Abstract
OBJECTIVE—It has recently been proposed that the peripheral taste organ is one of the targets for leptin. In lean mice, leptin selectively suppresses gustatory neural and behavioral responses to sweet compounds without affecting responses to other taste stimuli, whereas obese diabetic db/db mice with defects in leptin receptor lack this leptin suppression on sweet taste. Here, we further examined potential links between leptin and sweet taste in humans.
RESEARCH DESIGN AND METHODS—A total of 91 nonobese subjects were used to determine recognition thresholds using a standard stair-case methodology for various taste stimuli. Plasma leptin levels were determined by an enzyme-linked immunosorbent assay at several timepoints during the day under normal and restricted-meal conditions.
RESULTS—The recognition thresholds for sweet compounds exhibited a diurnal variation from 0800 to 2200 h that parallels variation for leptin levels, with the lowest thresholds in the morning and the highest thresholds at night. This diurnal variation is sweet-taste selective—it was not observed in thresholds for other taste stimuli (NaCl, citric acid, quinine, and mono-sodium glutamate). The diurnal variation for sweet thresholds in the normal feeding condition (three meals) was independent of meal timing and thereby blood glucose levels. Furthermore, when leptin levels were phase-shifted following imposition of one or two meals per day, the diurnal variation of thresholds for sweet taste shifted in parallel.
CONCLUSIONS—This synchronization of diurnal variation in leptin levels and sweet taste recognition thresholds suggests a mechanistic connection between these two variables in humans.
Leptin is a hormone primarily produced in adipose cells; it regulates food intake, energy expenditure, and body weight mainly via activation of the hypothalamic functional leptin receptor (Ob-Rb) (1–3). Recently, we found that the peripheral taste organ is also a target for leptin, where it acts on taste receptor cells via Ob-Rb expressed in these cells and specifically inhibits gustatory responses to sweet substances without affecting responses to sour, salty, and bitter substances in lean mice. Such selective sweet response inhibition by leptin was not observed in leptin receptor–deficient db/db mice (4–6), suggesting that leptin may be a sweet-sensation modulator involved in the regulation of food intake.
It has been shown in both rats and humans that there is a diurnal pattern in circulating leptin levels (7–10). In humans, leptin levels start rising before noon and peak between 2300 and 0100 h, after which the levels decline until morning (10,11). This diurnal pattern can be phase-shifted when meals are shifted (12,13). If leptin acts as a modulator for sweet taste sensitivity, and it shows diurnal variation, then it follows that the threshold for sweet taste may show correlated diurnal variation. We examined this possibility by measuring recognition thresholds for various taste stimuli and plasma leptin levels at several timepoints during the day in normal-weight adults. Because we found a positive correlation, we next sought to determine the tightness of this relationship. To accomplish this, we investigated whether phase shifts in leptin secretion induced by meal restriction would be followed by parallel changes in variation in sweet taste recognition thresholds.
RESEARCH DESIGN AND METHODS
A total of 91 healthy, nonobese, nondiabetic subjects (37 male/54 female, aged 21–30 years, BMI 16.7–24.8 kg/m2) participated in this study. The inclusion criteria were satisfactory state of oral hygiene, nonsmoking, regular work, sleep, and meal schedules. The purpose of the study as well as the methods and procedure were explained to the participants, and their informed consent was received. The study was performed in three steps according to feeding conditions, as follows. All protocols were approved by the institutional review board at Kyushu University. In all protocols, female subjects were tested between 3–16 days after the end of the menstrual phase in the menstrual cycle. All subjects were asked not to eat or drink anything other than water after 2200 h the evening before the experiment and to abstain from snacks and tooth paste during the day of experiment.
Protocol 1: three meals (normal meal condition).
Forty-seven healthy subjects (21 male/26 female, aged 21–30 years, BMI 16.7–24.0 kg/m2) participated in this experiment. A blood sample was taken 30 min before a meal, with taste recognition thresholds measured immediately thereafter. Blood samplings and measurement of taste recognition thresholds were repeated 1 h after the meal and at 2200 h. Blood samplings and measurement of taste recognition thresholds were identical in all three protocols. Meal times were as follows: breakfast 0830, lunch 1230, and dinner 1730. The caloric values for each meal were 400, 800, and 1,000 kcal for breakfast, lunch, and dinner, respectively.
Protocol 2: two meals (no breakfast).
Sixteen healthy subjects (3 male/13 female, aged 21–29 years, BMI 17.6–24.8 kg/m2) participated in this experiment. Three male and 12 female subjects also participated in the protocol 1 experiment. Thus, only one novel subject was tested with protocol 2. The subjects received only lunch at 1230 h and dinner at 1730 h the day of experiment.
Protocol 3: one meal (no breakfast, no lunch).
Twenty-four healthy subjects (9 male/15 female, aged 21–30 years, BMI 16.7–24.8 kg/m2) participated in this experiment. Three male and 11 female subjects also participated in protocols 1 and 2. Thus, 14 subjects were tested with all three protocols. Six male and three female subjects also participated in protocol 1 but not protocol 2. The subjects received only dinner at 1730 h the day of experiment.
Taste threshold measurement.
Recognition thresholds were measured for sweet, salty, sour, bitter, and umami taste qualities using different concentrations of sucrose (0.001–0.1 mol/l), glucose (0.001–0.56 mol/l), saccharin Na (0.001–1.0 mmol/l), NaCl (0.001–1.0 mol/l), citric acid (0.01–10.0 mmol/l), quinine HCl (QHCl) (0.0001–0.1 mmol/l), and monosodium glutamate (MSG) (0.1–100.0 mmol/l). The differences in concentrations in the present study were in 0.25 log steps. All solutions were made in distilled water and used at room temperature. Subjects were also taste tested with 1.0 mmol/l phenylthiocarbamide (PTC) and were classified into PTC tasters and nontasters, as previously described (14).
The testing procedure was the staircase method (modified from Pasquet et al. [15]). Each subject evaluated all seven tastants. The seven series of solutions were presented one after another in random order (see online appendix Methods, available at http://dx.doi.org/10.2337/db07-1103).
Assay.
Blood samples were drawn for determination of plasma leptin, insulin, and glucose. After blood samples were centrifuged at 4°C (3,000 rpm for 15 min), plasma samples were collected and stored at −80°C. Plasma leptin and insulin concentrations were determined using an ELISA kit (R&D Systems) and a sensitive ELISA method (LUMIPULSE, Fujirebio), respectively. All samples were within the linear detection range and analyzed in duplicate. Blood glucose levels were determined by the glucose dehydrogenase method (ACCU-CHEK; Roche Diagnostics).
Data analysis.
Leptin, blood glucose, and insulin levels and taste recognition thresholds, expressed as means ± SE, were used for statistical analyses. To evaluate circadian variation, only taste recognition thresholds were log transformed. Then, the values from 0800 to 2200 h were expressed as percent changes from 0800 h values (control = 100%). Statistical significance was determined by unpaired t tests. To evaluate the variation, a single factor and repeated-factor ANOVA were performed.
RESULTS
At 0800 h after overnight fasting, plasma leptin levels were significantly higher (P < 0.001) in female (8.84 ± 0.62 ng/ml) compared with male (3.58 ± 0.50 ng/ml) subjects, whereas sex difference was not evident in taste recognition thresholds for any stimulus, for blood glucose and insulin, or for BMI (P > 0.05) (online appendix Table 1). Recognition thresholds for seven taste test stimuli did not significantly differ between PTC nontaster and tasters (online appendix Table 2).
Figure 1 shows mean relative plasma leptin, insulin, and blood glucose levels and taste recognition thresholds for various taste substances at seven timepoints from 0800 to 2200 h in the three meal condition (normal), the two meal condition (no breakfast), and the one meal condition (no breakfast and no lunch). For plasma insulin and blood glucose, there were meal-related changes with increases evident after each meal in the three different feeding conditions.
FIG. 1.
Mean plasma leptin, blood glucose, and plasma insulin levels (A) and recognition thresholds for seven taste stimuli (B) measured at seven different time points during the day from 0800 to 2200 h in normal meal condition (three meals, n = 47 [44 for saccharin]) and restricted meal conditions (two meals with no breakfast, n = 16; one meal with no breakfast and no lunch, n = 24). The value at each point is a percentage of the value at 0800 h (control = 100%).
In the normal feeding condition, leptin concentrations started rising before noon and peaked at night. This rise in leptin occurred later in the two and one meal conditions, resulting in a phase shift of diurnal variation, as shown previously (12,13). Statistical analyses shown in Table 1 indicate significant time-dependent changes in leptin levels in each of the three different feeding conditions (P < 0.01) and differences in diurnal variation among three feeding conditions (P < 0.001). With regard to taste recognition thresholds, similar to plasma leptin levels, significant time-dependent increases in thresholds for sucrose, glucose, and saccharin (P < 0.05) were observed in the normal meal condition. That is, subjects needed higher concentrations of these sweeteners to detect the stimulus quality when they were tested in the evening compared with in the morning. There was also a phase shift in the one and two meal conditions eliminating the time-dependent changes in sweetener recognition threshold (P > 0.05). Diurnal variations in sweetener thresholds were significantly different among three meal conditions (P < 0.05). Unlike recognition thresholds for sweeteners, no clear diurnal variation was evident in recognition thresholds for other taste stimuli (P > 0.05) except for NaCl (P < 0.01). In the case of NaCl, thresholds had a tendency to decrease after each meal, a pattern that looked quite different from those of leptin and sugar thresholds. No significant differences among three meal conditions were observed in thresholds for NaCl, citric acid, and QHCl (P > 0.05), although differences were significant for the MSG thresholds (P < 0.05).
TABLE 1.
Results of statistical analyses on time-dependent changes in leptin levels and recognition thresholds for seven taste stimuli in three different feeding conditions
| Two-way repeated-measures ANOVA |
One-way repeated-measures ANOVA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect of meal condition |
Meal condition − time change interaction |
Three meals |
Two meals |
One meal |
||||||
| F | P | F | P | F | P | F | P | F | P | |
| Plasma leptin | (2, 84) = 13.67 | <0.001 | (12, 502) = 16.20 | <0.001 | (6, 322) = 22.50 | <0.001 | (6, 105) = 21.97 | <0.001 | (6, 159) = 3.04 | <0.01 |
| Sucrose | (2, 84) = 3.45 | <0.05 | (12, 498) = 1.92 | <0.05 | (6, 319) = 7.63 | <0.001 | (6, 105) = 1.09 | NS | (6, 158) = 0.56 | NS |
| Glucose | (2, 84) = 7.57 | <0.001 | (12, 499) = 2.76 | <0.05 | (6, 320) = 3.12 | <0.01 | (6, 105) = 1.90 | NS | (6, 158) = 0.88 | NS |
| Saccharin | (2, 81) = 4.59 | <0.05 | (12, 481) = 2.21 | <0.05 | (6, 300) = 2.15 | <0.05 | (6, 100) = 0.44 | NS | (6, 157) = 0.49 | NS |
| NaCl | (2, 84) = 1.69 | NS | (12, 498) = 1.42 | NS | (6, 318) = 3.09 | <0.01 | (6, 105) = 0.40 | NS | (6, 159) = 0.35 | NS |
| Citric acid | (2, 84) = 0.06 | NS | (12, 496) = 0.40 | NS | (6, 315) = 1.10 | NS | (6, 105) = 0.83 | NS | (6, 160) = 0.24 | NS |
| QHCl | (2, 83) = 0.05 | NS | (12, 488) = 1.54 | NS | (6, 313) = 1.08 | NS | (6, 103) = 0.89 | NS | (6, 155) = 0.70 | NS |
| MSG | (2, 84) = 3.76 | <0.05 | (12, 500) = 2.01 | <0.05 | (6, 319) = 0.96 | NS | (6, 105) = 0.12 | NS | (6, 160) = 1.36 | NS |
NS, not significant.
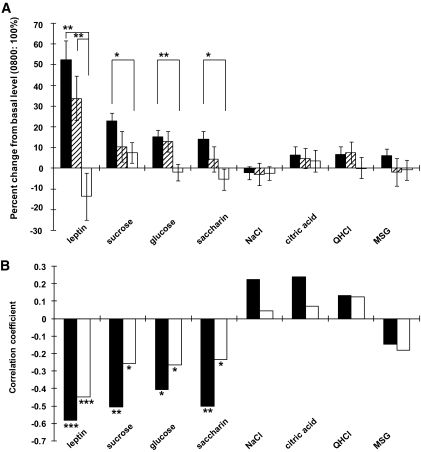
As shown in Table 2, there were significant increases in threshold values for sucrose, glucose, and saccharin as well as in leptin levels at 2200 h compared with the values at 0800 h (t tests, P < 0.05). This was not the case for the other taste stimuli (P > 0.05). Percent changes in thresholds (in log-transformed values) between the two time points (2200 vs. 0,800 h) in the normal feeding condition were ∼20% for sugars, which were significantly larger than those for other taste stimuli (t tests, P < 0.05) but smaller than that for leptin (∼50%, P < 0.01) (Fig. 2A). Skipping meals led to significant decreases in percent changes in leptin levels and recognition thresholds for sweeteners (P < 0.01–0.05). Increases in blood glucose and insulin levels of individuals after meals were negatively correlated with leptin levels and recognition thresholds for sweeteners but not for other tastes before meals (Fig. 2B). This suggests that leptin levels and sweet sensitivities before meals may influence postingestive increases in glucose and insulin levels.
TABLE 2.
Comparison between values at 0800 and 2200 h in normal feeding (three meals)
| 0800 h | 2200 h | P | |
|---|---|---|---|
| Leptin (ng/ml) | 6.43 ± 0.74 | 8.77 ± 0.87 | <0.001 |
| Female subjects | 8.83 ± 1.00 | 11.40 ± 1.00 | <0.001 |
| Male subjects | 3.47 ± 0.66 | 5.51 ± 1.16 | <0.001 |
| Taste recognition thresholds (mmol/l) | |||
| Sucrose | 23.0 ± 2.5 | 38.2 ± 4.0 | <0.001 |
| Glucose | 95.3 ± 7.7 | 156.9 ± 20.0 | <0.001 |
| Saccharin | 0.087 ± 0.011 | 0.15 ± 0.03 | <0.05 |
| NaCl | 24.8 ± 2.7 | 24.8 ± 4.0 | NS |
| Citric acid | 0.47 ± 0.06 | 0.57 ± 0.09 | NS |
| QHCl | 0.012 ± 0.001 | 0.013 ± 0.002 | NS |
| MSG | 4.2 ± 0.6 | 5.3 ± 0.8 | NS |
Data are means ± SE. n = 47 (21 male, 20 for saccharin; 26 female, 24 for saccharin). P < 0.05 and P < 0.001 by paired t test between values for male and female subjects. NS, not significant.
FIG. 2.
A: Percent changes in plasma leptin levels and taste recognition thresholds at 2200 h compared with basal values collected at 0800 h (control = 100%). Statistical analysis (t test) for differences in 2200 h values among meal conditions was done by using raw values. n = 47 subjects tested (44 for saccharin) for three meals (▪), n = 16 for two meals (▒), and n = 24 for one meal (□). **P < 0.01 (t test), *P < 0.05. B: Correlation coefficients between postingestive increases in blood glucose (□) or insulin (▪) levels after dinner (at 1900 h) versus leptin levels and recognition thresholds for sucrose, glucose, saccharin, NaCl, HCl, quinine, or MSG before dinner (at 1700 h) among individuals (n = 32–82). Correlation coefficients were obtained by using data for all subjects having a common dinner, thereby consuming the same calorie (1,000 kcal) and nutritional contents regardless of whether they had breakfast and/or lunch before dinner. ***P < 0.001 (t test), **P < 0.01, *P < 0.05.
DISCUSSION
The present study reveals that recognition thresholds for sweet substances are tightly linked with circulating leptin. The tight linkage between sweet taste thresholds and leptin levels was evident even in the restricted meal conditions with synchronized phase shifts of diurnal variations.
Previous studies in lean mice (4) demonstrated that exogenously applied leptin suppressed taste cells and nerve responses to sugars and non-nutritive saccharin. The strength of suppressive effects by leptin may be at most ∼30% of control responses, and the effect saturates when plasma leptin concentration reaches ∼15–20 ng/ml. In the present study, by using nonobese subjects with leptin levels <21 ng/ml, we found approximately a 20% increase in thresholds for sugars and saccharin (Fig. 2A), indicating consistency between human and mouse studies.
The circadian rhythm of taste sensitivity has not been fully investigated. One study indicated that recognition thresholds for salt taste and salivary sodium concentration exhibited similar circadian variations (16). With respect to sweet sensitivity, it has been shown in rats that the preference for high-sucrose pellets is most marked in the periods preceding lights-off (17), when leptin levels might be close to the lowest level of the day. To our knowledge, this report will be the first showing circadian variation in sweet taste sensitivity in humans.
A previous study in rats (18) showed that hyperglycemia was associated with decreased neural responses in the hindbrain and perceived taste intensity of glucose. Our present study and a previous one also in humans (19) revealed that taste recognition thresholds for sweet compounds were independent of changes in blood glucose levels. We have currently no persuasive explanation for this apparent species difference. Conversely, our data suggest that greater postingestive increases in blood glucose and insulin levels may be associated with lower leptin levels and higher sweet sensitivities before meals. With regard to this linkage, recent studies demonstrated that enteroendocrine cells in the gastrointestinal tract express sweet receptors (T1R2/T1R3) and leptin receptors and release glucagon-like peptide-1 in response to sugars and non-nutritive sweeteners, leading to an increase in expression of Na+/glucose cotransporter SGLT1, followed by increased glucose absorption in enterocytes (20,21). If enteroendocrine cells, like taste cells, possess a leptin modulatory system and comparable sweet sensitivities with diurnal variations and meal-related phase shifts, postingestive increases in glucose and insulin levels may be influenced by sweet sensitivities of both taste and gut cells. This possibility, together with potential links between sweet recognition thresholds and changes in BMI levels, must be clarified in future studies.
In summary, the present study shows that recognition thresholds for sweet compounds exhibit circadian variations in healthy human subjects. Furthermore, when leptin levels were phase-shifted following imposition of one or two meals per day, the diurnal variation of thresholds for sweet sugars shifted in parallel. This synchronization of diurnal variation in leptin levels and sweet taste recognition thresholds suggests a mechanistic connection between these two variables.
Supplementary Material
Acknowledgments
This work was supported by grant-in-aid 18077004, 18109013 (to Y.N.) for Scientific Research from the Japan Society for the Promotion of Science.
We thank Dr. Gary K. Beauchamp for valuable suggestions on the manuscript.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM: Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM: Abnormal splicing of the leptin receptor in diabetic mice. Nature 379:632–635, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP: Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y: Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A 97:11044–11049, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ninomiya Y, Shigemura N, Yasumatsu K, Ohta R, Sugimoto K, Nakashima K, Lindemann B: Leptin and sweet taste. Vitam Horm 64:221–248, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y: Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology 145:839–843, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Saladin R, Vos DP, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J: Transient increase in obese gene expression after food intake or insulin administration. Nature 377:527–529, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS: Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF: Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest 97:1344–1347, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havel PJ, Townsend R, Chaump L, Teff K: High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes 48:334–341, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Sinha MK, Sturis J, Ohannesian J, Magosin S, Thomas S, Heiman ML, Polonsky KS, Caro JF: Ultradian oscillations of leptin secretion in humans. Biochem Biophys Res Commun 228:733–738, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Schoeller DA, Cella LK, Sinha MK, Caro JF: Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest 100:1882–1887, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boden G, Chen X, Mozzoli M, Ryan I: Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 81:3419–3423, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Okada Y, Miyamoto T, Fujiyama R: Distribution of non-tasters for phenylthiocarbamide and high sensitivity to quinine hydrochloride of the non-tasters in Japanese. Chem Senses 22:547–551, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Pasquet P, Monneuse MO, Simmen B, Marez A, Hladik CM: Relationship between taste thresholds and hunger under debate. Appetite 46:63–66, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Fujimura A, Kajiyama H, Tateishi T, Ebihara A: Circadian rhythm in recognition threshold of salt taste in healthy subjects. Am J Physiol 259:R931–R935, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Kant GJ, Bauman RA: Effects of chronic stress and time of day on preference for sucrose. Physiol Behav 54:499–502, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Giza BK, Scott TR: Blood glucose level affects perceived sweetness intensity in rats. Physiol Behav 41:459–464, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Teff KL, Petrova M, Havel PJ, Townsend RR: 48-h glucose infusion in humans: effect on hormonal responses, hunger and food intake. Physiol Behav 90:733–743, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang H, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim B, Zhou J, Kim H, Xu X, Chan S, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM: Gut-expressed gustducin and taste receptors regulate secretion of glucagons-like peptide-1. Proc Natl Acad Sci U S A 104:15069–15074, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP: T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotranspoter 1. Proc Natl Acad Sci U S A 104:15075–15081, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.