Abstract
OBJECTIVE— Tall-like receptor (TLR)4 has been implicated in the pathogenesis of free fatty acid (FFA)-induced insulin resistance by activating inflammatory pathways, including inhibitor of κB (IκB)/nuclear factor κB (NFκB). However, it is not known whether insulin-resistant subjects have abnormal TLR4 signaling. We examined whether insulin-resistant subjects have abnormal TLR4 expression and TLR4-driven (IκB/NFκB) signaling in skeletal muscle.
RESEARCH DESIGN AND METHODS— TLR4 gene expression and protein content were measured in muscle biopsies in 7 lean, 8 obese, and 14 type 2 diabetic subjects. A primary human myotube culture system was used to examine whether FFAs stimulate IκB/NFκB via TLR4 and whether FFAs increase TLR4 expression/content in muscle.
RESULTS— Obese and type 2 diabetic subjects had significantly elevated TLR4 gene expression and protein content in muscle. TLR4 muscle protein content correlated with the severity of insulin resistance. Obese and type 2 diabetic subjects also had lower IκBα content, an indication of elevated IκB/NFκB signaling. The increase in TLR4 and NFκB signaling was accompanied by elevated expression of the NFκB-regulated genes interleukin (IL)-6 and superoxide dismutase (SOD)2. In primary human myotubes, acute palmitate treatment stimulated IκB/NFκB, and blockade of TLR4 prevented the ability of palmitate to stimulate the IκB/NFκB pathway. Increased TLR4 content and gene expression observed in muscle from insulin-resistant subjects were reproduced by treating myotubes from lean, normal-glucose-tolerant subjects with palmitate. Palmitate also increased IL-6 and SOD2 gene expression, and this effect was prevented by inhibiting NFκB.
CONCLUSIONS— Abnormal TLR4 expression and signaling, possibly caused by elevated plasma FFA levels, may contribute to the pathogenesis of insulin resistance in humans.
The mechanism(s) by which free fatty acids (FFAs) cause insulin resistance is not fully understood. Considerable evidence suggests that the deleterious effect of FFAs on insulin action is caused by intramyocellular FFA metabolites that stimulate inflammatory pathways leading to impaired insulin signaling/action (1). However, recent reports demonstrate that FFAs directly can stimulate plasma membrane receptors (2,3), suggesting an alternate model in which FFAs cause insulin resistance by stimulating inflammatory pathways through the direct activation of plasma membrane receptors. Consistent with this hypothesis, FFAs have been shown to bind to toll-like receptor (TLR)4 (4), a transmembrane receptor, and TLR4-driven inflammatory cascades, such as the inhibitor of κB (IκB)/nuclear factor κB (NFκB) pathway, are implicated in the pathogenesis of insulin resistance (5–7).
TLRs play an important role in the innate immune system by activating inflammatory pathways in response to microbial agents (8). TLR4 functions as the receptor for lipopolysaccharide (LPS) of gram-negative bacterial cell walls (8). Saturated FFAs acylated in the lipid A moiety of LPS are essential for the biological activity of LPS (9). In mononuclear cells/monocytes, saturated FFAs are potent activators of TLR4 signaling, whereas unsaturated FFAs do not stimulate this pathway (10). After LPS binds to TLR4, and its co-receptors CD14 and MD-2, the adaptor protein myeloid differentiation factor 88 (MyD88) is recruited to the Toll/interleukin (IL)-1 receptor (TIR) domain of the TLR4 receptor (11). The interaction between TLR4 and MyD88 leads to the autophosphorylation of IL-1R–associated kinase (IRAK) (11). When IRAK becomes activated, it interacts with tumor necrosis factor (TNF)-associated factor 6 (TRAF6), leading to stimulation of the kinase complex IκB kinase (IKK), which phosphorylates IκΒ. Phosphorylation of IκB by IKK triggers the degradation of IκΒ by the proteosome, causing the liberation of NFκB from IκB. NFκB then translocates into the nucleus, where it stimulates the transcription of numerous inflammatory genes including IL-6 (12) and superoxide dismutase (SOD)2 (13).
Most of the data implicating FFAs as ligands of TLR4 have been obtained in inflammatory cells and mononuclear cells/monocytes (10). However, recent studies have provided evidence that FFAs stimulate TLR4 signaling in conventional insulin-target tissues such as fat (14,15) and muscle (16,17). TLR4 was found to be highly expressed in 3T3 L1 adipocytes and fat tissue from mice (14,15). In addition, TLR4 gene expression was higher in adipose tissue from insulin-resistant (high-fat–fed, db/db, and ob/ob) mice compared with normal animals (14,15). Moreover, the ability of a lipid infusion to activate the IκB/NFκB pathway in adipose tissue (14) and to inhibit insulin-stimulated whole-body glucose disposal (14,17) is reduced in TLR4 null mice. Because muscle is the major site of insulin-stimulated glucose disposal (18), this important finding implies that TLR4 is also functional in skeletal muscle. Indeed, muscles from mice carrying an inactivating mutation on TLR4 are prevented from developing lipid-induced insulin resistance (16). Whereas these data obtained from animal and tissue culture models clearly implicate TLR4 in the pathogenesis of insulin resistance, it remains unknown whether this pathway functions abnormally and contributes to insulin resistance in muscle from human subjects. Therefore, the purpose of this study was to examine whether insulin-resistant subjects have abnormal TLR4 expression and signaling in muscle and to determine whether TLR4 plays a role in the stimulation of IκB/NFκB caused by FFAs. We hypothesize that 1) insulin-resistant subjects have abnormal function of TLR4 in muscle, and 2) TLR4 is involved in FFA-induced IκB/NFκB stimulation in human muscle.
RESEARCH DESIGN AND METHODS
We recruited 7 lean, 8 obese nondiabetic, and 14 obese subjects with type 2 diabetes. All subjects were sedentary and had stable body weight for 6 months before study. Each subject underwent a medical history, physical examination, screening laboratory tests, and an oral glucose tolerance test (OGTT). Lean and obese nondiabetic subjects had no family history of type 2 diabetes and were normal glucose tolerant. None of the lean or obese subjects were taking any medication. Three type 2 diabetic subjects were taking glipizide, which was withdrawn 3 days before the OGTT. Nine type 2 diabetic subjects were treated with diet alone. Other than glipizide, no subject was taking any medication known to affect glucose metabolism. The study was approved by the institutional review board of the University of Texas Health Science Center at San Antonio (UTHSCSA), and all subjects gave written consent.
OGTT.
Plasma glucose and FFA levels were measured at baseline and every 15 min for 2 h after the ingestion of 75 g glucose. Plasma insulin was measured at baseline, and the severity of insulin resistance was estimated using the homeostasis model assessment (HOMA) index (19).
Muscle biopsies.
Subjects reported to the clinical research center at 8:00 a.m. after an overnight fast and refrained from any exercise for 48 h before the muscle biopsy. Subjects rested for 30 min before a vastus lateralis muscle biopsy (20). The muscle was debrided of adipose and connective tissue and immediately (within 5 s after the biopsy) frozen in liquid nitrogen.
Generation of primary myotubes.
Primary skeletal muscle cells were grown from satellite cells obtained from the muscle tissue based on the protocol by Henry et al. (21), with minor modifications. All donors were lean and had a normal glucose tolerance test. After excision, the muscle tissue (∼100 mg) was immediately placed in 15 ml Ham's F-12 medium at 4°C. After removing excess fat and connective tissue, the samples were digested in 0.05% trypsin, 0.1% collagenase, and 1.5% BSA, pH 7.4, for 30 min at 37°C with agitation. After 30 min of incubation, 10% FBS was added and the tissue was centrifuged at 80g for 10 min. Cell pellets were resuspended with 20% α-minimal essential medium (MEM) containing penicillin (200 units/ml)/streptomycin (200 mg/ml). Cells were plated on 100-mm dishes and incubated for 1 h to remove fibroblasts. The myoblasts then were grown to confluence in 75 cm2 collagen-coated flasks with 20% α-MEM for 5–7 days and subsequently seeded in 75 cm2 flasks for another 5 days. For all individual experiments, myoblasts were seeded in six-well culture dishes (9.6 cm2/well) at a density of 20,000 cells/well. When myoblasts reached 80–90% confluence, the cells were fused for 4–7 days in α-MEM with 2% FBS. Cells displayed the typical features of differentiated myotubes: elongated, multinucleated cells expressing myosin heavy chain by Western blotting.
Preparation of palmitate solution.
Stocks of 8 mmol/l palmitate and 10.5% fatty acid–free BSA (low endotoxin grade; Sigma, St. Louis, MO) were prepared as described (22).
Acute IκB/NFκB pathway stimulation in myotubes.
Dose-response and time-course experiments were performed by treating human myotubes with 200 or 400 μmol/l palmitate at different time points. Palmitate at a 400-μmol/l concentration was used because it has been shown to activate the IκB/NFκB pathway in C2C12 cells (23). After treatment, cells were lysed in lysis buffer (20 mmol/l Tris, pH 7.5, 5 mmol/l EDTA, 10 mmol/l Na3PO4, 100 mmol/l NaF, 2 mmol/l Na3VO4, 1% NP 40, 10 μmol/l leupeptin, 3 mmol/l Benzamidine, 10 μg/ml aprotinin, and 1 mmol/l phenylmethylsulfonyl fluoride), and the lysates were stored in liquid nitrogen. To examine whether TLR4 is involved in palmitate-induced IκB/NFκB activation, myotubes were first incubated in media with a TLR4 neutralizing antibody (10 μg/ml) or an isotype control (10 μg/ml) (both from eBioscience, San Diego, CA) for 1 h at room temperature. The TLR4 neutralizing antibody (10 μg/ml) has been shown to block TLR4-driven inflammatory responses (16,24). After blocking TLR4, the myotubes were washed once with PBS, treated with 400 μmol/l palmitate for 1 h, and lysed. To determine whether other TLRs, such as TLR2, play a role in FFA stimulation of IκB/NFκB, similar experiments were done with a TLR2 neutralizing antibody (10 μg/ml) (eBioscience) (25). In addition, we determined the effect of a different saturated fatty acid, 400 μmol/l stearate (Sigma), as well as an unsaturated fatty acid, 400 μmol/l linoleate (Sigma), by treating myotubes for 1 h and measuring IκB phosphorylation.
IKK kinase activity assay.
Myotubes were treated with 400 μmol/l palmitate, 100 ng/ml LPS (Sigma), or 20 ng/ml human TNF-α (R&D Systems, Minneapolis, MN) for 1 h, harvested in lysis buffer, and frozen in liquid nitrogen. After thawing, lysates were then centrifuged at 14,000g for 20 min at 4°C and the supernatants (250 μg protein) were immunoprecipitated with an anti-human IKKβ antibody (Epitomics, Burlingame, CA) and protein A beads. Immunoprecipitates were washed twice in lysis buffer and twice in wash buffer (50 mmol/l Tris, pH 7.4, 0.5 mol/l NaCl, and 0.1 mmol/l EGTA). Kinase reactions were performed in 50 mmol/l Tris, pH 7.4, 0.2 mmol/l IKK peptide (Upstate Biotechnology, Lake Placid, NY), 1 mmol/l dithiothreitol, 10 mmol/l MgCl2, 0.1 mmol/l EGTA, and 0.1 mmol/l ATP (3 μCi [32P]ATP) in a final volume of 50 μl for 20 min at 30°C. At the end of the reaction, a 25-μl aliquot was removed and spotted on Whatman P81 paper. The papers were washed six times in ice-cold 1% phosphoric acid and once with acetone at room temperature. Radioactivity was quantitated with a scintillation counter.
Chronic palmitate treatment.
Myotubes were treated with 200 μmol/l palmitate, 100 nmol/l insulin (Humulin; Eli Lilly, Indianapolis, IN), 20 ng/ml human TNF-α, or 100 ng/ml IL-6 (R&D Systems) for 72 h. TNF-α and IL-6, at the concentrations used, have been shown to elicit an inflammatory response in muscle cells (26,27). Myotubes then were lysed, and TLR4 protein content was measured by Western blotting.
Simulation of TLR4 gene expression by palmitate and lipid A.
Myotubes were incubated for 6 h with 200 μmol/l palmitate or with the specific TLR4 agonist synthetic (from E. coli) monophosphoryl lipid A (10 μg/ml) (Invivogen, San Diego, CA), and mRNA expression was measured by real-time PCR.
Adenoviral-mediated transduction.
Myotubes were first infected with adeno-IκB-superrepressor (tagged with HA) or adeno–green fluorescent protein (GFP) (both at 1 × 108 plaque-forming units; Vector Biolabs, Philadelphia, PA) for 4 h, achieving a >95% transduction efficiency based on fluorescence microscopy. To examine the effect of palmitate on IL-6, SOD2, and TLR4 gene expression, 48 h after the transduction, myotubes were treated with 200 μmol/l palmitate for 6 h, and mRNA gene expression was measured by real-time PCR. The IκBSS32/36AA (superrepressor) mutant functions as a potent inhibitor of NFκB because mutating serines 32 and 36 for alanine prevents phosphorylation and dissociation of IκB from NFκB (28).
Quantitative real-time PCR.
Total RNA was extracted with Trizol solution (Sigma) and purified with RNeasy and DNase I treatment (Qiagen, Chatsworth, CA). An Agilent Bioanalyzer was used to check RNA quality. Quantitative real-time PCR was performed on an ABI PRISM 7900HT System (Applied Biosystems, Foster City, CA) using TaqMan One-Step RT-PCR Master Mix Reagents and Assay On-Demand primer/probes (TLR4: Hs00152939_m1, SOD2: Hs00167309_m1, EMR1: Hs00173562_m1). TLR2 expression was determined using the following primers/probe: forward primer: 5-CAATGATGCTGCCATTCTCAT-3, reverse primer: 5-ATTATCTTCCGCAGCTTGCA-3, probe: 5-CATTGAGAAAAAAGCCATTCCCCAGCG-3. IL-6 expression was determined using forward primer: 5-GGTACATCCTCGACGGCATCT-3, reverse primer: 5-GTGCCTCTTTGCTGCTTTCAC-3, probe: 5-TGTTACTCTTGTTACATGTCTCCTTTCTCAGGGCT-3. Each sample was run in duplicate, and the quantity of mRNA for each gene of interest was normalized to that of 18S ribosomal RNA using the comparative (2−ΔΔCT) method (29).
Western blotting.
Muscle tissue samples were homogenized as previously described (30). Proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. After blocking the membranes, these were incubated overnight with a primary antibody against TLR4 (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-IκB (Cell Signaling, Danvers, MA), IκBα (Cell Signaling), or β-actin (Cell Signaling). Bound antibodies were detected with a secondary antibody (anti-rabbit immunoglobulin–horseradish peroxidase–linked antibody) using enhanced chemiluminescence reagents. Bands were quantitated with ImageTool (UTHSCSA). Detection of TLR2 protein in muscle was attempted using antibodies from eBioscience, Cell Signaling, Santa Cruz Biotechnology, and Imgenex (San Diego, CA). Detection of the monocyte/macrophage marker epidermal growth factor–like module (EMR)-1 was performed using an anti-human EMR1 antibody (Santa Cruz Biotechnology).
NFκB activity.
Binding activity of the p65 NFκB subunit was measured in cell lysates using an enzyme-linked immunosorbent assay (ELISA) kit (Active Motif, Carlsbad, CA).
Laboratory analyses.
Plasma insulin was measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA), glucose by the glucose oxidase method on a Beckman analyzer, and A1C using a DCA2000 analyzer (Bayer, Tarrytown, NY). FFA concentration was determined with a colorimetric method (Wako, Nuess, Germany). Plasma TNF-α and IL-6 concentrations were measured using an ELISA (R&D Systems).
Statistical analysis.
All data are expressed as means ± SE. Comparison of data between the lean, obese, and type 2 diabetes groups was done using one-way ANOVA, followed by Tukey post hoc analysis. The effect of palmitate on IKK and IκB was assessed using one-way ANOVA with repeated measures. The effect of the TLR4-blocking antibodies and the IκB supperrepressor on palmitate-induced TLR4 pathway stimulation was assessed by two-way ANOVA. The effect of palmitate on TLR4 protein/gene expression and the effect of monophosphoryl lipid A on TLR4 gene expression were determined using the Student's t test. Analyses were performed using SigmaStat software.
RESULTS
Subject characteristics.
Clinical and laboratory characteristics of the subjects are shown in Table 1. All groups were closely matched for age. Type 2 diabetic and obese subjects were well matched for BMI, and both groups were more obese than the lean normal-glucose-tolerant group (P < 0.05). Subjects with type 2 diabetes had higher fasting plasma glucose concentrations, glucose area under the curve (AUC) during OGTT, and A1C levels compared with the obese and lean subjects (P < 0.05). Obese and type 2 diabetic subjects were more insulin resistant than lean subjects, based on a higher HOMA index and plasma insulin concentration (P < 0.05). The subjects from the obese and type 2 diabetes groups also had higher fasting plasma FFA concentrations and FFA AUC during the OGTT compared with lean control subjects (P < 0.05). Plasma concentrations of TNF-α and IL-6 were significantly elevated in the type 2 diabetic subjects (P < 0.05), and there was a tendency for higher TNF-α and IL-6 levels in obese versus lean subjects (P = 0.1 for both cytokines).
TABLE 1.
Clinical and laboratory characteristics
| Lean | Obese | Type 2 diabetes | |
|---|---|---|---|
| n | 7 | 8 | 14 |
| Sex (M/F) | 4/3 | 3/5 | 5/9 |
| Age (years) | 45 ± 2 | 44 ± 3 | 52 ± 3 |
| BMI (kg/m2) | 25.3 ± 0.7 | 30.5 ± 0.6* | 31.8 ± 1.2* |
| Fasting glucose (mmol/l) | 5.4 ± 0.2 | 5.6 ± 0.1 | 7.8 ± 0.6*† |
| Glucose AUC during OGTT (mg/dl · 2 h) | 120 ± 11 | 121 ± 8 | 230 ± 18*† |
| Fasting insulin (pmol/l) | 19 ± 4 | 92 ± 16* | 86 ± 16* |
| HOMA-IR | 0.6 ± 0.1 | 2.0 ± 0.3* | 2.1 ± 0.4* |
| Fasting FFAs (μmol/l) | 351 ± 37 | 479 ± 59* | 750 ± 29*† |
| FFA AUC during OGTT (μmol/l · 2 h) | 22.8 ± 1.1 | 31.3 ± 3.3* | 55.9 ± 3.7*† |
| TNF-α (pg/ml) | 1.26 ± 0.10 | 1.63 ± 0.30 | 2.13 ± 0.32* |
| IL-6 (pg/ml) | 1.17 ± 0.15 | 1.91 ± 0.40 | 2.42 ± 0.33* |
Data are means ± SE.
P < 0.05 vs. lean;
P < 0.05 vs. obese.
Elevated TLR4 gene expression and protein content in insulin-resistant subjects.
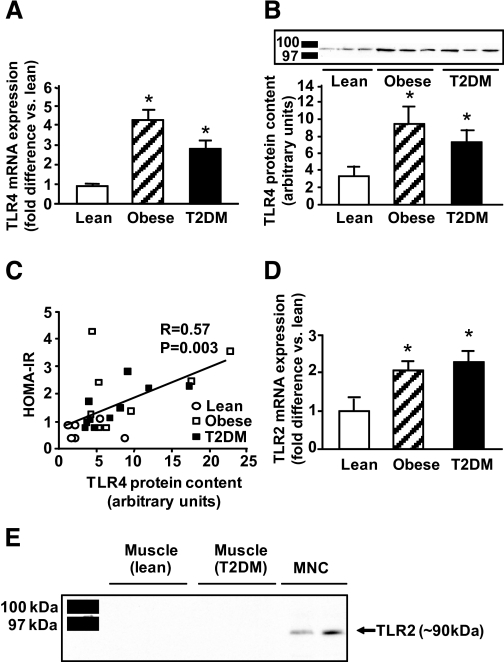
TLR4 gene expression was significantly increased in the muscle of the obese nondiabetic and type 2 diabetic subjects (4.8- and 3.2-fold, respectively; P < 0.05) compared with lean subjects (Fig. 1A). Collectively, in the three groups, absolute TLR4 mRNA levels correlated with fasting plasma FFA concentrations (r = 0.37, P = 0.05) and FFA AUC during OGTT (r = 0.41, P = 0.03). To examine whether increased TLR4 gene expression in obese and type 2 diabetic subjects was reflected at the protein level, we measured TLR4 muscle protein content by Western analysis using an antibody targeted against the epitope corresponding to amino acids 242–321 of the internal region of human TLR4. Using this antibody, TLR4 migrated as a single ∼100-kDa band. To confirm the specificity of the antibody, human embryonic kidney cell lysates overexpressing human TLR4 tagged with yellow fluorescent protein (provided by Dr. Andrei Medvedev, University of Maryland) were immunoprecipitated with an anti-GFP antibody (Invitrogen, Carlsbad, CA) and blotted with anti-TLR4 (not shown). Consistent with the increases in TLR4 gene expression, obese and type 2 diabetic subjects had 2.8- and 2.2-fold higher TLR4 muscle protein content than lean subjects (P < 0.05) (Fig. 1B), and there was a positive correlation (r = 0.57, P = 0.003) between the HOMA index of insulin resistance (HOMA-IR) and TLR4 content (Fig. 1C).
FIG. 1.
TLR4 and TLR2 gene expression and protein content. TLR4 mRNA expression (A) and protein content (B) were measured in lean, obese, and type 2 diabetic (T2DM) subjects. Data are means ± SE in 7 lean, 8 obese, and 14 type 2 diabetic subjects in A and 6 lean, 8 obese, and 10 type 2 diabetic subjects in B (protein extracts were not available for all subjects). C: Correlation between TLR4 content and HOMA-IR. D: TLR2 mRNA expression in 7 lean, 8 obese, and 14 type 2 diabetic subjects. *P < 0.05 vs. lean. E: Direct Western blotting in muscle from three lean and three type 2 diabetic subjects using an antibody against human TLR2 (eBiosciences). A band corresponding to TLR2 was detected in mononuclear cells (MNCs) isolated from two subjects.
Similar to TLR4, the gene expression of TLR2 was significantly elevated in muscle from obese and type 2 diabetic individuals (P < 0.05) (Fig. 1D). Nonetheless, we were not able to detect TLR2 protein in muscle from human subjects (diabetic and nondiabetic) by direct Western blotting, despite using several commercially available antibodies. Figure 1E shows that, in contrast to muscle, human mononuclear cells highly express TLR2 protein.
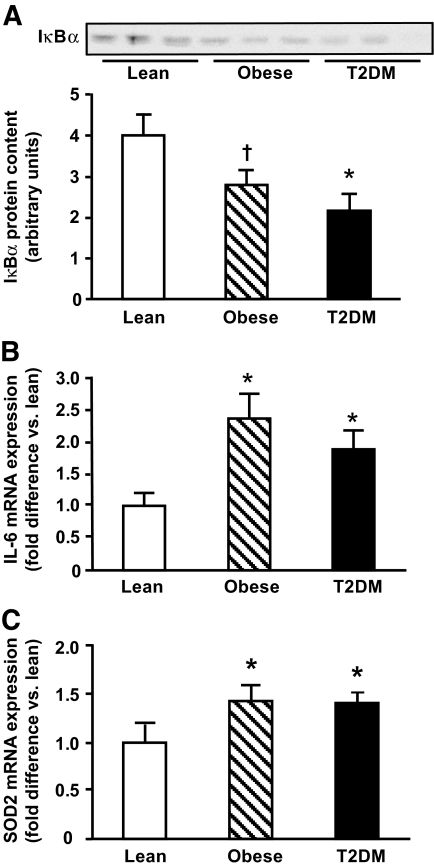
TLR4-driven signaling.
We examined whether increased TLR4 gene expression and protein content in insulin-resistant subjects was associated with abnormal TLR4 signaling by measuring abundance of IκBα. Obese and type 2 diabetic subjects had decreased IκBα content in muscle (Fig. 2A). Because phosphorylation of IκB by IKK leads to IκB degradation, a reduction in IκB abundance is considered to indicate activation of the IκB/NFκB pathway (31). IκB abundance inversely correlated with fasting plasma FFA concentrations (r = −0.6, P = 0.005) and FFA AUC during OGTT (r = −0.46, P = 0.03) and tended to correlate negatively with the HOMA index (r = −0.34, P = 0.1). Currently, we are investigating whether direct measurement of IKK activity (which was not measured because of insufficient protein) has a stronger (negative) correlation with insulin sensitivity measured with the insulin clamp.
FIG. 2.
TLR4 signaling. IκBα protein abundance (A) and mRNA expression of IL-6 (B) and SOD2 (C) were measured in lean, obese, and type 2 diabetic (T2DM) subjects. Data are means ± SE. *P < 0.05; †P = 0.05 vs. lean.
We also determined the expression of IL-6 and SOD2, genes that are highly regulated by NFκB (12,13), in muscle from obese and type 2 diabetic subjects. As shown in Figs. 2B and C, obese and type 2 diabetic subjects had elevated IL-6 and SOD2 gene expression compared with lean normal-glucose-tolerant subjects (P < 0.05). Collectively, these results indicate that insulin-resistant subjects have increased TLR4 expression/content and TLR4-driven signaling.
FFAs acutely stimulate TLR4-driven signaling.
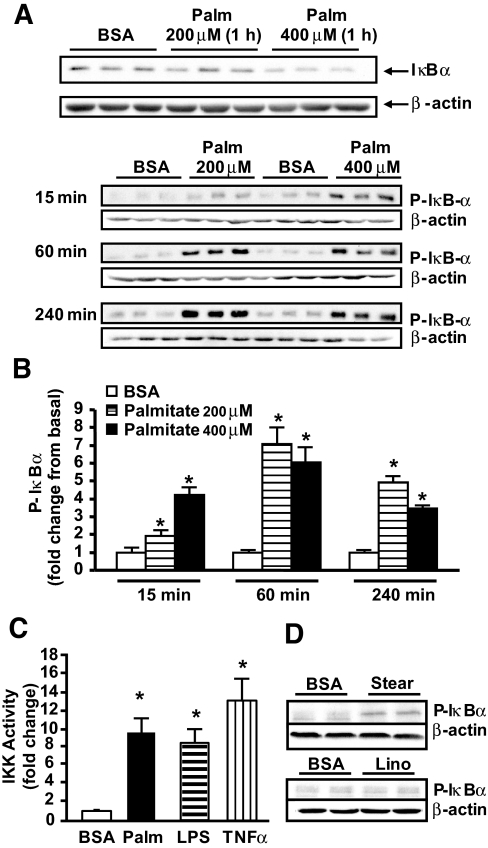
To explore whether FFAs directly stimulate TLR4-driven signaling in human muscle, we used a primary muscle cell culture system using satellite cells obtained from lean, normal-glucose-tolerant subjects. Treatment with 200 and 400 μmol/l palmitate decreased IκBα abundance (Fig. 3A) and increased IκB phosphorylation within 15 min (Fig. 3B), an effect that persisted after 60 and 240 min of palmitate treatment. Treatment with 100 μmol/l palmitate for up to 240 min did not consistently increase IκB phosphorylation (not shown). In line with the increases in IκB phosphorylation, palmitate significantly increased IKKβ kinase activity (Fig. 3C). Stearate, another saturated fatty acid, also stimulated IκB/NFκB signaling, whereas the unsaturated fatty acid linoleate did not affect this pathway (Fig. 3D). This finding indicates that the stimulatory effect of palmitate on TLR4-driven signaling in human muscle is common to other saturated fatty acids.
FIG. 3.
Effect of FFAs on IκB and IKK activity. Human myotubes were treated with palmitate (Palm), and IκBα content (A) and phosphorylation (B) were measured by Western blotting. IKK activity was measured after myotubes were treated with 400 μmol/l palmitate, 100 ng/ml LPS, or 20 ng/ml human TNF-α for 1 h (C). IκBα phosphorylation was measured after myotubes were treated with 400 μmol/l stearate (Stear) or 400 μmol/l linoleate (Lino) for 1 h (D). Data are means ± SE, n = 6–9 per group. *P < 0.05 vs. BSA.
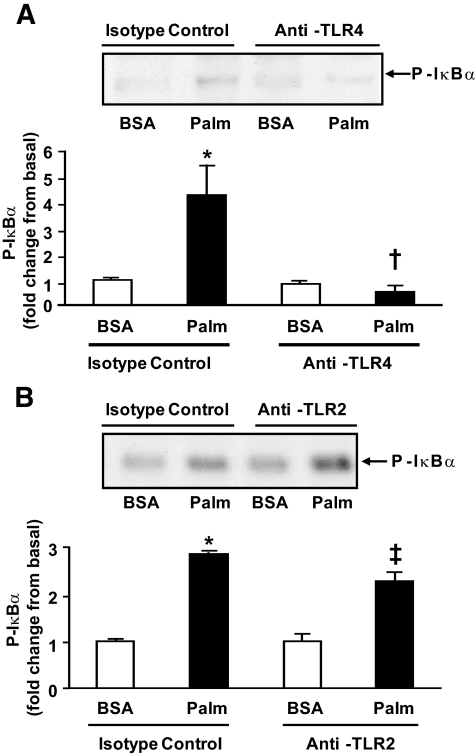
TLR4 mediates the effect of palmitate on IκB/NFκB signaling.
The role of TLR4 in the activation of IκB/NFκB by FFAs was assessed by blocking TLR4. Preincubation of human myotubes with TLR4 neutralizing antibodies prevented the ability of palmitate to phosphorylate IκB (Fig. 4A), whereas antibodies against TLR2 did not block the stimulatory effect of palmitate (Fig. 4B).
FIG. 4.
Effect of TLR neutralizing antibodies. Human myotubes were incubated with TLR4 (A) or TLR2 (B) neutralizing antibodies for 1 h and then stimulated with 400 μmol/l palmitate (Palm) for 1 h. Data are means ± SE, n = 6–9 per group. *P < 0.05 vs. BSA of isotype control group, †P < 0.05 vs. palmitate of isotype control group, and ‡P < 0.05 vs. BSA of anti-TLR2 group.
Prolonged FFA exposure increases TLR4 expression.
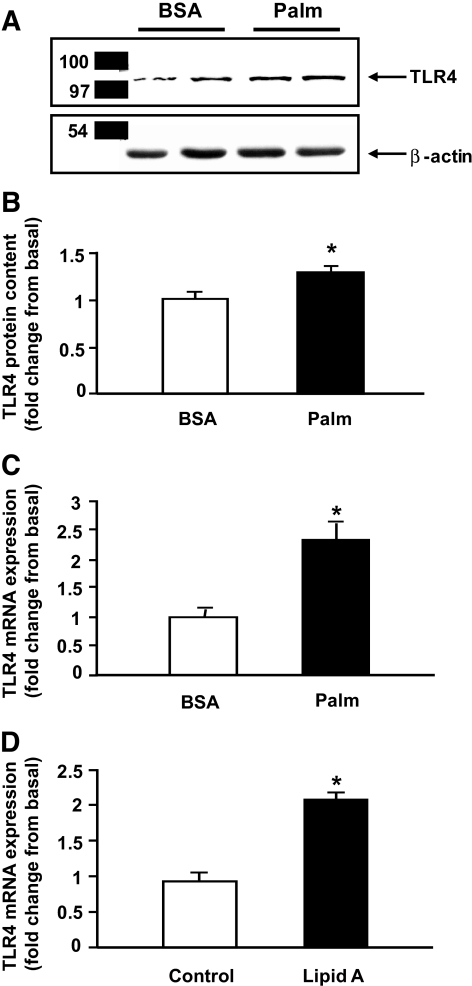
Because plasma FFA, insulin, TNF-α, and IL-6 concentrations are typically elevated in insulin-resistant subjects, we examined whether these factors had any effect on muscle TLR4 content. Figure 5A shows that a 3-day exposure to palmitate caused a significant increase in TLR4 protein content in human myotubes. In contrast to palmitate, chronic exposure to insulin, TNF-α, or IL-6 did not increase TLR4 protein content (not shown). Consistent with the elevation in TLR4 protein content, treatment with palmitate for 6 h also increased TLR4 gene expression (Fig. 5B). Importantly, this effect was reproduced by stimulating the cells with synthetic monophosphoryl-lipid A (Fig. 5C), a specific TLR4 agonist (32).
FIG. 5.
Palmitate (Palm) increases TLR4 content and gene expression. Human myotubes were incubated with 200 μmol/l palmitate for 3 days (A) or 6 h (B), and TLR4 protein content (A) and mRNA expression (B) were measured by Western blotting and real-time PCR, respectively. TLR4 gene expression was measured after treating myotubes with synthetic monophosphoryl lipid A (10 μg/ml) for 6 h (C). Data are means ± SE, n = 6–9 per group. *P < 0.05 vs. BSA/control.
The proinflammatory effect of palmitate is NFκB mediated.
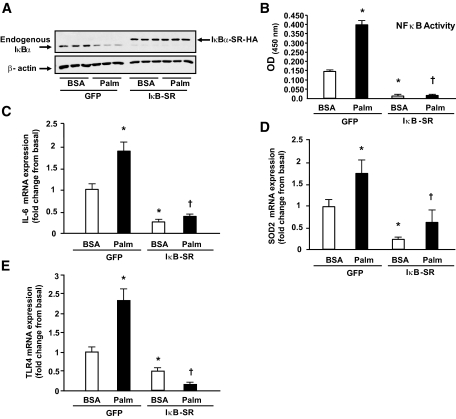
An IκB supperrepressor mutant was used to examine whether the effect of palmitate on IL-6, SOD2, and TLR4 mRNA expression is mediated by NFκB. As shown in Fig. 6, palmitate decreased IκBα abundance (Fig. 6A), stimulated NFκB activity (Fig. 6B), and increased the gene expression of IL-6 (Fig. 6C) and SOD2 (Fig. 6D), effects that were blocked by the IκB supperrepressor. Palmitate-induced TLR4 gene expression was also blocked by inhibiting NFκB through overexpression of the IκB supperrepressor (Fig. 6E), but not by preincubating the cells with TLR4-neutralizing antibodies (not shown).
FIG. 6.
Palmitate induces an inflammatory response via NFκB. Human myotubes were transduced with adenovirus-IκB supperrepressor (SR)-HA or adenovirus-GFP for 4 h, and 48 h later, cells were stimulated with 200 μmol/l palmitate for 6 h. IκBα was detected by Western blotting (A), NFκB p65 activity was measured by ELISA (B), and IL-6 (C), SOD2 (D), and TLR4 (E) gene expression were quantitated by real-time PCR. Data are means ± SE, n = 9 per group. *P < 0.05 vs. BSA of GFP group and †P < 0.05 vs. palmitate of GFP group.
DISCUSSION
The main finding of this study is that obese and type 2 diabetic subjects have increased TLR4 gene expression/content and TLR4-driven (IκB/NFκB) signaling in skeletal muscle. To examine whether FFAs directly activate TLR4 signaling in human muscle, myotubes were treated with palmitate, which rapidly stimulated the IκB/NFκB cascade. Furthermore, blocking TLR4 completely inhibited the ability of palmitate to stimulate this pathway. Because most obese nondiabetic and type 2 diabetic subjects have elevated plasma FFA concentrations (33), our results suggest that TLR4 may play an important pathogenic role in the mechanism by which FFAs induce an inflammatory response in insulin-resistant subjects.
To elucidate the cause for the elevation in TLR4 content present in the muscle from the obese and type 2 diabetic subjects, human myotubes were treated with palmitate for 3 days. Chronic palmitate treatment caused a significant increase in myotube TLR4 content, whereas prolonged exposure to insulin, TNF-α, or IL-6, whose plasma concentrations are typically elevated in insulin-resistant (obese and type 2 diabetic) subjects, had no effect. In line with this finding, palmitate increased TLR4 gene expression, an effect that was mediated via NFκB. Consistent with investigations performed in human endothelial cells that showed that LPS increases TLR4 expression (34), we found that treatment with the specific TLR4 agonist monophosphoryl lipid A also induces TLR4 gene expression in human myotubes. This suggests that palmitate-induced TLR4 gene expression is dependent on TLR4 activation. Nonetheless, this effect was not inhibited by TLR4-neutralizing antibodies, suggesting that, in addition to TLR4 stimulation, other mechanisms, such as accumulation of intracellular lipids (ceramides and diacylglycerol), may be involved in FFA-induced TLR4 expression. Because the elevations in TLR4 expression/content in the muscle from insulin-resistant subjects were reproduced in myotubes by prolonged exposure to palmitate, one could speculate that these increases in TLR4 expression/content are acquired defects, secondary to excess FFA supply. Nonetheless, future studies will be needed to establish whether there also is a genetic (inherited) basis underlying the elevation in TLR4 expression in insulin-resistant muscle.
Senn (35) reported that TLR2, another member of the TLR family, is involved in palmitate-induced insulin resistance in mouse-derived C2C12 myotubes. To examine this possibility in humans, we measured TLR2 gene expression in muscle from insulin-resistant subjects. Both obese and type 2 diabetic individuals displayed elevated TLR2 gene expression in muscle. However, TLR2 protein was not detected in muscle by direct Western blotting, although a signal (too weak for quantitation) corresponding to TLR2 was detected after immunoprecipitation. Taking into account that absolute mRNA levels of TLR4 versus TLR2 in human muscle were roughly similar (not shown), the low TLR2 protein content observed in human muscle is likely due to post-transcriptional regulation and/or protein instability (i.e., degradation). Poor antibody immunoefficiency could be another reason for the low TLR2 protein content observed in human muscle, although this seems unlikely considering that TLR2 protein was readily detectable in human mononuclear cells. Importantly, TLR2 neutralizing antibodies did not affect the ability of palmitate to activate IκB/NFκB signaling. Based on these results, it is unlikely that TLR2 plays a major role in lipid-induced insulin resistance in human muscle.
Another relevant finding in this study is the increased gene expression of the inflammatory proteins IL-6 and SOD2 in the muscle from obese and type 2 diabetic subjects. Experiments performed in monocyte-like (U937) cells (12) and fibroblasts (13) have demonstrated that the expression of these proteins is regulated by NFκB. Human myotubes were treated with palmitate to explore whether elevated circulating FFA levels in obese and type 2 diabetic subjects might be responsible for the increased gene expression of IL-6 and SOD2 in muscle. Palmitate robustly increased mRNA expression of IL-6 and SOD2 in the myotubes. Moreover, blockade of NFκB completely blocked the ability of palmitate to induce the gene expression of these inflammatory proteins. Therefore, elevation in plasma FFA concentration may contribute to increased gene expression of IL-6 and SOD2 in muscle from insulin-resistant subjects, and this effect is likely mediated by NFκB.
Accumulating evidence suggests that monocytes/macrophages play an important role in the pathogenesis of insulin resistance by infiltrating insulin-sensitive tissues, such as muscle and fat (36,37). Thus, it is possible that the increases in TLR4 expression and signaling observed in the muscle tissue from insulin-resistant subjects are due to inflammatory cell infiltration of the muscle, rather than from intrinsic upregulation of TLR4 expression/signaling within the myofibers. For this reason, we performed Western analysis and real-time PCR of EMR1, a marker for monocyte/macrophage infiltration, in muscle tissue of insulin-resistant subjects and in cultured myotubes. Unlike CD14+ monocytes (AllCells, Emeryville, CA), which expressed EMR1, neither EMR1 protein nor mRNA was detected in the muscle tissue or myotubes (not shown). It is therefore unlikely that the differences in TLR4 expression/signaling observed between groups are secondary to monocyte/macrophage infiltration of muscle.
In conclusion, insulin-resistant subjects have increased TLR4-driven signaling in muscle. We propose a model in which elevated plasma FFA levels in obese and type 2 diabetic subjects activates this signaling pathway by 1) directly interacting (binding) with TLR4 and 2) increasing TLR4 gene expression and protein content, leading to a net increase in the number of TLR4 receptors available for stimulation by elevated plasma FFA levels. Strategies aimed at reducing TLR4 expression, or at blocking TLR4 signaling, may prove useful in enhancing insulin sensitivity in insulin-resistant individuals.
Acknowledgments
This study was supported by grants from the American Diabetes Association (to E.C., R.A.D., and N.M.), the National Institutes of Health (AG030979 and DK080157 to N.M., DK24092 to R.A.D., DK067690 to C.P.J., and HL086089 to S.M.R.), the UTHSCSA Executive Research Committee (to N.M.), the South Texas Health Research Center (to N.M.), a Nathan Shock Center Pilot Grant (to N.M.), the U.S Department of Veterans Affairs (to R.A.D. and C.P.J.), the American Heart Association (to D.K.C.), the Faculty of Medicine Siriraj Hospital Mahidol University of Thailand (to A.S.), the Endocrine Fellows Foundation (to A.S.), and the Thai Ministry of Public Health (to P.T.). S.G. is supported by grant T32 HL007446.
We thank all the volunteers who participated in the study.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 July 2008.
S.M.R. and S.G. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Shulman GI: Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G: Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11:90–94, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M: Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422:173–176, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Hwang D: Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through toll-like receptor 4-derived signaling pathways. FASEB J 15:2556–2564, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI: Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108:437–446, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ, Musi N: Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes 55:760–767, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE: Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293:1673–1677, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R: Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Raetz CR: Biochemistry of endotoxins. Annu Rev Biochem 59:129–170, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH: Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem 279:16971–16979, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Zuany-Amorim C, Hastewell J, Walker C: Toll-like receptors as potential therapeutic targets for multiple diseases. Nat Rev Drug Discov 1:797–807, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Libermann TA, Baltimore D: Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10:2327–2334, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiningham KK, Xu Y, Daosukho C, Popova B, St Clair DK: Nuclear factor kappaB-dependent mechanisms coordinate the synergistic effect of PMA and cytokines on the induction of superoxide dismutase 2. Biochem J 353:147–156, 2001 [PMC free article] [PubMed] [Google Scholar]
- 14.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS: TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song MJ, Kim KH, Yoon JM, Kim JB: Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 346:739–745, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ: Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56:1986–1998, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Radin MS, Sinha S, Bhatt BA, Dedousis N, O'Doherty RM: Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia 51:336–346, 2008 [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J: Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 68:1468–1474, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy JC, Matthews DR, Hermans MP: Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21:2191–2192, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ: Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 105:311–320, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry RR, Abrams L, Nikoulina S, Ciaraldi TP: Insulin action and glucose metabolism in nondiabetic control and NIDDM subjects: comparison using human skeletal muscle cell cultures. Diabetes 44:936–946, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Mott DM, Stone K, Gessel MC, Bunt JC, Bogardus C: Palmitate action to inhibit glycogen synthase and stimulate protein phosphatase 2A increases with risk factors for type 2 diabetes. Am J Physiol Endocrinol Metab 294:E444–E450, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha S, Perdomo G, Brown NF, O'Doherty RM: Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem 279:41294–41301, 2004 [DOI] [PubMed] [Google Scholar]
- 24.MacRedmond R, Greene C, Taggart CC, McElvaney N, O'Neill S: Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir Res 6:116, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ: Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest 113:1473–1481, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladner KJ, Caligiuri MA, Guttridge DC: Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem 278:2294–2303, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A: Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20:3364–3375, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Baldwin AS: Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest 107:241–246, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, Thorell A, Goodyear LJ: AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50:921–927, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Mercurio F, Manning AM: NF-kappaB as a primary regulator of the stress response. Oncogene 18:6163–6171, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Ogawa T, Asai Y, Hashimoto M, Takeuchi O, Kurita T, Yoshikai Y, Miyake K, Akira S: Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int Immunol 14:1325–1332, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Reaven GM, Chen YD: Role of abnormal free fatty acid metabolism in the development of non-insulin-dependent diabetes mellitus. Am J Med 85:106–112, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M: Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol 166:2018–2024, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Senn JJ: Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281:26865–26875, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM: A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282:35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M: Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 117:1658–1669, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]