Abstract
OBJECTIVE—Visceral obesity and elevated plasma free fatty acids are predisposing factors for type 2 diabetes. Chronic exposure to these lipids is detrimental for pancreatic β-cells, resulting in reduced insulin content, defective insulin secretion, and apoptosis. We investigated the involvement in this phenomenon of microRNAs (miRNAs), a class of noncoding RNAs regulating gene expression by sequence-specific inhibition of mRNA translation.
RESEARCH DESIGN AND METHODS—We analyzed miRNA expression in insulin-secreting cell lines or pancreatic islets exposed to palmitate for 3 days and in islets from diabetic db/db mice. We studied the signaling pathways triggering the changes in miRNA expression and determined the impact of the miRNAs affected by palmitate on insulin secretion and apoptosis.
RESULTS—Prolonged exposure of the β-cell line MIN6B1 and pancreatic islets to palmitate causes a time- and dose-dependent increase of miR34a and miR146. Elevated levels of these miRNAs are also observed in islets of diabetic db/db mice. miR34a rise is linked to activation of p53 and results in sensitization to apoptosis and impaired nutrient-induced secretion. The latter effect is associated with inhibition of the expression of vesicle-associated membrane protein 2, a key player in β-cell exocytosis. Higher miR146 levels do not affect the capacity to release insulin but contribute to increased apoptosis. Treatment with oligonucleotides that block miR34a or miR146 activity partially protects palmitate-treated cells from apoptosis but is insufficient to restore normal secretion.
CONCLUSIONS—Our findings suggest that at least part of the detrimental effects of palmitate on β-cells is caused by alterations in the level of specific miRNAs.
Type 2 diabetes is the most frequent metabolic disorder and affects >150 million people worldwide. The disease results from a combination of unfavorable genetic and environmental factors that conspire to diminish sensitivity of insulin target tissues and insulin secretion from pancreatic β-cells. Under normal conditions, a feedback loop exists between insulin sensitivity and insulin secretion such that reduction in sensitivity of peripheral tissues is compensated by an increase in hormone release. Progression to hyperglycemia and diabetes occurs if the secretory activity of β-cells becomes inadequate to counterbalance insulin resistance (1,2). Visceral obesity and elevated plasma free fatty acid (FFA) concentrations are predisposing factors for the development of type 2 diabetes. Chronically elevated FFAs promote insulin resistance in target tissues and have detrimental effects on β-cells, resulting in reduction in insulin content, abnormally elevated insulin release in the absence of stimuli, diminished capacity to secrete insulin in response to glucose, and increased β-cell apoptosis (2,3). The combination of these adverse effects on β-cells causes failure to maintain blood glucose homeostasis and promotes the development of diabetes. The mechanisms underlying the negative impact of FFAs on insulin-secreting cells are still incompletely understood, but alterations in the expression of genes essential to accomplish specific β-cell tasks are believed to occur. We hypothesized that β-cell failure due to chronic exposure to FFAs could be linked to alterations in the level of microRNAs (miRNAs), a recently discovered class of small (∼22 nucleotides) noncoding RNAs that plays a key role in the development and function of mammalian cells (4). miRNAs act by pairing to sequences in the 3′-untranslated region (UTR) of target mRNAs and by inhibiting their translation (5). According to recent estimates, up to one-third of all human genes may be controlled by miRNAs. Although we are only beginning to appreciate the immense potential of miRNAs as controllers of gene networks, there is already substantial evidence indicating that these small noncoding RNA molecules play a central role in a variety of physiological processes, including tissue differentiation, cell proliferation, and apoptosis (4). Moreover, miRNAs have already been implicated in human diseases (6). Recent studies have highlighted a role for the miRNAs miR375, miR9, and miR124a in pancreatic islet development and in the execution of specialized β-cell functions, including insulin synthesis and insulin release in response to secretagogues (7–11).
In this study, we investigated the potential involvement of miRNAs in FFA-mediated β-cell dysfunction. We found that chronic exposure of β-cells to palmitate results in alterations in the expression of a specific set of miRNAs. Detailed analysis of the role of two of these miRNAs revealed that modification of their level has an important impact on different β-cell functions. Our data suggest that at least part of the harmful effects of FFAs on insulin-secreting cells may be mediated by alterations in the miRNA expression pattern.
RESEARCH DESIGN AND METHODS
Cell culture and FFA treatment.
The insulin-secreting cell line MIN6B1 (12) was cultured in 24- or 6-well dishes at a concentration of 1.3 × 105 cells/cm2 in Dulbecco's modified Eagle's medium (DMEM)–Glutamax medium supplemented with 15% FCS, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 70 μmol/l β-mercaptoethanol. Rat pancreatic islets were isolated by hand-picking after collagenase digestion of pancreas as described previously (13) and maintained in RPMI 1640 supplemented with 10% FCS, 10 mmol/l HEPES, pH 7.4, 1 mmol/l sodium pyruvate, 100 units/ml penicillin-streptomycin, 50 μmol/l β-mercaptoethanol, and 11 mmol/l glucose. Sodium palmitate, oleate, and linoleate (Sigma, St. Louis, MO) were dissolved in 33% ethanol at 33 mmol/l and added to the medium at the indicated concentration. Control cells were exposed to an equivalent concentration of ethanol. Pancreatic islets from 14- to 20-week-old wild-type and C57BL/KsJ db/db mice were isolated as previously described (14) and used immediately for RNA extraction.
RNA extraction, microarray, and quantitative RT-PCR assays.
Extraction of RNA was performed with the mirVana miRNA isolation kit (Ambion, Austin, TX) using the total RNA isolation option. For microarray profiling, total RNAs were sent to the miRNA Microarray Service of LC Sciences (Houston, TX). Conventional mRNA quantitative RT-PCR assays were carried out as previously described (15) using the insulin two (NM_008387) primers: SS, 5′-TGG CTT CTT CTA CAC ACC CA-3′, and AS, 5′-TCT AGT TGC AGT AGT TCT CCA-3′; the p53 primers: SS, 5′-GCA ACT ATG GCT TCC ACC TG-3′, and AS, AGC TTA TTG AGG GGA GGA GAG T; and, as control, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers: SS, 5′-TCC ATG ACA ACT TTG GCA TTG-3′, and AS, 5′-CAG TCT TCT GGG TGG CAG TGA-3′. Mature miRNA measurements were performed using the mirVana qRT-PCR miRNA Detection kit (Ambion) according to the manufacturer's instructions. U6 was chosen as control for small RNAs. All quantitative RT-PCR assays were carried out on a Bio-Rad MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories, Reinach, Switzerland).
miRNA overexpression and downregulation.
To increase the level of individual miRNAs, the cells were transfected with RNA duplexes containing the mature sequence of the miRNA (Dharmacon or Eurogentec). The sequences were the following: miR34a sense, UGGCAGUGUCUUAGCUGGUUGUU, and antisense, AACAACCAGCUAAGACACUGCCA; and miR146 sense, UGAGAACUGAAUUCCAUGGGUU, and antisense, AACCCAUGGAAUUCAGUUCUCA. Because assessing precisely the amount of miRNAs effectively entering the cells is difficult when using this approach, in a limited set of experiments, the level of miR34a and miR146 was increased by transfecting the cells with plasmids encoding the corresponding miRNA precursors (16). This technique led to an increase in the level of miR34a and miR146 of 10- to 30-fold and resulted in functional changes identical to those observed with RNA duplexes. Endogenous activity of miRNAs was blocked using Clear-MiR miRNA inhibitors (Eurogentec). Transient transfections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) using a concentration of 1.33 ng/μl plasmids and/or 60 pmol RNA duplexes. Cotransfection experiments were performed with a mass ratio of 1:10 plasmid:RNA duplex.
Luciferase reporter assays.
The luciferase reporter plasmid driven by the miR34a promoter (promo miR34a) and the corresponding construct in which the p53 binding site is mutated (promo miR34a mut) (17) were provided by Dr. M. Oren (Weizmann Institute, Rehovot, Israel). The p53 sensor plasmid bearing the Firefly luciferase cDNA under the control of p53-responsive elements and the plasmid encoding wild-type p53 have been described previously (18). To generate the 3′UTR–vesicle-associated membrane protein 2 (VAMP2) luciferase construct, the whole 3′UTR (1.4 kb) of the mouse VAMP2 gene (NM_009497) was amplified by PCR from genomic DNA and inserted in the psiCHECK-1 vector (Promega, Madison, WI) between the XhoI and EcoRI sites. The sequences of the primers were sense, 5′-CAC CCC TTC TCG AGG TTC CCA TC-3′, and antisense, 5′-GCG CCA CAG AAT TCG GGG CAT G-3′. The BclII 3′UTR construct was generated by inserting the nucleotides 179–211 of the 3′UTR of mouse BclII (BC095964) between the XhoI and EcoRI sites of psiCHECK-1. Luciferase activities were measured with a dual-luciferase reporter assay system (Promega). The Firefly luciferase activity was normalized for transfection efficiency with the SV40-driven Renilla activity generated by the psiCHECK-1 vector (Promega). The Renilla activity from the 3′UTR-VAMP2 plasmid was normalized with the Firefly activity of the SV40 PGL3 promoter plasmid (Promega).
Secretion assay.
For the assessment of hormone secretory capacity, MIN6B1 cells (2.5 × 105) plated in 24-well dishes were transiently cotransfected with RNA duplexes or antisense miR oligonucleotides and with a construct encoding the human growth hormone (hGH). Three days later, the cells were washed and preincubated for 30 min in Krebs buffer (127 mmol/l NaCl, 4.7 mmol/l KCl, 1 mmol/l CaCl2, 1.2 mmol/l KH2PO4, 1.2 mmol/l MgSO4, 5 mmol/l NaHCO3, 25 mmol/l HEPES, and 0.1% BSA) containing 2 mmol/l glucose. The medium was then discarded, and the cells were incubated during 45 min either in the same Krebs buffer (basal condition) or in a Krebs buffer containing 20 mmol/l glucose, 10 μmol/l forskolin, and 100 μmol/l isobutylmethylxanthine (IBMX) (stimulated condition). After collecting the supernatant, the cells were lysed in PBS containing 0.5% Triton X-100 to evaluate the remaining hGH content. The amount of hGH in the samples was assessed using an hGH ELISA kit (Roche Diagnostics, Rotkreuz, Switzerland).
Western blots.
For Western blot analysis, the cells were washed once in PBS, and total extracts were obtained by lysing the cells by brief sonication. Thirty micrograms protein was separated on acrylamide gels and transferred on polyvinylidine fluoride membranes. The membranes were incubated overnight at 4°C with the primary antibodies. Immunoreactive bands were visualized using a chemiluminescent substrate (Amersham Biosciences) after incubation with a secondary horseradish peroxidase antibody. The antibodies against VAMP2, Rab3A, and Rab27A were purchased from Synaptic Systems (Goettingen, Germany). The antibodies against Syntaxin-1A and SNAP25 were from Sigma and BD Transduction Laboratories (Lexington, KY), respectively. The antibody against Noc2 (19) was provided by Dr. M. Fukuda (Tohoku University, Sendai, Japan). The antibody against Granuphilin was described previously (20). Actin antibody was from Chemicon International (Temecula, CA).
Apoptosis assay.
For the assessment of the apoptotic activity, MIN6B1 cells (2.5 × 105) plated in 24-well dishes were transiently cotransfected with oligonucleotides. The apoptotic activity of the cells was evaluated after 3 days by scoring the cells displaying pycnotic nuclei (visualized with Hoechst 33342 dye).
Statistical analysis.
The data were tested by ANOVA for statistical differences. The experiments including more than two groups were first analyzed by ANOVA. Multiple comparisons of the means were then carried out using the post hoc Bonferroni (Dunn) test with a P value discriminating limit of 0.05.
RESULTS
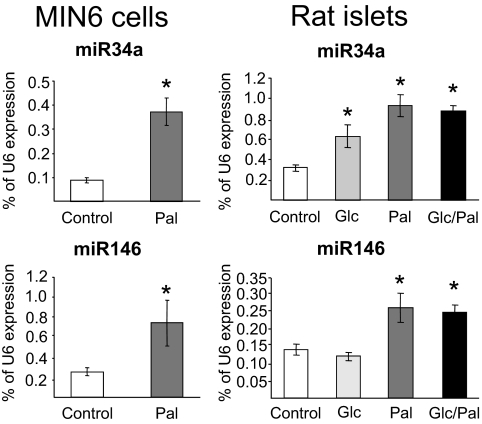
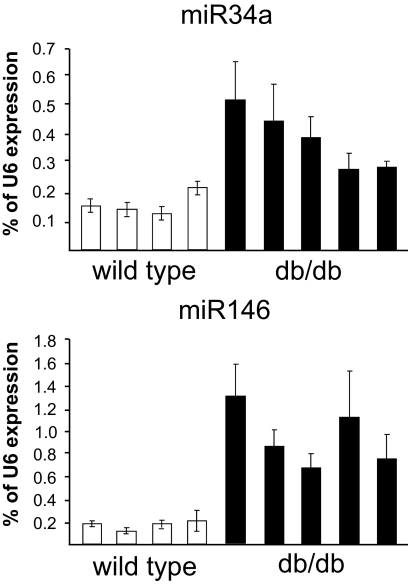
Prolonged exposure to FFAs has deleterious impacts on pancreatic β-cell function, including alterations in insulin secretion and sensitization toward apoptosis (2,3). Three-day exposure to palmitate resulted in analogous defects in the well-differentiated mouse insulin-secreting cell line MIN6B1 (12) (see below). miRNAs are newly discovered gene regulators that have been shown to control insulin secretion (7,9–11). These noncoding RNAs have also been demonstrated to regulate apoptosis in different cell systems (21). We therefore investigated whether changes in the level of miRNAs can contribute to the effects of FFAs. For this purpose, we initially compared the global miRNA expression profile of MIN6B1 cells cultured in the presence or absence of 1 mmol/l palmitate for 3 days. Microarray analysis permitted the detection of 132 miRNAs expressed in MIN6B1 cells (supplemental Table 1, which is available in an online appendix at http://dx.doi.org 10.2337/db07-1252). With prolonged incubation with palmitate, we observed no significant changes in the level of most miRNAs, including miR375, miR9, and miR124a, the three miRNAs previously involved in the regulation of pancreatic β-cell functions (7,9–11) (supplemental Table 1). However, after chronic exposure to palmitate, the level of a small group of miRNAs appeared to be altered. The expression of this subset of miRNAs, including miR34a, miR96, miR145, miR146, miR195, and miR210, was analyzed in more detail by quantitative RT-PCR in a large series of samples obtained from MIN6B1 cells and freshly isolated rat pancreatic islets. The relatively small variations in the levels of miR96, miR145, and miR195 detected in the microarray profiling could not be replicated in all samples (supplementary Fig. 1), whereas the decrease in miR210 expression was confirmed in MIN6B1 cells but could not be reproduced in rat pancreatic islets (supplementary Fig. 2). In view of these findings, these miRNAs were not further analyzed, and we focused on miR34a and miR146, which are increased in MIN6B1 cells and in rat pancreatic islets (Fig. 1). Exposure of pancreatic islets to elevated glucose concentrations have been shown to exacerbate the effect of FFAs (22). Incubations of rat pancreatic islets in the presence of 25 mmol/l glucose resulted in a small increase of miR34a but did not potentiate the effect of palmitate (Fig. 1). The presence of supraphysiological concentrations of glucose did not modify the expression of miR146. The relevance of our in vitro observations was verified by measuring the expression of these two miRNAs in pancreatic islets from a mouse model of type 2 diabetes. Elevated plasma FFA concentrations, β-cell dysfunction, and diabetes have been displayed in 14- to 20-week-old db/db obese mice (14). We found that in pancreatic islets from diabetic db/db mice, the levels of miR34a and miR146 were significantly higher compared with age-matched wild-type mice (Fig. 2), whereas the level of miR124a was unchanged (not shown).
FIG. 1.
Effect of palmitate on miR34a and miR146 expression. MIN6B1 cells (left) were cultured for 72 h in normal DMEM (25 mmol/l glucose concentration; Control) or in DMEM supplemented with 1 mmol/l palmitate (Pal). Freshly isolated rat pancreatic islets (right) were cultured for 72 h in normal RPMI 1640 (11 mmol/l glucose concentration; Control), in RPMI 1640 containing 25 mmol/l glucose (Glc), in RPMI 1640 containing 1 mmol/l palmitate (Pal), or in RPMI 1640 containing 25 mmol/l glucose and 1 mmol/l palmitate (Glc/Pal). The expression of miR34a and miR146 was assessed by quantitative RT-PCR. The level of U6 measured in parallel in the same samples was used to normalize the data. The asterisks indicate the conditions significantly different (P < 0.05) from control.
FIG. 2.
miR34a and miR146 expression is increased in pancreatic islets of db/db mice. Pancreatic islets were isolated from four wild-type mice and five diabetic db/db mice. Blood glucose level was 6.8 ± 0.4 mmol/l in control animals and 21.8 ± 1.4 mmol/l in db/db mice (P < 0.001). RNA extracts from each islet preparation were analyzed by quantitative RT-PCR for the expression of the indicated miRNAs. Data are expressed as percent U6 content measured in the same sample.
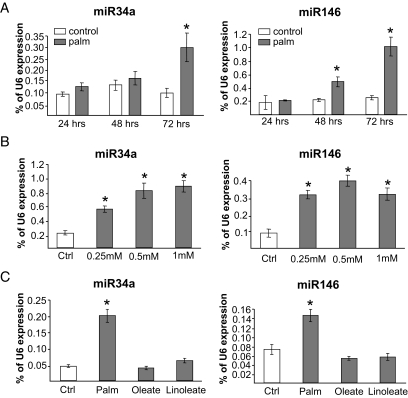
In MIN6B1 cells, differences in miR34a and miR146 expression were already seen after 24–48 h of culture in the presence of palmitate but were more pronounced after 72 h, consistent with a potential involvement in long-term effects of the fatty acid (Fig. 3A). Incubation for 72 h with different doses of palmitate revealed that the effect of the lipid was already maximal at concentrations between 0.25 and 0.5 mmol/l (Fig. 3B). Treatment of the cells with the unsaturated FFAs oleate and linoleate did not significantly affect the expression of miR34a and miR146 (Fig. 3C).
FIG. 3.
miR34a and miR146 expression is induced in a time- and dose-dependent manner by palmitate but not by unsaturated FFAs. A: MIN6B1 cells were cultured for the indicated time in normal medium (control) or in the presence of 1 mmol/l palmitate (palm). After RNA extraction, miR34a and miR146 levels were determined by quantitative RT-PCR. Data are expressed as percent U6 content measured in the same samples. *Conditions significantly different (P < 0.05, n = 3) from control. B: The cells were incubated for 72 h in normal DMEM (control) or in DMEM containing the indicated concentrations of palmitate. miR34a, miR146, and U6 levels were determined by quantitative RT-PCR. The results are expressed as percent U6 content in each sample. *Conditions significantly different (P < 0.05, n = 3) from control. C: The cells were incubated for 72 h in normal medium (control) or in the presence of 0.5 mmol/l palmitate (Palm), 0.5 mmol/l oleate, or 0.5 mmol/l linoleate. miR34a and miR146 levels were determined by quantitative RT-PCR and normalized to the U6 content. *Conditions significantly different (P < 0.05, n = 3) from control.
To evaluate the possible contribution of miR34a and miR146 to palmitate-mediated β-cell dysfunction, we either mimicked the changes elicited by the fatty acid by overexpressing the miRNAs or inhibited their function with modified antisense oligonucleotides (23) that can efficiently reduce the level of each miRNA (supplementary Fig. 3).
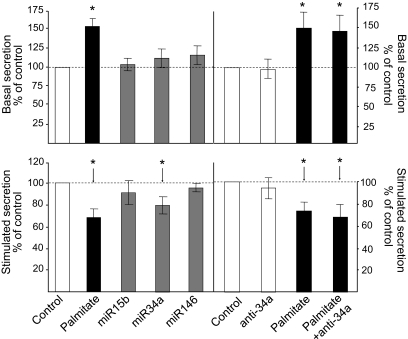
We first assessed the impact of the three miRNAs on the secretory properties of MIN6B1 cells. For this purpose, the cells were transfected with a plasmid leading to constitutive expression of the hGH. When introduced in β-cells, hGH is packaged in dense-core granules and is coreleased with insulin, enabling us to monitor exocytosis from transiently transfected insulin-secreting cells independently from their capacity to produce insulin (24). As shown in Fig. 4, 3-day exposure to 1 mmol/l palmitate led to the release of an exaggerated amount of hormone under basal conditions (2 mmol/l glucose) and to a reduced capacity of the cells to respond to stimulatory concentrations of 20 mmol/l glucose and cAMP-raising agents (10 μmol/l forskolin and 100 μmol/l IBMX). Overexpression of miR146 or miR15b, a miRNA whose expression is not affected by palmitate (supplemental Table 1), did not affect the secretory properties of MIN6B1 cells (Fig. 4, left). Blockade of miR146 function with an antisense oligonucleotide was also without effect (not shown). Overexpression of miR34a resulted in a significant reduction in the amount of hormone released under stimulatory conditions without modification of the secretion rate at low glucose (Fig. 4, left). Antisense miR34a treatment did not interfere with hormone secretion under basal or stimulated conditions (Fig. 4, right). Blockade of miR34a function was not sufficient to rescue a normal secretory response in cells treated with palmitate (Fig. 4, right).
FIG. 4.
Effect of miR34a and miR146 on hormone secretion. The mouse insulin-secreting cell line MIN6B1 was transiently transfected with a plasmid encoding hGH and with control RNA duplexes (control, palmitate); with RNA duplexes with the mature sequence of miR15b, miR34a, or minR146; or with antisense miR34a (anti-miR34a). The cells were cultured for 3 days in normal DMEM (□ and □) or with DMEM containing 1 mmol/l palmitate (▪). hGH secretion under basal conditions (top) and in the presence of stimulatory concentrations of glucose and cAMP-raising agents (bottom) was measured by ELISA. The figure shows the results of three to five independent experiments performed in triplicate. *Conditions significantly different from controls (P < 0.05).
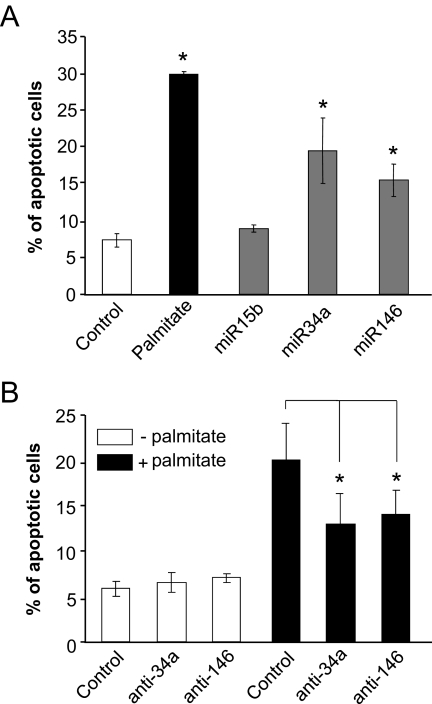
FFAs are known to sensitize β-cells to apoptosis (3). Under our experimental conditions, incubation of MIN6B1 cells with palmitate increased the number of apoptotic cells by about fourfold (Fig. 5A). A rise in miR34a or miR146 expression significantly augmented the fraction of cells undergoing apoptosis, suggesting that at least part of the effect of palmitate may be mediated through changes in the level of these miRNAs (Fig. 5A). Inhibition of miR34a or miR146 activity was found to partially protect palmitate-treated cells against apoptosis (Fig. 5B).
FIG. 5.
Effect of miR34a and miR146 on apoptosis. A: MIN6B1 cells were transfected with a control RNA duplex or RNA duplexes containing miR15b, miR34a, or miR146. The cells were cultured for 3 days in normal DMEM (□ and □) or with DMEM containing 1 mmol/l palmitate (▪). The number of cells displaying apoptotic nuclei was scored and divided by the total number of cells analyzed. Data are means ± SE of three independent experiments. *Conditions significantly different (P < 0.05, n = 3) from control. B: MIN6B1 cells were transfected with control oligonucleotides, anti-miR34a (anti-34a), or anti-miR146 (anti-146). The cells were then cultured for 3 days in normal DMEM (□) or in DMEM supplemented with 1 mmol/l palmitate (▪). The number of cells displaying apoptotic nuclei was scored and divided by the total number of cells analyzed. Data are means ± SE of four to five independent experiments. *Conditions that are significantly different (P < 0.05).
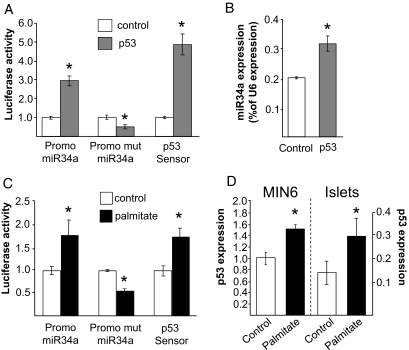
We then investigated in more detail the mechanisms responsible for the induction and action of miR34a, the miRNA with a broader impact on β-cell functions. Very recently, different independent studies have shown that the expression of miR34a is controlled by the transcription factor p53 (17,25–29). In agreement with these reports, overexpression of p53 in MIN6B1 cells increased the expression of a luciferase reporter construct driven by the miR34a promoter but not the expression of an analogous construct in which the p53 recognition site is mutated (Fig. 6A). In agreement with these findings, overexpression of p53 led to an increase in miR34a levels (Fig. 6B). We then investigated whether prolonged incubation in the presence of palmitate can activate the p53 pathway. Quantitative RT-PCR measurements revealed that under our experimental conditions, the mRNA level of p53 is increased in MIN6B1 cells and in rat pancreatic islets (Fig. 6D). In addition, we found that in cells treated with palmitate, the p53 transcriptional activity assessed using a luciferase reporter construct (p53 sensor) is enhanced (Fig. 6C), confirming the activation of the p53 pathway. As expected, prolonged incubation in the presence of palmitate augmented the activity of the miR34a (30) promoter but not the activity of the construct lacking the p53 binding site (Fig. 6C).
FIG. 6.
Identification of the signaling pathway leading to the induction of miR34a. A: MIN6B1 cells were transfected with luciferase reporter plasmids driven by the wild-type miR34a promoter (Promo miR34a), by a miR34a promoter lacking the p53 binding site (Promo mut miR34a), or by p53-responsive elements (p53 Sensor). Each of these luciferase reporters was cotransfected with an empty vector (control) or with a p53-overexpressing plasmid (p53). Luciferase activities were measured 3 days later and normalized to controls. *Conditions significantly different (P < 0.05, n = 3) from controls. B: MIN6B1 cells were transfected with an empty vector or with a plasmid encoding p53. The expression of miR34a was measured by quantitative RT-PCR 2 days later. Data represent the mean of three independent experiments. *P < 0.05 (n = 3). C: MIN6B1 cells were transfected with luciferase reporter plasmids as in A and were then cultured in the absence (control) or in the presence of 1 mmol/l palmitate. Luciferase activities were measured 3 days later and normalized to controls. The results are representative of three independent experiments. *Conditions significantly different (P < 0.05) from controls. D: MIN6B1 cells (left) and isolated rat pancreatic islets (right) were cultured for 3 days in the absence (Control) or in the presence of 1 mmol/l palmitate. p53 mRNA levels were assessed by quantitative RT-PCR and normalized to the level of housekeeping gene GAPDH. Data are means ± SE of three independent experiments. *P < 0.05.
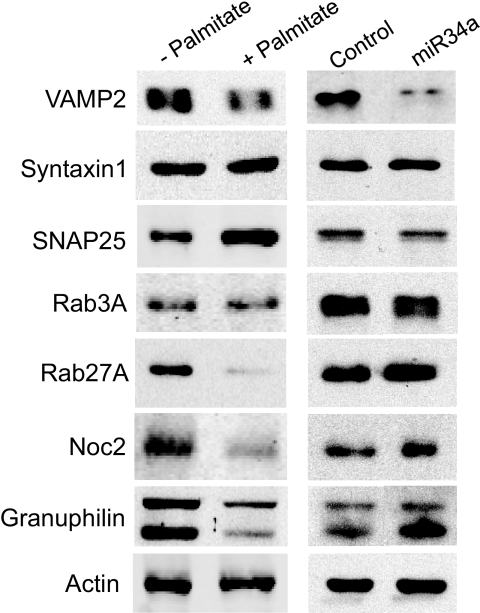
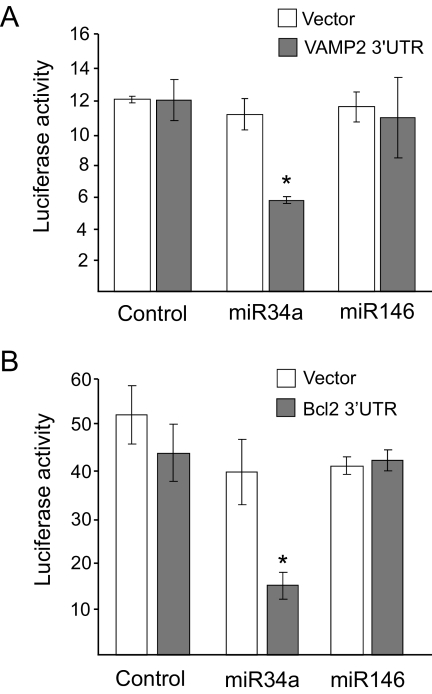
Chronic exposure to palmitate leads to changes in the expression of several proteins that play an essential role in β-cell exocytosis (30–32), including VAMP2, SNAP25, Rab27A, Noc2, and Granuphilin (Fig. 7). Interestingly, VAMP2 is among the putative targets of miR34a (33,34). In agreement with the computational prediction, Western blot analysis revealed that miR34a overexpression in MIN6B1 cells results in a decrease in the level of VAMP2 (Fig. 7). In contrast, miR34 did not affect the expression of all the other components of the machinery of exocytosis tested (Fig. 7). To verify whether VAMP2 is a direct miR34a target in insulin-secreting cells, we generated a luciferase reporter construct containing the 3′UTR of VAMP2. As shown in Fig. 8A, miR34a overexpression diminished the luciferase activity produced from this construct but not from a control luciferase construct with a 3′UTR that does not contain the sequences of VAMP2 mRNA. As expected, overexpression of miR146 that has no impact on hormone secretion did not affect the activity of the control luciferase reporter or of the vector containing the 3′UTR of VAMP2 (Fig. 8A). These findings confirm that VAMP2 is a direct target of miR34a and suggest that diminished expression of this SNARE may potentially contribute to the secretory defects observed with increased expression of this miRNA.
FIG. 7.
Comparison of the effects of palmitate and miR34 on the expression of components controlling insulin exocytosis. Left: MIN6B1 cells were incubated for 72 h in the absence (−) or in the presence of 1 mmol/l palmitate (+). Right: MIN6B1 cells were transfected with a control RNA duplex or with an RNA oligonucleotide corresponding to the mature form of miR34a. The expression of the indicated components of the machinery controlling insulin exocytosis was assessed by Western blotting. The figure shows representative blots. Similar results were obtained in at least three independent experiments.
FIG. 8.
VAMP2 and BclII are direct targets of miR34a in insulin-secreting cells. A: MIN6B1 cells were cotransfected with a constitutively expressed Firefly luciferase construct; a Renilla luciferase reporter plasmid containing (▪) or lacking (Vector, □) the sequence of the 3′UTR of mouse VAMP2; and a control RNA duplex (control), miR34a, or miR146. Luciferase activities were measured 3 days later. Renilla luciferase activities were divided by the Firefly luciferase activities to correct for differences in transfection efficiencies. *Condition is significantly different (P < 0.05, n = 3) from control. B: MIN6B1 cells were cotransfected with a constitutively expressed Firefly luciferase construct; a Renilla luciferase reporter plasmid containing (▪) or lacking (Vector, □) the putative miR34 recognition sequence in the 3′UTR of BclII; and a control RNA duplex (control), miR34a, or miR146. Luciferase activities were measured 3 days later. Renilla luciferase activities were divided by the Firefly luciferase activities to correct for differences in transfection efficiencies. *Condition that is significantly different (P < 0.05, n = 3) from control.
BclII is a key antiapoptotic factor that has been shown to be a direct target of miR34a in other cell types (29). To assess whether BclII is a direct miR34a target in insulin-secreting cells as well, we investigated the effect of this miRNA on the activity of a luciferase reporter construct containing the 3′UTR target sequence of BclII mRNA. As shown in Fig. 8B, miR34a diminished the luciferase activity produced from the BclII 3′UTR construct but not from a control vector lacking the miR34a target motif. In contrast, overexpression of miR146 was without effect.
DISCUSSION
Acute exposure of pancreatic β-cells to FFAs sustains insulin secretion in the fasted state and potentiates hormone release in the presence of glucose. However, chronic exposure to elevated concentrations of FFAs has detrimental impacts on β-cell functions and is believed to favor the development of type 2 diabetes (2). Although prolonged incubation with FFAs is known to lead to alterations in the expression of several genes playing key roles in the accomplishment of β-cell functions (2), the precise mechanisms causing the lipotoxic effects are still incompletely understood. In this study, we investigated the potential involvement of miRNAs, a recently discovered family of regulators of gene expression, in FFA-mediated β-cell dysfunction. We found that the expression of most miRNAs, including those previously shown to be involved in the control of specialized β-cell functions (7,9–11), is not affected by palmitate. However, the level of two miRNAs, miR34a and miR146, was increased with sustained exposure of insulin-secreting cell lines or freshly isolated rat pancreatic islets to the FFAs. In this study, we did not investigate in detail the effect of glucose on the expression of miR34a and miR146. MIN6 cells are cultured at 25 mmol/l and are not ideally suited to address this issue. In rat pancreatic islets, the effects of glucose and palmitate on miR34a expression were not additive, and miR146 expression was not affected by glucose. Loss of β-cell function is known to be more severe when the cells are exposed concomitantly to elevated concentrations of glucose and palmitate (2,3). Our observations in rat islets indicate that alone, changes in the level of these two miRNAs cannot account for the synergistic impact of glucose and palmitate on β-cell dysfunction.
Interestingly, a strong increase in the levels of miR34a and miR146 was detected in pancreatic islets from a mouse model of type 2 diabetes as well, suggesting that the effects observed in vitro may recapitulate events occurring in vivo. Detailed analysis of the role of these miRNAs in insulin-secreting cells revealed that their expression level can directly affect the execution of specialized β-cell tasks, raising the possibility of a possible involvement of these noncoding RNAs in the toxic effects triggered by palmitate.
In MIN6B1 cells, 3-day incubation with concentrations of palmitate ranging from 0.25 to 1 mmol/l raised the cellular level of miR34a. We provide evidence indicating that the increase in miR34a expression is linked to activation of the p53 pathway. p53 is a tumor suppressor and an inducer of apoptosis that can be activated by several stressful conditions (35). p53 can induce apoptosis through different mechanisms, including repression of transcription of antiapoptotic genes and induction of proapoptotic genes. Interestingly, in addition to its well-established role in preventing cancer development, p53 was recently discovered to have much broader cellular functions, including the regulation of glucose metabolism and oxidative stress (35,36). The role of p53 in β-cells has been poorly investigated. The p53 signaling pathway has been shown to be activated by cytokines (37) and to potentiate FFA-induced apoptosis in insulinoma cell lines (38). Our data show that the p53 pathway is activated with chronic exposure to FFAs and identify the rise of miR34a as an additional mechanism through which p53 can trigger apoptosis in β-cells.
miR34a and miR146 have been predicted, using bioinformatic approaches, to target numerous mRNAs (http://www.targetscan.org/; http://www.microrna.org/microrna/home.do). In view of the complexity of the impact of miRNAs on gene expression, a precise definition of the mode of action of miR34a and miR146 in β-cells was beyond the scope of the present study. However, here, we were able to highlight some possible mechanisms leading to β-cell dysfunction in cells expressing elevated levels of miRNAs.
miR34a is known to regulate directly or indirectly the expression of a large set of genes and to favor apoptosis by inhibiting the expression of the antiapoptotic protein BclII (29). Our data suggest that a similar mechanism could contribute to the apoptotic effect of this miRNA in insulin-secreting cells because miR34a reduced the expression of a reporter construct containing the 3′UTR of BclII. Interestingly, a decrease in BclII expression has been associated with FFA-induced apoptosis of human pancreatic islet cells (39).
The molecular events responsible for the miR34a-mediated defects in insulin exocytosis are likely to concern highly specialized genes selectively expressed in β-cells. Here, we were able to elucidate at least one mechanism through which miR34a can affect hormone secretion. In fact, the predicted targets of miR34a include VAMP2, a protein associated with secretory vesicles and that plays an essential role in insulin exocytosis (40–42). By direct experimental testing, we could demonstrate that miR34a can modulate the expression of VAMP2 in insulin-secreting cells, providing a potential explanation for the secretory defects observed in cells expressing increased levels of miR34a.
The precise mechanism through which miR146 can affect β-cell function and, in particular, β-cell survival remains to be elucidated. In other cell systems, miR146 has been shown to target interleukin-1 receptor–associated kinase 1 (IRAK1) and tumor necrosis factor receptor–associated factor 6 (TRAF6) (43), two key components of interleukin-1β and Toll-like receptor signaling that mediate activation of nuclear factor-κB (NF-κB) and activation protein-1 (AP-1) pathways (44). In pancreatic β-cells, activation of these pathways can lead to cell death (45,46); although under certain experimental conditions, inhibition of the NF-κB pathway has been reported to increase the susceptibility to cell death and accelerate the development of diabetes (47). Whether miR146 operates via a similar mechanism in β-cells is not yet known. A set of experiments performed in our laboratory did not reveal changes in TRAF6 protein expression in MIN6B1 cells (R.R., E. Rogglie, unpublished observations). Recently, several other genes have been shown to be directly or directly influenced by miR146 (48,49). Future experiments will have to assess whether the effect of miR146 on β-cell survival is linked to changes in the expression of one of these genes or is due to other, yet-to-be-identified, molecular mechanisms.
Having demonstrated the impact of miR34a or miR146 on different β-cell activities, we attempted to determine to which extent the changes in the level of these two small RNAs could contribute to fatty acid-mediated functional impairments. For this purpose, palmitate-treated cells were transfected with antisense oligonucleotides that specifically block the activity of each miRNA. Using this approach, we were able to demonstrate that the rise of miR34a and miR146 contributes to FFA-induced β-cell death. We found that reduction of miR34a and miR146 partially protects the cells against palmitate-induced apoptosis. In contrast, it was more difficult to determine the precise contribution of miR34a to the deleterious effects of palmitate on insulin secretion. We showed that the secretory dysfunction observed in miR34a-overexpressing cells is most probably mediated by a decrease in the expression of the SNARE protein VAMP2. Reduction of VAMP2 expression occurs in cells chronically exposed to palmitate as well. However, in this case, the effect is rather small, and palmitate treatment is associated with major changes in the level of several other components of the machinery of exocytosis that are not reproduced by miR34a overexpression (Fig. 7). Part of these changes may be linked to chronic induction of a transcriptional repressor, inducible cAMP early repressor, that we previously demonstrated to target Rab27A, Noc2, and Granuphilin (50). Thus, although the rise of miR34a may contribute to perturbing the exocytotic process by reducing VAMP2 expression, it is unlikely that the alteration of the level of this miRNA is the main cause of the secretory dysfunction in palmitate-treated cells.
To our knowledge, this is the first study reporting changes in β-cell miRNA expression under physiopathological conditions. Chronic hyperglycemia, prolonged exposure to cytokines, or oxidized LDLs are all known to cause pancreatic β-cell dysfunctions (46,51). These adverse conditions may also be associated with distinct modifications of the miRNA expression profile. A precise definition of the impact of these different physiopathological conditions on miRNA expression will help in elucidating the causes of β-cell failure and favor the definition of new directions for the development of better tools for prevention and treatment of diabetes.
Supplementary Material
Acknowledgments
D.R.L. has received National Health and Medical Research Council of Australia Grant 427616. C.W. has received Swiss National Science Foundation Grant 3100A0-107819. A.A. has received Swiss National Science Foundation Grant 3100A0-105425. R.R. has received Swiss National Science Foundation Grant 3100A0-113421 and grants from the Fondation Romande pour la Recherche sur le Diabète, the Association de Langue Française pour l’Etude du Diabète et des Maladies Métaboliques, and CardioMet Lausanne.
We are indebted to Guy Niederhauser for technical assistance in preparing rat islets. We thank Dr. M. Oren (Weizmann Institute) for supplying the luciferase reporter plasmids driven by the miR34a promoter and Dr. M. Fukuda (Tohoku University, Sendai, Japan) for providing the antibody against Noc2.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 July 2008.
P.L. and E.R. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Weir GC, Bonner-Weir S: Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 53 (Suppl. 3): S16–S21, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Prentki M, Nolan CJ: Islet beta cell failure in type 2 diabetes. J Clin Invest 116: 1802–1812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newsholme P, Keane D, Welters HJ, Morgan NG: Life and death decisions of the pancreatic beta-cell: the role of fatty acids. Clin Sci (Lond) 112: 27–42, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bushati N, Cohen SM: microRNA Functions. Annu Rev Cell Dev Biol 23: 175–205, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP: MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Chang TC, Mendell JT: The roles of microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet 8: 215–239, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, Rutter GA, Van Obberghen E: MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cells lines. J Biol Chem 282: 19575–19588, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RH: Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol 5: e203, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plaisance V, Abderrahmani A, Perret-Menoud V, Jacquemin P, Lemaigre F, Regazzi R: MicroRNA-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells. J Biol Chem 281: 26932–26942, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M: A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432: 226–230, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Lovis P, Gattesco S, Regazzi R: Regulation of the expression of components of the machinery of exocytosis of insulin-secreting cells by microRNAs. Biol Chem 389: 305–312, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Lilla V, Webb G, Rickenbach K, Maturana A, Steiner DF, Halban PA, Irminger JC: Differential gene expression in well-regulated and dysregulated pancreatic beta-cell (MIN6) sublines. Endocrinology 144: 1368–1379, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Sutton R, Peters M, McShane P, Gray DW, Morris PJ: Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation 42: 689–691, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR: Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of β-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 54: 2755–2763, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Plaisance V, Niederhauser G, Azzouz F, Lenain V, Haefliger JA, Waeber G, Abderrahmani A: The repressor element silencing transcription factor (REST)-mediated transcriptional repression requires the inhibition of Sp1. J Biol Chem 280: 401–407, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH, Agami R: A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 124: 1169–1181, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M: Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26: 731–743, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Michod D, Widmann C: TAT-RasGAP317–326 requires p53 and PUMA to sensitize tumor cells to genotoxins. Mol Cancer Res 5: 497–507, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Imai A, Yoshie S, Nashida T, Shimomura H, Fukuda M: Functional involvement of Noc2, a Rab27 effector, in rat parotid acinar cells. Arch Biochem Biophys 455: 127–135, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Coppola T, Frantz C, Perret-Menoud V, Gattesco S, Hirling H, Regazzi R: Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell 13: 1906–1915, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jovanovic M, Hengartner MO: miRNAs and apoptosis: RNAs to die for. Oncogene 25: 6176–6187, 2006 [DOI] [PubMed] [Google Scholar]
- 22.El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M: Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology 144: 4154–4163, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Meister G, Landthaler M, Dorsett Y, Tuschl T: Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA 10: 544–550, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varadi A, Ainscow EK, Allan VJ, Rutter GA: Involvement of conventional kinesin in glucose-stimulated secretory granule movements and exocytosis in clonal pancreatic beta-cells. J Cell Sci 115: 4177–4189, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT: Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26: 745–752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ: A microRNA component of the p53 tumour suppressor network. Nature 447: 1130–1134, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H: Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 6: 1586–1593, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Welch C, Chen Y, Stallings RL: MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene 26: 5017–5022, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, Macdougald OA, Cho KR, Fearon ER: p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 17: 1298–1307, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Lang J: Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem 259: 3–17, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Cheviet S, Waselle L, Regazzi R: Noc-king out exocrine and endocrine secretion. Trends Cell Biol 14: 525–528, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Izumi T, Gomi H, Kasai K, Mizutani S, Torii S: The roles of Rab27 and its effectors in the regulated secretory pathways. Cell Struct Funct 28: 465–474, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB: Prediction of mammalian microRNA targets. Cell 115: 787–798, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N: Combinatorial microRNA target predictions. Nat Genet 37: 495–500, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Vousden KH, Lane DP: p53 in health and disease. Nat Rev Mol Cell Biol 8: 275–283, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Bensaad K, Vousden KH: p53: new roles in metabolism. Trends Cell Biol 17: 286–291, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Kim WH, Lee JW, Gao B, Jung MH: Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal 17: 1516–1532, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ: Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J Biol Chem 277: 49676–49684, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P: Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that β-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51: 1437–1442, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Regazzi R, Sadoul K, Meda P, Kelly RB, Halban PA, Wollheim CB: Mutational analysis of VAMP domains implicated in Ca2+-induced insulin exocytosis. EMBO J 15: 6951–6959, 1996 [PMC free article] [PubMed] [Google Scholar]
- 41.Regazzi R, Wollheim CB, Lang J, Theler JM, Rossetto O, Montecucco C, Sadoul K, Weller U, Palmer M, Thorens B: VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J 14: 2723–2730, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler MB, Sheu L, Ghai M, Bouquillon A, Grondin G, Weller U, Beaudoin AR, Bennett MK, Trimble WS, Gaisano HY: Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology 137: 1340–1348, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Taganov KD, Boldin MP, Chang KJ, Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103: 12481–12486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunne A, O’Neill LA: The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2003: re3, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ortis F, Cardozo AK, Crispim D, Storling J, Mandrup-Poulsen T, Eizirik DL: Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-kappaB activation. Mol Endocrinol 20: 1867–1879, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Abderrahmani A, Niederhauser G, Favre D, Abdelli S, Ferdaoussi M, Yang JY, Regazzi R, Widmann C, Waeber G: Human high density lipoprotein-particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein-particles in pancreatic beta cells. Diabetologia 50: 1304–1314, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS: NF-kappa B prevents beta cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci U S A 104: 1913–1918, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin SL, Chiang A, Chang D, Ying SY: Loss of mir-146a function in hormone-refractory prostate cancer. RNA 14: 417–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, Wang X, Lin Z, Schaefer BC, Flemington EK: Epstein-Barr virus latent membrane protein 1 induces cellular MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol 82: 1946–1958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abderrahmani A, Cheviet S, Ferdaoussi M, Coppola T, Waeber G, Regazzi R: ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis. EMBO J 25: 977–986, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson RP, Harmon JS: Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med 41: 177–184, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.