Abstract
OBJECTIVE—Zinc transporter eight (SLC30A8) is a major target of autoimmunity in human type 1A diabetes and is implicated in type 2 diabetes in genome-wide association studies. The type 2 diabetes nonsynonymous single nucleotide polymorphism (SNP) affecting aa325 lies within the region of highest ZnT8 autoantibody (ZnT8A) binding, prompting an investigation of its relationship to type 1 diabetes.
RESEARCH DESIGN AND METHODS—ZnT8A radioimmunoprecipitation assays were performed in 421 new-onset type 1 diabetic Caucasians using COOH-terminal constructs incorporating the known human aa325 variants (Trp, Arg, and Gln). Genotypes were determined by PCR-based SNP analysis.
RESULTS—Sera from 224 subjects (53%) were reactive to Arg325 probes, from 185 (44%) to Trp325probes, and from 142 (34%) to Gln325probes. Sixty subjects reacted only with Arg325 constructs, 31 with Trp325 only, and 1 with Gln325 only. The restriction to either Arg325 or Trp325 corresponded with inheritance of the respective C- or T-alleles. A strong gene dosage effect was also evident because both Arg- and Trp-restricted ZnT8As were less prevalent in heterozygous than homozygous individuals. The SLC30A8 SNP allele frequency (75% C and 25% T) varied little with age of type 1 diabetes onset or the presence of other autoantibodies.
CONCLUSIONS—The finding that diabetes autoimmunity can be defined by a single polymorphic residue has not previously been documented. It argues against ZnT8 autoimmunity arising from molecular mimicry and suggests a mechanistic link between the two major forms of diabetes. It has implications for antigen-based therapeutic interventions because the response to ZnT8 administration could be protective or immunogenic depending on an individual's genotype.
Human type 1A diabetes results from autoimmune destruction of pancreatic β-cells targeted at a restricted number of autoantigens, many of which show high β-cell specificity of expression (1). Susceptibility to the disease is associated with multiple genetic loci, most prominently HLA alleles encoding particular major histocompatibility complex class II glycoproteins (2).
ZnT8 is a newly discovered target of type 1 diabetes autoimmunity (3) localized to the insulin granule of the pancreatic β-cell. It is encoded by SLC30A8, one of nine human genes for multispanning transmembrane proteins facilitating Zn2+ efflux from the cell and sequestration into intracellular compartments (4,5). Recent genome-wide association studies demonstrate association of ZnT8 gene polymorphisms with human type 2 diabetes (6–9), notably a nonsynonymous SNP encoding either Arg or Trp at aa325. The major, Arg325-encoding C-allele confers a minor risk (odds ratio 1.07–1.18) of disease. In nondiabetic subjects with a family history of type 2 diabetes, the C-allele was associated with increased insulin sensitivity (10), increased circulating proinsulin-to-insulin ratio (11), and decreased insulin responses in intravenous glucose tolerance tests (12), indicating a dominant effect on insulin secretion, β-cell mass, or both.
We report here that the type 1 diabetes autoimmune response to ZnT8 is focused on a few key epitopes, two of which are defined by the polymorphic aa325 residue. To our knowledge, this is the first reported instance where a polymorphic variant determines the specificity of the autoimmune response. It indicates that the autoreactive B-lymphocyte repertoire is restricted to a few ZnT8 epitopes and is truly self-reactive as opposed to arising as a bystander response to a foreign antigen.
RESEARCH DESIGN AND METHODS
Serum samples (n = 421) were obtained within 2 weeks of type 1 diabetes diagnosis from patients attending the Barbara Davis Center (median age 11.3 years [range 0.6–58]), 87% Caucasian, and 6.3% Hispanic). The 150 control subjects (median age 13.1 years [1–55]), 72% Caucasian, and 15.1% Hispanic) were parents and children in the Diabetes Autoimmunity Study in the Young (DAISY) general population cohort and parents of the sibling/offspring cohort (13). The male-to-female sex ratio in both groups was 0.8. Informed consent was obtained under approved institutional review board oversight.
Genomic DNA was extracted from heparinized blood from 352 of the above type 1 diabetes patients using standard procedures. Polymorphic variations in the SLC30A8 gene were determined by qPCR using Taqman probes and an ABI7000 (ABI, Waltham, MA) targeting the nonsynonymous SNPs rs13266634, rs2466295 in the 3′ untranslated region, and rs6469675 in intron 2. Ascertainment rates were >99%.
ZnT8 autoantibody (ZnT8A) radioimmunoprecipitation assays used 35S-Met–labeled in vitro transcribed and translated probes of hZnT8 COOH-terminal cytosolic segments (aa268–369) encoding the aa325 codon variants CCG (Arg), TCG (Trp), and CAG (Gln) (supplementary Fig. 1, available in an online appendix at http://dx.doi.org/10.2337/db08-0522). Assay procedures have previously been described (3,14). ZnT8A assay data were normalized to a panreactive positive control sera (1:50) generated in rabbits to a glutathione-S-transferase/C-term Trp325 fusion protein and 16 human control sera in the same assay (3). Recombinant NUS-ZnT8 fusion proteins were generated in pET43.1 (EMD Biosciences, San Diego, CA), expressed in BL-21(DE3) Escherichia coli, and purified by Ni-NTA agarose chromatography (Qiagen, Hilden, Germany). Synthetic 20-mer peptides were from Sigma Genosys (Woodlands, TX). For preabsorption, sera (5 μl) were preincubated with 10 μg protein or peptide in 40 μl PBS at 20°C for 2 h before addition of the radiolabeled antigen to initiate the assay. Results are expressed as means ± SD; statistical analyses were performed with the Prism 4.0 software package (www.graphpad.com).
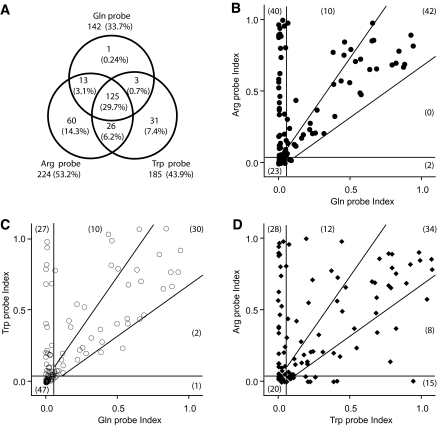
FIG. 1.
Relationship between autoantibody responses to Arg325, Trp325, and Gln325 constructs. A: Venn diagram illustrating the overlap of antibody detection with each of the polymorphic probes in the entire population (n = 421) in the study. The prevalence in each sector is expressed as a percentage of the population total. B–D: Assays were performed on the same set of 117 new-onset type 1 diabetic individuals and stratified as indicated; the numbers in each sector are shown in parentheses. The cutoff for positive responses was set at 0.05 (vertical and horizontal lines). Responses judged to be equivalent are set by the boundaries indicated by the angled lines, which correspond to a 3-SD excursion from the diagonal, assuming an intrassay coefficient of variation of 12.5% for each sample. Data are expressed as the immunoprecipitation index (sample-control)/(positive sample [BUNE]-control).
RESULTS
The present study was initiated to resolve a paradoxical finding that two constructs bearing the COOH-terminal antigenic region of ZnT8 with or without the NH2-terminus (supplementary Fig. 1) were recognized in a differential fashion by subsets of type 1 diabetes new-onset sera (3). The constructs were derived from different cDNAs and subsequently shown to encode the Arg (C-probe) or Trp (NC-probe) variants of aa325. To further explore this phenomenon, assays were performed in sera from newly diagnosed type 1 diabetic patients using COOH-terminal ZnT8 probes bearing Arg, Trp, or Gln at aa325: 259 of 421 individuals (61.5%) reacted to at least one probe, with the highest response recorded in reaction to the Arg variant (53.2%) followed by Trp (43.9%) and Gln (33.7%) (P < 0.0001, χ2). Analysis of the overlap in responses (Fig. 1A) shows that some individuals react to the Arg or Trp probes alone and very rarely to Gln alone; 29.7% of individuals reacted to all three probes.
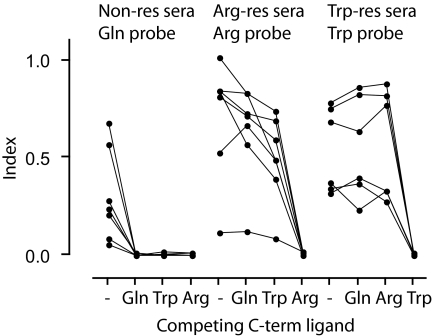
A comparison of the levels of autoantibody reactivity to the Arg and Gln probes (Fig. 1B) showed that the majority of individuals either reacted equivalently to the probes (falling within the bounds of the diagonal of the x-y plot ± 3 SD) or responded to the Arg probe alone. Trp and Gln reactivities (Fig. 1C) were similarly separated. Of the 34 patients who reacted equivalently to Arg and Trp probes (within the bounds of the diagonal ± 3 SD of Fig. 1D), 29 (85.3%) had an equivalent response as determined by the Gln probe. This indicated that for these individuals, the aa at position 325 was not a determinant of autoantibody reactivity. A series of preabsorption experiments was therefore performed using peptides and recombinant proteins as competing ligands (Fig. 2). Selected type 1 diabetes sera that reacted with the Arg probe alone were blocked by recombinant NUS-C-term Arg325 protein but not by NUS-C-term Trp325 or NUS-C-term Gln325. Similarly, Trp-only responses were blocked by NUS-C-term Trp325 but not NUS-C-term Arg325 or NUS-C-term Gln325. Sera that reacted equivalently to Arg, Trp, and Gln probes were blocked by any of the NUS-C-term ZnT8 recombinants. Overlapping 20-mer peptides spanning the ZnT8 COOH-terminal from 268–369 did not compete for reactivity, suggesting that the epitopes were conformational rather than linear. Overall, these results suggest that ZnT8A reactivity could be accounted for by three classes of conformational epitopes: one for which Arg325 was an essential determinant, a second Trp325 restricted, and a third not affected by aa325.
FIG. 2.
Preabsorption of autoantibodies with recombinant proteins. Single sera samples were selected from hCArg-restricted sera, hCTrp-restricted sera, or samples that react equivalently with hCGln, hCArg, and hCTrp probes. Samples were preincubated without (-) or with 10 μg of the indicated affinity-purified NUS-C-term ZnT8 fusion protein for 2 h at room temperature before addition of the designated labeled probe and then processed by the usual procedure.
The relationship between ZnT8 autoantibody reactivity and genetic variation at the SLC30A8 locus was examined using the SNP (rs13266634) encoding the Arg/Trp325 variant and two adjacent noncoding SNPs identified in a type 2 diabetes genome-wide association study (6), rs2466295, located 259 bp distally in the 3′ UTR, and rs6469675, located 19635 bp proximally in intron 2. The minor allele frequency (MAF) for rs13266634 in our type 1 diabetic population of 0.266 (n = 351) approximated the reference frequency of 0.256 (n = 168) for Europeans in the NLM SNP database (http://www.ncbi.nlm.nih.gov/SNP/snpref.cgi?rs=13266634). The distribution of genotypes (55.3% CC, 36.2% CT, and 8.5% TT) was consistent with Hardy-Weinberg distribution (53.9, 39.0, and 7.1, respectively). Similar correlations were observed for the MAF for rs6469675 (0.285 vs. 0.220 in our study vs. the NLM SNP database, respectively) and rs2466295 (0.361 vs. 0.407).
The specificity of the ZnT8A response reflected the rs13266634 genotype (Table 1), with little or no association observed with the adjacent rs2466295 and rs6469675 SNPs. The ZnT8A response assessed by the Gln probe showed no significant variation with the rs13266634 genotype, whereas responses to the Arg probe were highest in CC homozygotes, lowest in TT homozygotes, and intermediate in the heterozygote group. Conversely, responses to the Trp probe were highest in TT homozygotes, lowest in CC, homozygotes, and intermediate in heterozygotes. An even stronger relationship with genotype was seen in the groups having only Arg325- and Trp325-restricted responses. Arg325-only responses were observed only in individuals bearing the rs13266634 C-allele, with a 4.2-fold higher frequency in homozygotes than heterozygotes. With one exception, all Trp325-restricted responses were associated with the rs13266634 T-allele, with a 10.2-fold higher frequency in homozygotes than heterozygotes. The single Gln325-restricted ZnT8A patient (Fig. 1A) was genotyped CC, confirmed by sequencing. The small number of sera positive for both Trp325 and Arg325 but not Gln325 probes were associated with heterozygote genotypes (11 of 13 cases) as expected (data not shown). The prevalence of insulin autoantibody, GAD antibody, or IA-2 antigen response did not change as a function of rs13266634 genotype.
TABLE 1.
Prevalence of autoantibody in relation to rs13266634 genotype
| XX | rs13266634 genotype |
||||
|---|---|---|---|---|---|
| CC | CT | TT | P | ||
| n | 351 | 194 | 127 | 30 | |
| Any probe+ | 220 (62.7) | 128 (66.0) | 70 (55.1) | 22 (73.3) | 0.07 |
| All probes+ | 107 (30.5) | 63 (32.5) | 37 (29.1) | 7 (23.3) | 0.55 |
| Gln probe | 125 (35.6) | 75 (38.7) | 42 (33.1) | 8 (26.7) | 0.33 |
| Arg probe | 196 (55.8) | 124 (63.9) | 63 (49.6) | 9 (30.0) | 0.0005 |
| Trp probe | 154 (43.9) | 71 (36.6) | 61 (48.0) | 22 (73.3) | 0.0004 |
| Gln only | 1 (0.3) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0.67 |
| Arg only | 52 (14.8) | 45 (23.2) | 7 (5.5) | 0 (0.0) | <0.0001 |
| Trp only | 18 (5.1) | 1 (0.5) | 5 (3.9) | 12 (40.0) | <0.0001 |
| Insulin | 171 (48.7) | 89 (45.9) | 68 (53.5) | 14 (46.7) | 0.39 |
| GAD65 | 204 (58.1) | 108 (55.7) | 82 (64.6) | 14 (46.7) | 0.12 |
| IA-2 | 254 (72.4) | 144 (74.2) | 88 (69.3) | 22 (73.3) | 0.62 |
Data are n (%) unless otherwise indicated. Serum from each type 1 diabetic subject was assayed with ZnT8 C-term probes incorporating Gln, Arg, or Trp at aa325 or insulin, GAD65, or IA-2. P values were calculated by a 3 × 2 Fisher exact test comparing the seropositivitity (index >0.02) to the number of subjects, stratified by rs13266634 genotypes. +, positive.
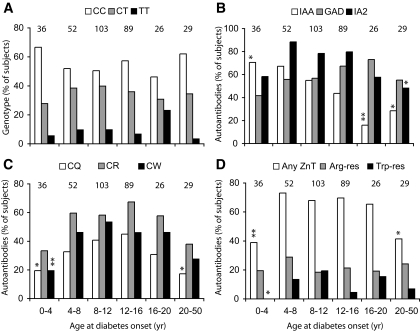
The median age of onset of disease in the genotyped individuals was 11.2 years (range 0.6–58), with more than half (57.3%) diagnosed between ages 8 and 16 years and 88.9% before age 18 years. A frequency distribution analysis based on binning at 4-year intervals showed no statistically significant difference in the representation of the CC, CT, and TT genotypes at any age of onset, though a trend was observed for a higher CC and lower CT genotype frequency in the youngest onset group (0.6–4 years) compared with that of a 5- to 15-year-old reference group (Fishers exact test, P = 0.24; n = 219) (Fig. 3A).
FIG. 3.
Prevalence of genotypes, autoantibodies to ZnT8, and other antigens in relation to age ofclinical diagnosis. Data from 335 individuals were subdivided in groups 4 years apart and analyzed with respect to distribution of genotypes (A); presence of insulin, GAD, and IA-2 autoantibodies (B); ZnT8 autoreactivity as defined by Gln, Arg, and Trp probes (C); and ZnT8 autoreactivity relative to isoepitope classification (D). Statistical significance relative to the 5- to 15-year-old reference group was determined by Fisher's exact test: *P < 0.05; **P < 0.01. IAA, insulin autoantibody.
The prevalence of ZnT8A measured with Gln325, Arg325, and Trp325 COOH-terminal probes increased with increasing age of onset, reached a plateau at 8–16 years, and then fell (Fig. 3C and D). Arg325- and Trp325-restricted responses were observed in all age-groups, but the low numbers of positive individuals did not permit ascertainment of changes in prevalence and levels of reactivity in association with age (Fig. 3D). The autoantibody responses to insulin, GAD65, and IA-2 showed characteristic changes in prevalence relative to age of onset of disease, insulin autoantibody prevalence being highest in younger onset patients, IA-2 antibody tending to be higher in adolescents than children, and GAD antibody showing little variation (Fig. 3B).
DISCUSSION
The COOH-terminal domain of ZnT8 to which type 1 diabetes autoantibodies bind (3) incorporates a conserved protein fold found in the large family of cation diffusion facilitator efflux carriers and has orthologs in all cellular organisms (15). Autoantibodies to ZnT8 in human type 1 diabetic patients, however, show little cross-reactivity to other human Zn transporters or even to mouse ZnT8, which is 82% identical in sequence. Even more remarkable, we show here that the amino acid encoded by a common polymorphism in human ZnT8 at aa325 is a key determinant of two of the three major conformational epitopes in the protein. Given that antibody responses in any individual are polyclonal and the structural variation in the antibody repertoire that occurs between genetically identical individuals, such epitope restriction is remarkable and, in light of this, are termed iso-epitopes. Polymorphic variants of other diabetes-related autoantigens exist, but to our knowledge none have been implicated as determinants of humoral autoreactivity, though clearly this bears further scrutiny.
The autoantibody responses to the ZnT8 Arg- and Trp-restricted isoepitopes segregated with the alleles encoding the respective variant amino acids, indicating that humoral type 1 diabetes autoimmunity to ZnT8 is directed against self and not nonself epitope determinants. This argues against the molecular mimicry hypothesis that suggests that autoimmunity is triggered by an initial immune response to a infectious agent that in turn triggers reactivity to self because of sequence homology between the pathological agent and a self protein (16–20). Our results favor the idea that ZnT8 autoreactivity arises because of a defect in induction of self-tolerance, since the mimicry model would more likely favor one isoepitope over another, which in turn would be manifest as genetic dissociation of the encoding allele with the disease. The MAF of the rs13266634 SNP in the type 1 diabetic population under study was, however, similar to reference populations, and no association of the SNP was seen with age of diabetes onset or the prevalence of antibodies to ZnT8 or other diabetes autoantigens. We cannot, however, preclude a role for molecular mimicry in T-cell recognition of antigenic peptides or in antigen presentation to CD4+ T-cells because antigen/antibody binding can directly influence the peptides presented from the antigen by virtue of altering intracellular proteolytic processing (21,22).
While the rs13266634 genotype or ZnT8 isoepitope specificites may not markedly affect type 1 diabetes susceptibility or age of onset, their measurement will be important in a number of clinical settings. Since ZnT8 autoantibodies provide an independent marker of disease susceptibility in pre-diabetic individuals (3), measurements based on a single aa325 probe would underestimate ZnT8 autoimmunity by as much as 20% given the differences in rs13266634 SNP allele frequency (12) and thus affect inclusion in clinical trials. Given its high tissue specificity, ZnT8 is an attractive candidate as a component of a DNA- or peptide-based vaccine (23,24) to prevent or retard the onset of type 1 diabetes. In this context, it is likely to be important to match the molecular form of the antigen to the recipient because mismatching the isoepitope might lead to immunization and acceleration of disease rather than induction of tolerance.
Supplementary Material
Acknowledgments
This study was supported by the Childhood Diabetes Foundation in Denver, the University of Colorado Health Sciences Diabetes and Endocrinology Research Center (National Institutes of Health [NIH] P30 DK57516), NIH R01 DK052068, and a Juvenile Diabetes Research Foundation Center grant. Y.L. and R.S. were recipients of American Diabetes Association (ADA) mentor-based postdoctoral awards, and K.F. received an ADA mentor-based minority postdoctoral award. We thanks Marian Rewers and Pam Fain for helpful discussion; Sunanda Babu, Randall Wong, and Erin Stewart for technical support; and the patients and families for their participation in this study.
Published ahead of print at http://diabetes.diabetesjournals.org on 30 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Lieberman SM, DiLorenzo TP: A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens 62:359–377, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alarcon-Riquelme ME: Recent advances in the genetics of autoimmune diseases. Ann N Y Acad Sci 1110:1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC: The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 104:17040–17045, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmiter RD, Huang L: Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch 447:744–751, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chimienti F, Devergnas S, Favier A, Seve M: Identification and cloning of a β-cell–specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53:2330–2337, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P: A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M: A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S: Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT: Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staiger H, Machicao F, Stefan N, Tschritter O, Thamer C, Kantartzis K, Schafer SA, Kirchhoff K, Fritsche A, Haring HU: Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS ONE 2:e832, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirchhoff K, Machicao F, Haupt A, Schafer SA, Tschritter O, Staiger H, Stefan N, Haring HU, Fritsche A: Polymorphisms in the TCF7L2, CDKAL1, and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 51:597–601, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Boesgaard TW, Zilinskaite J, Vanttinen M, Laakso M, Jansson PA, Hammarstedt A, Smith U, Stefan N, Fritsche A, Haring H, Hribal M, Sesti G, Zobel DP, Pedersen O, Hansen T: The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients: the EUGENE2 study. Diabetologia 51:816–820, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie RS Jr, Hamman RF, Klingensmith G, Eisenbarth GS, Erlich HA: Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 39:807–812, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Cuthbertson DD, Maclaren N, Jackson R, Palmer JP, Orban T, Eisenbarth GS, Krischer JP: Expression of GAD65 and islet cell antibody (ICA512) autoantibodies among cytoplasmic ICA+ relatives is associated with eligibility for the Diabetes Prevention Trial—Type 1. Diabetes 50:1735–1740, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Paulsen IT, Saier MH Jr.: A novel family of ubiquitous heavy metal ion transport proteins. J Membr Biol 156:99–103, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK: Environmental triggers and determinants of type 1 diabetes. Diabetes 54 (Suppl 2):S125–S136, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N: Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med 4:781–785, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Hiemstra HS, Schloot NC, van Veelen PA, Willemen SJ, Franken KL, van Rood JJ, de Vries RR, Chaudhuri A, Behan PO, Drijfhout JW, Roep BO: Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc Natl Acad Sci U S A 98:3988–3991, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osame K, Takahashi Y, Takasawa H, Watanabe S, Kishimoto M, Yasuda K, Kaburagi Y, Nakanishi K, Kajio H, Noda M: Rapid-onset type 1 diabetes associated with cytomegalovirus infection and islet autoantibody synthesis. Intern Med 46:873–877, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Honeyman MC, Brusic V, Stone NL, Harrison LC: Neural network-based prediction of candidate T-cell epitopes. Nat Biotechnol 16:966–969, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Davidson HW, Watts C: Epitope-directed processing of specific antigen by B lymphocytes. J Cell Biol 109:85–92, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts C, Antoniou A, Manoury B, Hewitt EW, McKay LM, Grayson L, Fairweather NF, Emsley P, Isaacs N, Simitsek PD: Modulation by epitope-specific antibodies of class II MHC-restricted presentation of the tetanus toxin antigen. Immunol Rev 164:11–16, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Haller MJ, Gottlieb PA, Schatz DA: Type 1 diabetes intervention trials 2007: where are we and where are we going? Curr Opin Endocrinol Diabetes Obes 14:283–287, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Skyler JS: Prediction and prevention of type 1 diabetes: progress, problems, and prospects. Clin Pharmacol Ther 81:768–771, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.