanied by cerebral hypoperfusion before treatment and suggest that blocking Na-K-Cl cotransport may reduce cerebral cell swelling.
Diabetic ketoacidosis (DKA) occurs frequently in children with type 1 diabetes. A total of 25–40 percent of children with new-onset type 1 diabetes present with DKA, and DKA can occur in children with known diabetes during episodes of illness, poor compliance, or malfunction of diabetes care equipment such as insulin pumps (1,2). Cerebral edema (CE) is the most feared complication of DKA in children (3–5). CE has a high mortality rate (21–24%), and survivors often have permanent neurological deficits (15–35%) (5,6). CE is the major diabetes-related cause of mortality in children with type 1 diabetes and is responsible for 50–85% of diabetes-related deaths (7–9).
Asymptomatic CE is thought to occur with much greater frequency than clinically apparent CE and may be present in the majority of DKA episodes in children (10–12). Studies utilizing sequential computed tomography or magnetic resonance (MR) scanning in children with DKA without significant neurological abnormalities have shown evidence of CE at presentation (10), demonstrated by decreased size of the cerebral ventricles, and that this edema likely worsens during therapy (10–12). Thus, the development of symptomatic CE in 1% of children with DKA may represent the most severe presentation of a more common pathophysiological phenomenon.
Osmotic fluctuations during DKA treatment have been presumed to play a central role in causing CE, but preliminary work by our group suggests instead that DKA-related CE may be mechanistically similar to that which occurs in the setting of strokes or other ischemic brain injury. Rats with DKA manifest a reduction of apparent diffusion coefficient (ADC) values for brain water, indicating cytotoxic edema, and administration of the Na-K-Cl cotransport inhibitor bumetanide, which has been shown to reduce both astrocyte and brain microvascular endothelial cell swelling under hypoxic and ischemic conditions (13–17), returns ADC values to normal (18). These findings are similar to those in experimental models of stroke (18–20). Furthermore, epidemiological studies in children suggest that the extent of dehydration and hypocapnia at presentation of DKA are the most important risk factors for CE, again suggesting that cerebral hypoperfusion before DKA treatment may play a role (5). Although there is indirect evidence that cerebral hypoperfusion is involved in DKA-related CE, reduced cerebral blood flow (CBF) during untreated DKA has not been demonstrated. We undertook the current studies to test the hypothesis that DKA is associated with a reduction in CBF. We also investigated the role of hypocapnia in modulating CBF during DKA and determined the effects of treatment with saline and insulin, as well as treatment with bumetanide, on CBF and ADCs.
RESEARCH DESIGN AND METHODS
Induction of DKA.
Four-week-old Sprague Dawley rats (150 g; Charles River Laboratories, Wilmington, MA) were given an intraperitoneal injection of streptozotocin (STZ) (150 mg/kg) or STZ vehicle, as described previously (18). Rats were given unlimited access to D10W (water with 10% dextrose; Fisher Scientific, Santa Clara, CA) in the first 24-h period after STZ injection to prevent hypoglycemia and were subsequently allowed unlimited access to tap water and standard rat diet. Rats were weighed daily and urine glucose and ketoacids (assessed as acetoacetate) determined using Multistix urinalysis strips (Bayer; Fisher Scientific, Santa Clara, CA) as described previously (18). Rats included in the DKA group were identified as those having urine glucose and acetoacetate concentrations ≥2,000 and 160 mg/dl, respectively. To induce dehydration and ensure acidosis, rats were deprived of drinking water 24 h before imaging. This study was conducted in accordance with the animal use and care guidelines issued by the National Institutes of Health using a protocol approved by the animal use and care committee at University of California Davis.
MR imaging analysis of CE and CBF.
Both DKA and control rats were anesthetized using sodium pentobarbital (65 mg/kg i.p.), and the left femoral vein and artery were subsequently cannulated with PE-50 polyethylene tubing as described previously (18). The femoral vein cannula was used for additional anesthesia, as needed, and for administering compounds to be tested. The femoral artery cannula was used for blood sampling. Body temperature was monitored via rectal probe (Cole-Parmer Instruments, Vernon Hills, IL), and a heating pad with circulating water (Gaymar, Orchard Park, NY) was used to maintain body temperature at 36.8–37.0°C throughout surgery and brain imaging. Rats were also subjected to tracheal intubation and ventilated (Harvard Small Animal Ventilator; Harvard Apparatus, Holliston, MA) throughout surgery and imaging. Ventilation was done to offset the tendency toward respiratory depression in the anesthetized rats and thereby ensure that the animal model closely mimicked DKA in humans. Blood samples were taken for analysis of pCO2 and pH immediately after intubation, and the respiratory rate and tidal volume were adjusted to maintain the pCO2 level within the range expected for a normal physiological response to the degree of acidosis (21). Using this method, we were able to adjust pCO2 levels such that they were significantly correlated with pH (correlation coefficient 0.35, P = 0.04; n = 33). MR diffusion–weighted spin echo images (DWIs) were acquired using a 7-Tesla Bruker Biospec MRS/MRI system as described previously (18,19,22). ADC values (10−6 cm2/s) were determined from 6 × 4–pixel regions of interest (ROIs) for eight brain regions (six cortex and two striatum) using Paravision 3.0.2 software, with four gradient strengths of 5–95 mT/m (23). In each rat, CBF (ml · 100 gm−1 · s−1) was also determined, using perfusion-weighted imaging analysis with continuous arterial spin labeling (24) and a standard Bruker PERFPACK2 protocol (Bruker, Billerica, MA). Arterial spin-labeling data were acquired using the same field of view and slice thickness as for DWIs, and the arterial spin-labeling ROIs were chosen from the 128 × 32 matrix so as to measure CBF and ADCs on the same voxels (18,19). Images from perfusion-weighted imaging were acquired in 11 min using TE/TR 12.77/1,291 ms with a 1-s Adiabatic-Fast-Passage labeling pulse in the presence of a 10 mT/m gradient to obtain inversion ±8,515 Hz (±2 cm) from the isocenter (also slice center) for control and labeled images, respectively. T1 maps for the same voxels were also acquired from selected rats in each treatment group in order to correct CBF measurements for possible location- and treatment-dependent variations in T1.
Experimental treatments: saline/insulin infusion.
For treatment with saline and insulin, rats were infused via cannulated femoral vein with regular Humulin insulin at 1.5 units · kg−1 · h−1 (Lilly and Company, Indianapolis, IN) and 0.9% NaCl at 80 ml · kg−1 · h−1 for 1 h followed by infusion with insulin and saline at 1.5 units · kg−1 · h−1 and 40 ml · kg−1 · h−1, respectively, for the remainder of the imaging experiment. These rates were calculated based on comparisons of human versus rat metabolic rate, body surface area, and percentage dehydration during DKA and were determined in initial studies to result in biochemical changes during DKA treatment (decline in serum glucose and urea nitrogen and resolution of acidosis) at rates similar to those observed in children with DKA.
Bumetanide treatments.
For experiments designed to determine the effects of bumetanide on ADCs, bumetanide (30 mg/kg) was administered in one of two ways. For rats not receiving intravenous infusion of saline/insulin, bumetanide was injected into femoral vein cannula (0.8 ml total volume) immediately before the start of imaging, as described previously (18). For rats treated with saline/insulin infusion, bumetanide was given via femoral vein cannula at the start of the saline/insulin infusion. Bumetanide (ICN Biomedicals, Costa Mesa, CA) was prepared as we described (18).
Blood chemistry.
Blood samples were withdrawn from the femoral artery and abdominal artery before and after imaging, respectively, at the conclusion of the experiment. Samples were analyzed for electrolytes, pH, blood urea nitrogen (BUN), and glucose using an I-STAT Portable Clinical Analyzer (I-STAT; Sensor Devices, Waukesha, WI). Blood ketoacids were assessed by measuring β-hydroxybutyrate in the blood sample using MediSense Precision Xtra blood β-ketone test strips (Abbott Laboratories, Bedford, MA).
Statistical analysis.
All values are presented as means ± SE. Biochemical values were compared using Student's t test. Within-animal changes in each of the cortical and striatal ADCs and CBF outcomes were assessed using paired t tests. For each of these four outcomes, a separate mixed-effects ANOVA model of the repeated measures from each animal was used to compare within-animal ADC and CBF changes between treatment groups. Raw (untransformed) values of ADCs and CBF were used in the statistical testing procedures for these outcomes. Bivariate correlations between MR findings (ADCs and CBF) and biochemical values were evaluated by calculating Pearson correlation coefficients. Associations between MR findings and biochemical values were also assessed in multivariable models using multiple linear regression. We used SAS version 9.1 for paired t tests and repeated-measures ANOVA (Stata version 8.0; Stata, College Station, TX) for correlation and regression analyses and Statview 5.01 for Student's t tests. P values <0.05 were considered to indicate significant differences, and P values between 0.05 and 0.10 were considered to represent a trend. We used 33 DKA rats for correlation and regression analyses of baseline measures. Of these, 22 rats were also evaluated for effects of treatments.
RESULTS
Using the methods described previously, we were able to reliably induce DKA in the rats, with levels of acidosis and hyperglycemia similar to those observed in children with DKA (Table 1). Treatment for 2 h with saline/insulin infusion resulted in improvements in acidosis and hyperglycemia at rates similar to those observed in human DKA. β-Hydroxybutyrate values shown were obtained using test strips (MediSense Precision), which allowed us to use a small volume of blood. In two experiments, we analyzed control and DKA rat blood using a Randox Veterinary Analyzer (Randox Laboratories, Antrim, U.K.). We found no difference between control rat values obtained with these two methods but twofold higher values for DKA rats with the veterinary analyzer (not shown), suggesting that the human test strip method used in the present study underestimates the DKA β-hydroxybutyrate values. Thus, while further tests are needed to determine the full extent of reduction in β-hydroxybutyrate values following saline/insulin infusion of DKA rats, our present findings suggest that a relative decrease in β-hydroxybutyrate occurs with saline/insulin infusion.
TABLE 1.
Blood chemistry parameters
| Control | DKA preinfusion | DKA saline/insulin infusion | |
|---|---|---|---|
| Glucose (mmol/l) | 8.9 ± 0.5 | 36.6 ± 1.2* | 24.8 ± 2.5† |
| Sodium (mmol/l) | 136.7 ± 0.7 | 141.0 ± 1.8* | 156.5 ± 2.0† |
| BUN (mmol/l) | 6.0 ± 0.3 | 35.0 ± 3.9* | 29.9 ± 4.5 |
| Total CO2 mmol/l | 28.92 ± 0.69 | 9.55 ± 1.52* | 12.45 ± 1.84† |
| pH | 7.44 ± 0.02 | 7.07 ± 0.04* | 7.12 ± 0.05 |
| β-Hydroxybutyrate (mmol/l) | 0.25 ± 0.05 | 4.16 ± 0.24* | 2.78 ± 0.52† |
Data are means ± SE for 12 control and 11 DKA rats. Plasma values of the parameters shown were determined for control (non-DKA) rats and DKA rats before and after 2 h of saline/insulin infusion.
Significantly different from control (P < 0.05).
Significantly different from DKA preinfusion (P < 0.05). Plasma values of these parameters when evaluated following 2 h of saline/insulin/bumetanide infusion did not differ significantly from those here for saline/insulin infusion alone.
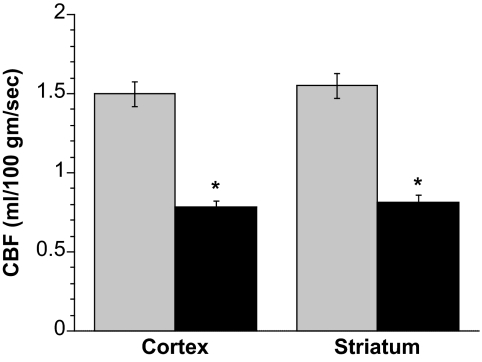
Figure 1 shows that DKA rats had significantly reduced CBF, as measured by perfusion-weighted imaging. Specifically, CBF was reduced by 48% in both cortex and striatum in the DKA rats compared with control non-DKA rats. For each rat, we evaluated ADC values in addition to CBF and found that, consistent with our previously reported observation of reduced ADCs in nonventilated DKA rats (18), the ventilated DKA rats had significantly reduced ADC values, in both the cortex and striatum, compared with control rats. The values observed were comparable with those we reported previously. Thus, ADC values were 7.00 ± 0.10 and 5.94 ± 0.10 for control cortex and striatum, respectively (n = 12), and 6.43 ± 0.13 and 4.99 ± 0.16 for DKA cortex and striatum, respectively (n = 6). DKA ADC values were found to be significantly different from control ADC values in both cortex and striatum (P < 0.005 and P < 0.001, respectively, by two-tailed unequal variance T tests). Overall, the findings presented in Fig. 1 indicate that reduction of CBF occurs in our rat model of DKA.
FIG. 1.
Reduction of CBF in DKA rats. CBF values of cortex and striatum in control and DKA rats were determined by perfusion-weighted imaging. Values are means ± SE; n = 12 for control and n = 38 for DKA rats. *Significantly different from control rat CBF values. P < 0.0001 for both cortex and striatum.  , control; ▪, DKA.
, control; ▪, DKA.
In the DKA rats, cortex ADC values were significantly correlated with BUN concentrations (correlation coefficient −0.65; P < 0.001) and with pH (correlation coefficient 0.39; P = 0.02) at the time of imaging. ADC values were not significantly correlated with serum glucose or sodium concentrations or with pCO2. When the biochemical values were included in a multivariable linear regression model, only BUN maintained a significant association with ADCs (correlation coefficient −0.017 [95% CI −0.026 to −0.007]; P = 0.001), with higher BUN concentrations being associated with lower ADC values. In bivariate analyses, CBF was not significantly correlated with any of the biochemical measures. In a multivariable linear regression model, however, there was significant association of higher BUN with lower CBF (−0.004 [−0.007 to −0.0003]; P = 0.04).
We also evaluated the effect of bumetanide on ADCs and CBF in both control and DKA rats (Fig. 2). Bumetanide (intravenous injection) significantly increased ADC values in the DKA rats to 7.17 ± 0.20 and 5.8 ± 0.46 for cortex and striatum, respectively (n = 6) (Fig. 2A). These values are not significantly different from the ADC values observed for control non-DKA rats of 6.84 ± 0.11 and 5.72 ± 0.06 for cortex and striatum, respectively, without bumetanide (n = 6) and 6.82 ± 0.11 and 5.71 ± 0.11 for cortex and striatum, respectively, with bumetanide (n = 6) (control values not represented in Fig. 2A). Although bumetanide increased ADCs in DKA rats, it had no significant effect on CBF of cortex or striatum in DKA rats (Fig. 2B) or in control rats (1.48 ± 0.13 vs. 1.53 ± 0.13 ml · 100 g−1 · s−1 for cortex pre- and postbumetanide treatment, respectively, and 1.43 ± 0.10 vs. 1.51 ± 0.08 ml · 100 g−1 · s−1 for striatum pre- and postbumetanide treatment, respectively; n = 6 for all, data not represented in Fig. 2B). For all data shown in Fig. 2A and B, we also evaluated mean absolute increases as well as mean percent increases in both ADC and CBF values relative to baseline values within each experiment (i.e., each animal). These alternate analysis methods produced the same results with respect to presence or absence of significant changes in ADCs and CBF, as shown in Fig. 2A and B (data not shown).
FIG. 2.
Administering intravenous bumetanide to DKA rats normalizes ADC values without significantly altering CBF. Rats were administered bumetanide (30 mg/kg) immediately before the start of imaging as described in research design and methods. A: ADC values of DKA rat cortex and striatum were determined by diffusion-weighted imaging before and 37 min after intravenous administration of bumetanide ( , −Bumet; ▪, +Bumet). All values are means ± SE, n = 6. *Significantly different from pretreatment ADC values (−Bumet). P < 0.05 for both cortex and striatum. B: CBF values of DKA rat cortex and striatum were determined by perfusion-weighted imaging before and 44 min after intravenous administration of bumetanide. All values are means ± SE, n = 6.
, −Bumet; ▪, +Bumet). All values are means ± SE, n = 6. *Significantly different from pretreatment ADC values (−Bumet). P < 0.05 for both cortex and striatum. B: CBF values of DKA rat cortex and striatum were determined by perfusion-weighted imaging before and 44 min after intravenous administration of bumetanide. All values are means ± SE, n = 6.  , −Bumet; ▪, +Bumet.
, −Bumet; ▪, +Bumet.
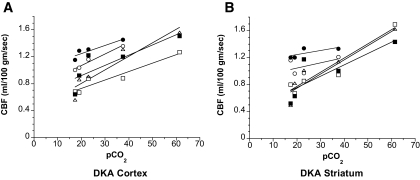
Hypocapnia induces vasoconstriction in cerebral blood vessels, but it is unclear whether this effect occurs via local direct action of pCO2 or is mediated by changes in brain pH (25). It has been proposed previously that the vasoconstrictive effect of hypocapnia may be absent during acidosis (26). Thus, in the present study we tested the effects of hypocapnia on CBF in DKA rats. pCO2 was varied by altering the ventilation rate and tidal volume of the anesthetized rats. Multiple measurements of CBF made on each of five rats at varying pCO2 levels revealed that CBF varied directly with pCO2 in DKA rats (Fig. 3). As predicted by the Henderson-Hasselbalch equation, we also found in these studies that plasma [H+] increased with pCO2 in the DKA rats (not shown).
FIG. 3.
Effect of pCO2 on CBF in DKA rats. DKA rats (n = 5) were ventilated with varying respiratory rates and tidal volumes to vary the pCO2 level. CBF was then measured in the cortex and striatum at these varying pCO2 levels using perfusion-weighted imaging as described in research design and methods. Data for each individual rat are plotted with a linear fit for ease of identification.
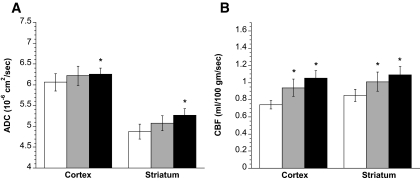
We next evaluated ADC and CBF values in DKA rats before saline/insulin infusion and also 1 and 2 h after the start of infusion. Figure 4 shows that saline/insulin infusion caused a modest increase in ADC values of the cortex and striatum after 2 h (3.3 and 8.0%, respectively) while having no significant effect after 1 h. In contrast, saline/insulin infusion significantly increased CBF in the cortex of DKA rats (27 and 42% after 1 and 2 h, respectively) as well as in the striatum (19 and 28% in the striatum after 1 and 2 h, respectively). Evaluating these data for mean absolute increases and mean percent increases in ADC and CBF values relative to baseline values for each animal revealed the same results as shown in Fig. 4 with respect to presence or absence of significant changes (not shown).
FIG. 4.
Effect of saline/insulin infusion on ADC values and CBF in DKA rats. Rats were infused with saline and insulin intravenously via cannulated femoral vein, as described in research design and methods. A: ADC values of DKA rat cortex and striatum were determined by diffusion-weighted imaging before, as well as 1 and 2 h after, the start saline/insulin fusion. Values are means ± SE, n = 6. *Significantly different from preinfusion ADC values by paired t test. P < 0.05 for both cortex and striatum at 2 h. One-hour values are not significantly different from pretreatment values. B: CBF values of DKA rat cortex and striatum were determined by perfusion-weighted imaging before, as well as 1 and 2 h after, the start of saline/insulin infusion. Values are means ± SE, n = 6. *Significantly different from preinfusion CBF values by paired t test. P < 0.01 and P < 0.001 for cortex at 1 and 2 h, respectively; P < 0.05 and P < 0.01 for striatum at 1 and 2 h, respectively. □, before saline/insulin;  , 1 hr after saline/insulin; ▪, 2 hr after sasline/insulin.
, 1 hr after saline/insulin; ▪, 2 hr after sasline/insulin.
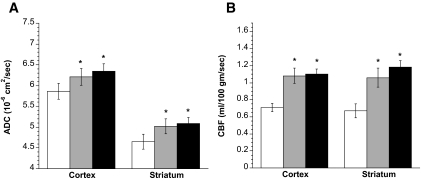
Figures 5 shows the results of experiments conducted to evaluate the effects of administering saline/insulin infusion in combination with intravenous bumetanide injection. We found that combined administration of bumetanide and saline/insulin infusion caused significant increases in ADC (Fig. 5A) and CBF values (Fig. 5B) of the DKA rats after 1 and 2 h, in both cortex and striatum. When we evaluated these data for mean absolute increases and mean percent increases in ADCs and CBF relative to baseline for each animal, we found the same results as shown in Fig. 5 with respect to presence or absence of significant changes (not shown).
FIG. 5.
Effect of saline/insulin/bumetanide infusion on ADC values and CBF in DKA rats. Rats were administered bumetanide (30 mg/kg) at the start of saline/insulin infusion as described in research design and methods. A: ADC values of DKA rat cortex and striatum were determined by diffusion-weighted imaging before, as well as 1 and 2 h after, the start of insulin/saline infusion containing bumetanide. Values are means ± SE, n = 11. For one rat, valid imaging data were not collected 1 h postinfusion due to technical problems. *Significantly different from preinfusion ADC values by paired t test. P < 0.01 and P < 0.0001 for cortex at 1 and 2 h, respectively; P < 0.01 and P < 0.001 for striatum at 1 and 2 h, respectively. B: CBF values of DKA rat cortex and striatum were determined by diffusion-weighted imaging before, as well as 1 and 2 h after, the start of insulin/saline infusion containing bumetanide. Values are means ± SE, n = 11. *Significantly different from preinfusion CBF values by paired t test. P < 0.001 and P < 0.01 for cortex at 1 and 2 h, respectively; P < 0.001 for striatum at 1 and at 2 h. □, before saline/insulin/bumet;  , 1 h after saline/insulin/bumet; ▪, 2 h after saline/insulin/bumet.
, 1 h after saline/insulin/bumet; ▪, 2 h after saline/insulin/bumet.
Mixed-effects ANOVA models comparing changes in cortical and striatal ADC and CBF values before and 1 and 2 h after the start of saline/insulin infusion alone (Figs. 4A and B) versus saline/insulin infusion with bumetanide (Figs. 5A and B) revealed that the latter treatment resulted in a significantly greater elevation of striatal CBF after 1 h (between-group contrast of mean 1-h gain for saline/insulin with bumetanide versus saline/insulin alone = 0.25 [95% CI 0.03–0.46]; P = 0.027) and after 2 h (contrast of mean 2-h gains = 0.27 [0.06–0.48]; P = 0.014). Between-group comparisons trended toward statistical significance for 1-h gains in cortical CBF (between-group contrast = 0.17 [−0.03 to 0.36]; P = 0.089) and for 2-h gains in cortical ADCs (contrast = 0.28 [−0.003 to 0.56]; P = 0.053).
DISCUSSION
The mechanism responsible for DKA-related CE continues to be a subject of much debate. An early case report postulated that hypoxia/ischemia might be involved (27). Subsequent hypotheses, however, focused mainly on the role of osmotic fluctuations during DKA treatment (28–31). More recently, evidence has been accumulating to again suggest that cerebral hypoperfusion may play a role. The current study is the first to provide evidence that CBF is substantially reduced in rats with untreated DKA. Furthermore, the current data demonstrate that CBF in rats with DKA is responsive to pCO2, suggesting that local pCO2 is an important factor controlling cerebral vascular diameter in DKA, consistent with the hypothesis that hypocapnia contributes to cerebral hypoperfusion and cerebral injury in DKA. This study also confirms our previous finding of low ADC values in untreated DKA and demonstrates that both ADCs and CBF are negatively correlated with BUN concentrations. These data suggest that dehydration may contribute to cerebral hypoperfusion during DKA, as has been suggested in humans (5). In sum, the current data are consistent with the hypothesis that cerebral hypoperfusion during untreated DKA may be a factor in the pathogenesis of CE and that DKA-related CE is mechanistically similar to CE resulting from other types of ischemic brain injury.
The present study also demonstrates that treating rats with intravenous bumetanide increases ADCs without significant effects on CBF. In contrast, in DKA rats treated with a saline/insulin infusion protocol similar to that used clinically, CBF values return toward normal levels, while a more gradual increase in ADCs is observed. When bumetanide was added to saline/insulin infusion, greater increases in CBF were observed in the striatum and there was a trend toward greater ADC increase in the cortex. The implications of these findings are not yet clear and bear further study. More rapid increases in ADCs may indicate more rapid resolution of cytotoxic CE with bumetanide treatment, but whether this improves the cerebral metabolic state and decreases the likelihood of cerebral injury has not been established. Vasogenic edema has been found to occur after several hours of DKA treatment in children. Whether the initial cytotoxic edema occurring during untreated DKA or the later development of vasogenic edema during prolonged DKA treatment is more important in causing cerebral injury is unknown. Furthermore, the effects of bumetanide on the development of vasogenic edema later in the course of DKA treatment have not been investigated. Additional investigations of more prolonged DKA treatment, and the effects of bumetanide on cerebral metabolic alterations, will help to resolve these questions.
The present study provides additional information about the pathophysiology of DKA-related CE, a topic that has puzzled clinicians for decades. Because symptoms of increased intracranial pressure often become apparent during DKA treatment, many investigators hypothesized that rapid changes in serum osmolality and/or rapid fluid infusion during DKA treatment are responsible (28,32,33). This hypothesis has been questioned, however, because studies (5,6,10) have documented the occurrence of both subclinical CE and overt, symptomatic CE before DKA treatment. Recent studies (3,5,6) have also demonstrated that dehydration and hypocapnia during DKA, rather than changes in osmolality, are the factors most strongly correlated with risk for CE. These associations imply that cerebral hypoperfusion during DKA, before DKA treatment, may play a role in causing cerebral injury and edema formation. A reduction in baseline CBF, before onset of DKA, may also contribute, as reduced CBF in children with type 1 diabetes without DKA has been demonstrated (34). The current study further reinforces these associations because elevated BUN concentrations (indicating greater dehydration) correlated with lower CBF and ADC values in our rat model, suggesting greater CE in association with lower circulatory volume.
Beyond epidemiological studies of risk factors, additional support for an association between cerebral hypoperfusion and DKA-related CE is suggested by the fact that DKA-related CE shares several characteristics with edema caused by cerebral hypoxia/ischemia. Our previous studies, as well as our present findings, demonstrate that ADC values are decreased in rats with untreated DKA, similar to decreased ADCs observed in both animal models and humans with stroke or other ischemic brain injuries (18). Similarly, in a previous study (18), as well as in the present study, we found that bumetanide treatment of rats with DKA increases ADCs, suggesting decreased cytotoxic edema. Finally, MR imaging studies of children with DKA suggest that after several hours of DKA treatment with intravenous fluids and insulin, ADC values are elevated above those of normal control subjects and cerebral perfusion is increased, consistent with vasogenic edema (35). These findings likewise parallel those of hypoxic/ischemic cerebral injury, in which a period of hyperemia and vasogenic edema generally develops after reperfusion of ischemic cerebral tissue (36–38). Our findings demonstrate that CBF remains responsive to pCO2 during DKA, suggesting that the rise in pCO2 during DKA treatment, independent of fluid resuscitation, might result in reperfusion with hyperemia. Most importantly, the current study demonstrates that CBF is indeed substantially decreased in rats with untreated DKA, consistent with our hypothesis that DKA-related CE is caused by cerebral hypoperfusion. Although the reduced CBF values in most DKA rats were not in the range known to cause ischemic injury, it is likely that CBF reduction may reach these extremes only in the most severely ill animals. This situation parallels that of human DKA, in which only a small minority of children (<1%) has cerebral injury resulting from DKA, although many, if not most, have some evidence of asymptomatic CE. It is likely that this reduction in CBF is the result of multiple factors, including reduced circulatory volume due to dehydration and cerebral vasoconstriction caused by hypocapnia.
The present study provides preliminary information, suggesting potential therapies to reduce CE and cerebral injury during DKA. Studies of ischemia and ischemia/reperfusion in rat models of stroke have shown that bumetanide reduces both CE and infarct (19,39). In those studies, it was found that the blood-brain barrier (BBB) Na-K-Cl cotransporter is present in the luminal BBB membrane (19) and is stimulated by ischemic conditions (19,40,41). It was also found that in the early hours of permanent middle cerebral artery occlusion, before breakdown of the BBB occurs (42–44), intravenous administration of bumetanide to inhibit the BBB Na-K-Cl cotransporter reduces CE, as assessed by DWI determination of ADCs as well as by gravimetric evaluation of brain water (19). Bumetanide also reduces the middle cerebral artery occlusion–induced brain infarct, as assessed by 2,3,5-triphenyltetrazolium chloride staining of brain slices (19). In other studies (39,45) using reversible middle cerebral artery occlusion to produce ischemia/reperfusion in rats, bumetanide administered via dialysis to the cortical parenchyma was similarly found to reduce brain water and infarct, as determined by gravimetry and 2,3,5-triphenyltetrazolium chloride staining. Evidence has been provided that the BBB cotransporter participates in ischemia-induced hypersecretion of sodium, chloride, and water from blood into brain during stroke (19,40,41) such that inhibiting the BBB cotransporter attenuates CE. However, the Na-K-Cl cotransporter can also cause hypoxia-induced cell swelling of both BBB endothelial cells (40) and astrocytes (41,46). The present finding that bumetanide increases cerebral ADC values in the DKA rats is consistent with the hypothesis that in DKA, cell swelling is diminished by inhibition of Na-K-Cl cotransport. Further study is needed to more fully evaluate the therapeutic potential of bumetanide and to determine whether, in fact, bumetanide attenuates CE by reducing swelling of the BBB and astrocytes in DKA.
The current study has some limitations. As previously noted, rats with DKA tended to have respiratory depression with administration of anesthesia. For this reason, rats were intubated and mechanically ventilated, and we adjusted ventilator settings to approximate physiological hyperventilation in response to acidosis. Because of the inherent limitations of the ventilator equipment, however, it was difficult to precisely adjust pCO2 levels to correlate with pH levels within narrow ranges. Therefore, pCO2 levels in some rats may have been somewhat higher or lower than expected physiologically, and we were limited in our ability to evaluate correlations between hypocapnia and changes in ADCs or CBF. Furthermore, CBF values, even in normal control rats, varied considerably among individual rats. This baseline variation limited our ability to detect associations between biochemical variables and differences in CBF in DKA rats. For example, although we observed a clear association between pCO2 and CBF when individual rats were evaluated over several different pCO2 values, no association between pCO2 and CBF could be detected when only single measurements were made for each individual rat. In addition, barbiturate anesthesia typically causes a decline in CBF and likely had some effect in the current studies. In general, however, DKA rats required a lower dosage of anesthetic compared with that required by control rats. Therefore, it would be unlikely that anesthetic effect alone could explain the decrease in CBF in DKA rats. In addition, in our rat DKA model, the severity of DKA varied among rats, similar to human DKA. Because of variations in DKA severity, however, mean initial CBF and ADC values for the different conditions studied also differed among experiments. Finally, in these studies, induction of DKA in rats resulted in the development of mild, subclinical CE, modeling that which develops in most children with DKA. By studying this condition, we aimed to gain insights into the causes of more severe, clinically manifest CE that occurs in a small minority of children and may represent the most extreme end of a continuum of manifestations. It is possible, however, that clinically manifest CE in these children results from additional factors not studied in these experiments. Nonetheless, determining the cerebral alterations commonly associated with DKA provides initial information important to the eventual understanding of DKA-related CE and cerebral injury.
In summary, untreated DKA in rats is associated with reduced CBF, as well as with low ADC values indicating CE. Both ADC and CBF values were lowest in the animals with the greatest dehydration, and CBF values in individual rats varied in response to changes in pCO2. These findings strengthen the hypothesis that dehydration and hypocapnia during DKA result in diminished CBF and that cerebral hypoperfusion may be important in the pathogenesis of DKA-related CE. The present study also provides further evidence for the hypothesis that Na-K-Cl cotransport participates in CE formation during DKA and suggests that treatment with bumetanide to reduce edema formation should be further investigated.
Acknowledgments
Supported by the National Institutes of Health RO1 NS048610 (to N.G.). This investigation was conducted in part in a facility constructed with support from the Research Facilities Improvement Program Grant no. C06 RR17348-01 from the National Center for Research Resources, National Institutes of Health. Funding for nuclear magnetic resonance equipment was provided in part by National Science Foundation Grant OSTI 97-24412.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Faich G, Fishbein H, Ellis E: The epidemiology of diabetic acidosis: a population-based study. Am J Epidemiol 117:551, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Pinkney J, Bingley P, Sawtell P: Presentation and progress of childhood diabetes mellitus: a prospective population-based study. Diabetologia 37:70–74, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Mahoney C, Vlcek B, Aguila MD: Risk factors for developing brain herniation during diabetic ketoacidosis. Pediatric Neurol 21:721–727, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Edge J, Hawkins M, Winter D, Dunger D: The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child 85:16–22, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, Kaufman F, Quayle K, Roback M, Malley R, Kupperman N: Risk factors for cerebral edema in children with diabetic ketoacidosis. N Engl J Med 344:264–269, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Lawrence S, Cummings E, Gaboury I, Daneman D: Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J Pediatr 146:688–692, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Daneman D: Diabetes-related mortality. Diabetes Care 24:801–802, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Edge J, Ford-Adams M, Dunger D: Causes of death in children with insulin-dependent diabetes 1990–1996. Arch Dis Child 81:318–323, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scibilia J, Finegold D, Dorman J, Becker D, Drash A: Why do children with diabetes die? Acta Endocrinol Suppl 279:326–333, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Hoffman W, Steinhart C, Gammal TE, Steele S, Cuadrado A, Morse P: Cranial CT in children and adolescents with diabetic ketoacidosis. Am J Neuroradiol 9:733–739, 1988 [PMC free article] [PubMed] [Google Scholar]
- 11.Krane E, Rockoff M, Wallman J, Wolfsdorf J: Subclinical brain swelling in children during treatment of diabetic ketoacidosis. N Engl J Med 312:1147–1151, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Glaser N, Wooton-Gorges S, Buonocore M, Marcin J, Rewers A, Strain J, DiCarlo J, Neely E, Barnes P, Kuppermann N: Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr Diab 7:75–80, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Brillault J, Lam TI, Rutkowsky JM, Foroutan S, O'Donnell ME: Hypoxia effects on cell volume and ion uptake of cerebral microvascular endothelial cells. Am J Physiol Cell Physiol 294:C88–C96, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Su G: Regulation of Na+-K+-Cl-cotransporter in primary astrocytes by dibutyryl cAMP and high [K+]o. Am J Physiol Cell Physiol 279:C1710–C1721, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Iadecola C: Mechansims of cerebral ischemic damage. In Cerebral Ischemia: Molecular and Cellular Pathophysiology. Walz W, Ed. Totowa, NJ, Humana Press, 1999, p. 3–34
- 16.Kempski O, Rosen Sv, Weight H, Staub F, Peters J, Baethmann A: Glial ion transport and volume control. In Glial-Neuronal Interaction. Abbott NJ, Ed. New York, New York Academy of Sciences, 1991, p. 306–317 [DOI] [PubMed]
- 17.Kimelberg HK: Cell swelling in cerebral ischemia. In Cerebral Ischemia: Molecular and Cellular Pathophysiology. Walz W, Ed. Totowa, NJ, Humana Press, 1999, p. 45–68
- 18.Lam TI, Anderson SE, Glaser N, O'Donnell ME: Bumetanide reduces cerebral edema formation in rats with diabetic ketoacidosis. Diabetes 54:510–516, 2005 [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE: Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab 24:1046–1056, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Rudin M, Baumann D, Ekatodramis D, Stirnimann R, McAllister KH, Sauter A: MRI analysis of the changes in apparent water diffusion coefficient, T2 relaxation time, and cerebral blood flow and volume in the temporal evolution of cerebral infarction following permanent middle cerebral artery occlusion in rats. Exp Neurol 169:56–63, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Robinson J: The Harriet Lane Handbook. 17th ed. Robinson J, Shilkofski N, Eds. Philadelphia, Mosby, 2005, p. 627–628
- 22.O'Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE: Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab 26:1234–1249, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Tatlisumak T, Takano K, Carano RAD, Fisher M: Effect of basic fibroblast growth factor on experimental focal ischemia studied by diffusion-weighted and perfusion imaging. Stroke 27:2292–2298, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Williams DS, Detre JA, Leigh JS, Koretsky AP: Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 89:212–216, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albuquerque ML, Leffler CW: pHo, pHi and pCO2 in stimulation of IP3 and [Ca2+] in piglet cerebrovascular smooth muscle. Proc Soc Exp Biol 219:226–234, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Kety SS, Polis BD, Nadler CS, Schmidt CF: The blood flow and oxygen consumption of the human brain in diabetic acidosis and coma. J Clin Invest 27:500–510, 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young E, Bradley RF: Cerebral edema with irreversible coma in severe diabetic ketoacidosis. N Engl J Med 276:665–669, 1967 [DOI] [PubMed] [Google Scholar]
- 28.Harris G, Fiordalisi I, Harris W, Mosovich L, Finberg L: Minimizing the risk of brain herniation during treatment of diabetic ketoacidemia: a retrospective and prospective study. J Pediatr 117:22–31, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Duck S, Wyatt D: Factors associated with brain herniation in the treatment of diabetic ketoacidosis. J Pediatr 113:10–14, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Hammond P, Wallis S: Cerebral oedema in diabetic ketoacidosis: still puzzling-and often fatal. BMJ 305:203–204, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsin JP, Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, Kaufman F, Quayle K, Roback M, Malley R, Kuppermann N: Factors associated with adverse outcmes in children with diabetic ketoacidosis related cerebral edema. J Pediatr 141:793–797, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Edge JA: Cerebral oedema during treatment of diabetic ketoacidosis: are we any nearer finding a cause? Diabetes Metab Res Rev16:316–324, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Hoorn EJ, Carlotti AP, MacMahon B, Bohn G, Zietse R, Halperin ML, Bohn D: Preventing a drop in effective plasma osmolality to minimize th elikelihood of cerebral edema during treatment of children with diabetic ketoacidosis. J Pediatr 150:467–473, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Salem MS, Matta LF, Tantawy AA, Hussein M, Gad GI: Single photon emission tomography (SPECT) study of regional cerebral blood flow in normoalbuminuric children and adolescents with type 1 diabetes. Pediatr Diab 3:155–162, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Glaser NS, Wootton-Gorges SL, Marcin JP, Buonocore MH, DiCarlo J, Neely EK, Barnes P, Bottomly J, Kuppermann N: Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr 145:164–171, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Marchal G, Young A, Baron J: Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab 19:467–482, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Yang G-Y, Betz AL, Chenevert T, Brunberg JA, Hoff JT: Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg 81:93–102, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Aronowski J, Strong R, Grotta JC: Reperfusion injury: demonstration of brain damage by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab 17:1048–1056, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Yan Y, Dempsey RJ, Sun D: Na-K-Cl cotransporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab 21:711–721, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Foroutan S, Brillault J, Forbush B, O'Donnell ME: Moderate to severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na-K-Cl cotransporter. Am J Physiol Cell Physiol 289:C1492–C1501, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Pedersen SF, O'Donnell ME, Anderson SE, Cala PM: Physiology and pathophysiology of Na+/H+ exchange and Na+-K+-2Cl− cotransporter in the heart, brain and blood. Am J Physiol Reg Integr Comp Physiol 291:R1–R25, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Menzies SA, Betz AL, Hoff JT: Contributions of ions and albumin to the formation and resolution of ischemic brain edema. J Neurosurg 78:257–266, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Schielke GP, Moises HC, Betz AL: Blood to brain sodium transport and interstitial fluid potassium concentration during focal ischemia in the rat. J Cereb Blood Flow Metab 11:466–471, 1991 [DOI] [PubMed] [Google Scholar]
- 44.Gotoh O, Asano T, Koide T, Takakura K: Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: the time course of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke 16:101–109, 1985 [DOI] [PubMed] [Google Scholar]
- 45.Yan Y, Dempsey RJ, Flemmer A, Forbush B, Sun D: Inhibition of Na-K-Cl cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res 961:22–31, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Su G, Kintner DB, Flagella M, Shull GE, Sun D: Astrocytes from Na(+)-K(+)-Cl(-) cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am J Physiol Cell Physiol 282:C1147–C1160, 2002 [DOI] [PubMed] [Google Scholar]