Abstract
OBJECTIVE—Despite tremendous progress in vitreoretinal surgery, certain postsurgical complications limit the success in the treatment of proliferative vitreoretinal diseases (PVDs), such as proliferative diabetic retinopathy (PDR) and proliferative vitreoretinopathy (PVR). One of the most significant complications is the cicatricial contraction of proliferative membranes, resulting in tractional retinal detachment and severe vision loss. Novel pharmaceutical approaches are thus urgently needed for the management of these vision-threatening diseases. In the current study, we investigated the inhibitory effects of statins on the progression of PVDs.
RESEARCH DESIGN AND METHODS—Human vitreous concentrations of transforming growth factor-β2 (TGF-β2) were measured by enzyme-linked immunosorbent assay. TGF-β2–and vitreous-dependent phosphorylation of myosin light chain (MLC), a downstream mediator of Rho-kinase pathway, and collagen gel contraction simulating cicatrical contraction were analyzed using cultured hyalocytes. Inhibitory effects of simvastatin on cicatrical contraction were assessed both in vitro and in vivo.
RESULTS—Human vitreous concentrations of TGF-β2 were significantly higher in the samples from patients with PVD compared with those without PVD. Simvastatin inhibited TGF-β2–dependent MLC phosphorylation and gel contraction in a dose- and time-dependent manner and was capable of inhibiting translocation of Rho protein to the plasma membrane in the presence of TGF-β2. Vitreous samples from patients with PVD enhanced MLC phosphorylation and gel contraction, whereas simvastatin almost completely inhibited these phenomena. Finally, intravitreal injection of simvastatin dose-dependently prevented the progression of diseased states in an in vivo model of PVR.
CONCLUSIONS—Statins might have therapeutic potential in the prevention of PVDs.
Proliferative vitreoretinal diseases (PVDs), such as proliferative diabetic retinopathy (PDR) and proliferative vitreoretinopathy (PVR), are common causes of severe vision loss (1). Surgical approaches for the treatment of these diseases have evolved significantly in the recent past, but the occurrence of postoperative complications, such as cicatricial contraction, limit the therapeutic success (2). Therefore, there is an urgent need for alternate pharmacological treatments of PVDs that can complement or potentially replace surgical intervention. In PDR and PVR, excessive wound healing and fibrosis induce the formation of proliferative membranes on the retinal surface. The proliferative membrane then extends into the vitreous and contracts, causing tractional detachment (3). The proliferative membrane consists of various cells, including hyalocytes, retinal pigment epithelial cells, glial cells, and fibroblast-like cells (4–7).
Hyalocytes morphologically resemble macrophages and are considered to originate from peripheral blood monocytes (8). Under physiological conditions, hyalocytes are mainly located in the cortical vitreous and are considered to maintain its transparency (9,10). Under pathological conditions, hyalocytes are thought to be critical in vitreoretinal interface diseases, such as idiopathic epiretinal membrane formation, macular hole, and diabetic macular edema (11). Hyalocytes in diabetic eyes are higher in number and of different shape compared with those in normal eyes (12).
Transforming growth factor-β (TGF-β) is pivotal to tissue fibrosis. Among the three isoforms of TGF-β, TGF-β2 is the predominant isoform in the vitreous (13,14). We and others have shown that TGF-β2 is overexpressed in the epiretinal membrane and vitreous of PDR and PVR patients and that its expression correlates with the presence of intraocular fibrosis (14–17). TGF-β2 modulates the differentiation of various cell types and is considered to increase the production of extracellular matrix, resulting in the formation and contraction of proliferative membranes (18,19). Thus, it is possible that the combination of hyalocytes and TGF-β2 may contribute to the pathogenesis of PVDs.
Statins, inhibitors of the 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, are widely used to reduce endogenous cholesterol synthesis and improve hypercholesterolemia (20). HMG-CoA reductase is an upstream enzyme in the mevalonate biosynthetic pathway that catalyzes the conversion of HMG-CoA into mevalonate and then, in a number of steps, into farnesylpyrophosphate (FPP), a precursor of cholesterol (21). By inhibiting HMG-CoA reductase, statins block the mevalonate pathway, resulting in reduced synthesis of FFP and cholesterol. Statins are divided into three categories: the natural (i.e., lovastatin and pravastatin), the semisynthetic (i.e., simvastatin), and the synthetic (i.e., atorvastatin, fluvastatin, and cerivastatin). Statins decrease the risk for cardiovascular events even among high-risk individuals with coronary disease and diabetes (22). Moreover, statins may be useful in the treatment of other conditions, such as osteoporosis (23) and cancer growth and metastasis (24,25).
Another product of the mevalonate pathway is geranylgeranylpyrophosphate (GGPP), which is synthesized from FPP. Both FPP and GGPP are important isoprenoid intermediates and serve as lipid attachments for a variety of intracellular proteins to the plasma membrane, including the γ-subunit of heterotrimeric G-proteins and the small GTP-binding proteins, such as Ras and Rho, resulting in their activation (26). Rho translocation from the cytoplasm to the plasma membrane is dependent on geranylgeranylation (GGPP attachment), whereas Ras translocation is dependent on farnesylation (FPP attachment) (27). Rho in the plasma membrane is implicated in the cytoskeletal responses to extracellular signals and is converted to an active GDP-bound state (28). Rho-kinase, one of the effector molecules of Rho, is involved in a variety of cellular events related to cell morphology, adhesion, and motility (28–30), especially through phosphorylation of the myosin light chain (MLC). MLC phosphorylation induces actin-myosin interaction and, consequently, smooth muscle contraction and stress fiber formation in nonmuscle cells (31,32). Activation of the Rho/Rho-kinase pathway is therefore indispensable for smooth muscle contraction. GGPP is one of the downstream components of the mevalonate pathway and plays an important part in the Rho/Rho-kinase pathway activation. Thus, statins, which regulate the mevalonate pathway, also regulate the Rho/Rho-kinase pathway and, consequently, MLC phosphorylation.
Previously, we reported that the Rho-kinase pathway is involved in TGF-β2–induced MLC phosphorylation and contraction of hyalocyte-containing collagen gels and that hydroxyfasudil, a potent Rho-kinase inhibitor, significantly diminishes these TGF-β2–induced effects (18). In the present study, we investigate the regulatory effects of statins on TGF-β2–and vitreous-dependent MLC phosphorylation and contraction of hyalocyte-containing collagen gels and their therapeutic potential for prevention of PVDs in vivo.
RESEARCH DESIGN AND METHODS
Reagents.
Simvastatin, fluvastatin, pravastatin, and cerivastatin were purchased from CalBiochem (La Jolla, CA). Lovastatin was purchased from Funakoshi (Tokyo). Simvastatin was used as an active form after treatment with NaOH.
Vitreous samples and enzyme-linked immunosorbent assay.
This study was carried out with approval from the Institutional Review Board and performed in accordance with the ethical standards of the 1989 Declaration of Helsinki. We obtained written informed consent from the patients. Vitreous samples were collected from patients who underwent pars plana vitrectomy because of non-PVD (macular hole) or PVD (PDR and PVR). Concentrations of TGF-β2 were measured by a human TGF-β2 immunoassay kit (R&D Systems, Minneapolis, MN).
Cell culture.
Bovine hyalocytes were isolated as we previously reported (33). Cultured hyalocytes obtained between passages 5 and 7 were used in experiments.
Collagen gel contraction assay.
The contraction assay was performed as we previously described (33). Type I collagen (Koken, Tokyo), a reconstitution buffer, hyalocytes suspension, and distilled water were mixed and added to a 24-multiwell plate (Nunc, Roskilde, Denmark). After 1-h pretreatment of vitreous samples with 1 μg/ml anti–TGF-β2 antibody (R&D Systems) or 1 μg/ml mouse IgG (Sigma-Aldrich) that was added for confirming the absence of nonspecific suppression of the gel contraction by anti–TGF-β2 antibody, the gels were treated with the vitreous samples (400 μl). The diameter of the collagen gel was measured at 5 days after the treatment. For quantitative purposes, contraction data are presented as the reduction in diameter of the collagen gels.
In the same way, collagen gels containing hyalocytes were starved, pretreated with statins for 24 h, and then treated with 3 ng/ml TGF-β2 (Sigma-Aldrich) or vitreous samples.
Western blot analysis.
Total cell lysates were subjected to 15% SDS-PAGE, and the blots were incubated with an antibody against phosphorylated-MLC (p-MLC; 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA). Visualization was performed with an enhanced chemiluminesence (ECL; Amersham, Arlington Heights, IL) detection system. Lane loading differences were normalized by reblotting the membranes with an antibody against MLC (Santa Cruz) (1:1,000).
Plasma membrane proteins from the cells were isolated by Qproteome Plasma Membrane Protein kit (Qiagen, Hilden, Germany) and were subjected to Western blot analysis with an antibody against Rho (1:1,000; Upstate, Charlottesville, VA).
Counting viable cells in collagen gels.
After 5 days of treatment, the collagen gels were dissolved by collagenase, and the cell suspension was collected. The viable cell number was counted with hemocytometer after trypan blue staining.
In vivo model of PVR.
All experimental procedures using animals adhered to the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Ophthalmic and Vision Research. We caused experimental PVR in rabbit eyes as previously described (34). Homologous conjunctival fibroblasts were prepared by harvesting the conjunctival tissue. Twenty eyes from 20 Dutch rabbits weighing 2–2.5 kg were assigned into three groups, and fibroblasts (100,000 cells) were injected into the right eye in each rabbit. Eyes in the first group (six eyes) were administered an intravitreal injection of 0.1 ml vehicle (balanced salt solution). Eyes in the second group (seven eyes) were administered an intravitreal injection of 0.1 ml vehicle containing simvastatin (5 μmol/l final intravitreal concentration). Eyes in the last group (seven eyes) were administered an intravitreal injection of 0.1 ml vehicle containing simvastatin (15 μmol/l final intravitreal concentration). The clinical observations were performed carefully as long as 28 days after surgery and categorized according with the PVR classification of Fastenberg et al. (35).
Electroretinography.
Nine eyes from 10 Dutch rabbits weighing 2–2.5 kg were assigned into three groups. Eyes in the first group (three eyes) were administered an intravitreal injection of 0.1 ml vehicle. Eyes in the second group (three eyes) were administered an intravitreal injection of 0.1 ml vehicle containing simvastatin (5 μmol/l final intravitreal concentration). Eyes in the third group (three eyes) were administered an intravitreal injection of 0.1 ml vehicle containing simvastatin (15 μmol/l final intravitreal concentration). Electroretinogram was performed on day 28 as previously described (36).
Light microscopy.
The eyes were enucleated and fixed in 4% paraformaldehyde on day 28. Whole eyes were cut approximately along the vertical meridian. Paraffin-embedded sections were stained with hematoxylin-eosin, and each section was examined using light microscopy.
TdT-dUTP terminal nick-end labeling assay.
Apoptotic and potentially necrotic cell death was detected by TdT-dUTP terminal nick-end labeling (TUNEL). The eyes were fixed in 4% paraformaldehyde and embedded in paraffin. TUNEL staining was performed with the TdT Fluorescein in situ apoptosis detection kit (R&D Systems), according to the manufacturer's protocols. The sections were costained with propidium iodide (Molecular Probes, Eugene, OR) to observe the cell nuclei by fluorescence microscopy (KEYENCE, BIORAVO, and BZ-9000). As a TUNEL-positive control, we used the retina of rabbit PVR model on day 7 after the onset.
Transmission electron microscopy.
The eyes were fixed in 1% glutaraldehyde and 1% paraformaldehyde in PBS. The specimens were postfixed in veronal acetate buffer osmium tetroxide (2%), dehydrated in ethanol and water, and embedded in Epon. Ultrathin sections were cut from blocks and mounted on copper grids. The specimens were observed with an electron microscope (H7650; Hitachi, Tokyo).
Statistical analysis.
Statistical differences were assessed using nonparametric test (Kruskal-Wallis variance analysis) for the data of TGF-β2 concentrations and ANOVA for the other groups. P values <0.05 were considered significant. To adjust for inflated α-error due to multiple comparisons, the corrected significant P value was defined using the Bonferroni correction.
RESULTS
TGF-β2 in the vitreous and its impact on membrane contraction.
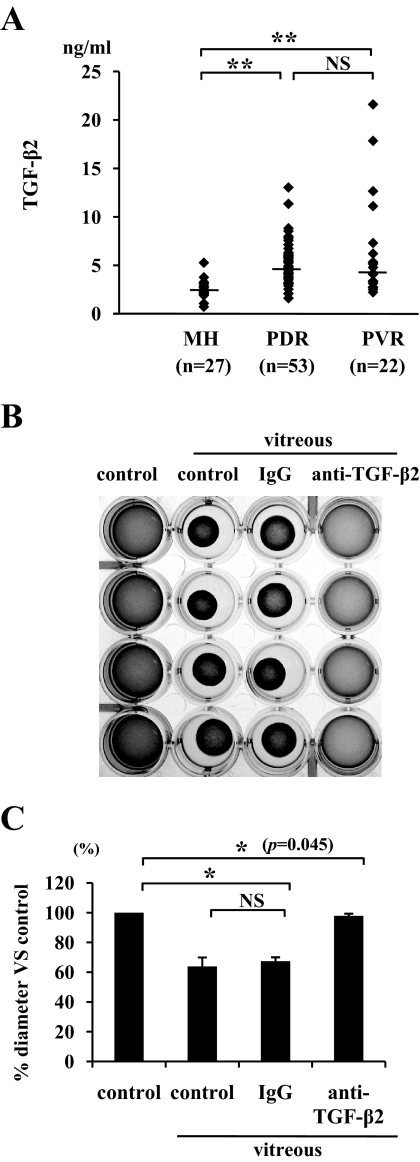
The median TGF-β2 protein concentrations, 2.35 ng/ml (range 0.72–5.26) in macular hole (n = 27), 4.74 ng/ml (1.60–13.0) in PDR (n = 53), and 4.48 ng/ml (2.52–21.6) in PVR (n = 22) patients, were significantly higher in PDR or PVR patients than in patients with macular hole (P < 0.01) (Fig. 1A). The median TGF-β2 protein concentration in the vitreous samples from patients with PDR or PVR showed no significant difference (P = 0.6).
FIG. 1.
TGF-β2 expression in the vitreous. A: Vitreous samples were collected from patients with non-PVD (macular hole) and PVD (PDR and PVR). Concentrations of TGF-β2 in the vitreous were measured by enzyme-linked immunosorbent assay (macular hole, n = 27; PDR, n = 53; PVR, n = 22). **P < 0.01 compared with macular hole. B: Hyalocytes were embedded in type I collagen gels (n = 4). After starvation and pretreatment with 1 μg/ml anti–TGF-β2 antibody or 1 μg/ml IgG for 1 h, the collagen gels were treated with vitreous samples from patients with PVD. Five days after the treatment, the gels were photographed. C: The diameter of the collagen gels was measured and expressed as percentage of the average diameter of the control group. *P < 0.05.
Vitreous samples from patients with PVD and those containing nonspecific IgG induced significant contraction of hyalocyte-containing collagen gels (63 and 67% vs. control, respectively). In comparison, the vitreous-induced contraction was virtually suppressed by specific anti–TGF-β2 antibody (P < 0.05) (97% vs. control) (Fig. 1B and C), suggesting a key role for TGF-β2 in the vitreous-induced collagen gel contraction.
Role of simvastatin in TGF-β2–dependent MLC phosphorylation.
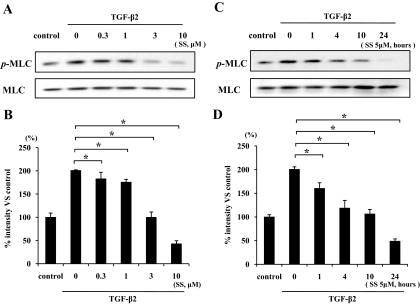
TGF-β2 enhanced MLC phosphorylation to about two times that seen with control (Fig. 2A and B). TGF-β2–dependent MLC phosphorylation showed a significant reduction at 0.3 μmol/l simvastatin or higher concentrations (up to 10 μmol/l simvastatin) compared with TGF-β2 alone (P < 0.05). The level of MLC phosphorylation at 10 μmol/l simvastatin concentration was lower than in untreated control, suggesting that simvastatin at higher concentrations may block the constitutive level of MLC phosphorylation. Because 3 μmol/l simvastatin was sufficient to reverse the effect of TGF-β2 on MLC phosphorylation, we chose the slightly higher concentration of 5 μmol/l to study the time-dependent effect of simvastatin on TGF-β2–dependent MLC phosphorylation. Treatment of the hyalocytes with 5 μmol/l simvastatin for 1 h or longer (up to 24 h) significantly suppressed the MLC phosphorylation compared with TGF-β2 alone, and treatment with 5 μmol/l simvastatin for 24 h sufficiently suppressed the MLC phosphorylation below that of untreated control (Fig. 2C and D).
FIG. 2.
Inhibitory effect of simvastatin on TGF-β2–dependent MLC phosphorylation. Hyalocytes were starved in Dulbecco's modified Eagle's medium containing 3% calf serum for 24 h. A: Hyalocytes were pretreated for 30 min with or without the indicated concentrations of simvastatin (0.3, 1, 3, and 10 μmol/l) and subsequently treated with 3 ng/ml TGF-β2 for 24 h. Total cell lysates were subjected to Western blot analysis with an antibody against p-MLC. Lane loading differences were normalized by reblotting the membranes with an antibody against MLC. C: Hyalocytes were pretreated with or without 5 μmol/l simvastatin for the indicated time (1, 4, 10, and 24 h) and subsequently treated with 3 ng/ml TGF-β2 for 24 h. p-MLC and MLC were also examined in the same way as in Fig. 1A. B and D: Signal intensity ratios (p-MLC to MLC) were expressed as percentage of control intensity ratio. *P < 0.05 compared with TGF-β2 alone.
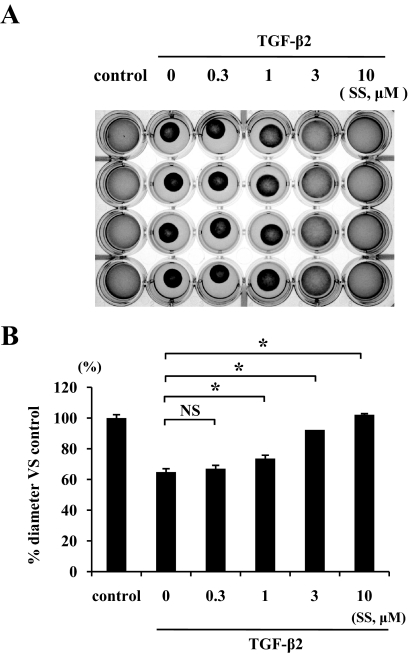
TGF-β2–dependent collagen gel contraction and its inhibition by simvastatin.
The control gels showed no apparent contraction up to 5 days, whereas TGF-β2 caused substantial collagen gel contraction in a time-dependent manner in the first 5 days (57.6% vs. control). TGF-β2–dependent collagen gel contraction was significantly reduced by simvastatin starting at a concentration of 1 μmol/l, and the reduction was greater at 3 and 10 μmol/l (92 and 100% vs. control, respectively) (Fig. 3).
FIG. 3.
The effect of simvastatin on TGF-β2–induced contraction of hyalocyte-containing collagen gels. Hyalocytes were embedded in type I collagen gels (n = 4). A: After starvation and pretreatment with indicated concentrations of simvastatin for 24 h, the collagen gels were stimulated with 3 ng/ml TGF-β2. Five days after the stimulation, the gels were photographed. B: The diameter of the collagen gels was measured and expressed as a percentage of the average diameter of control group. *P < 0.05; NS, not significant compared with TGF-β2 alone.
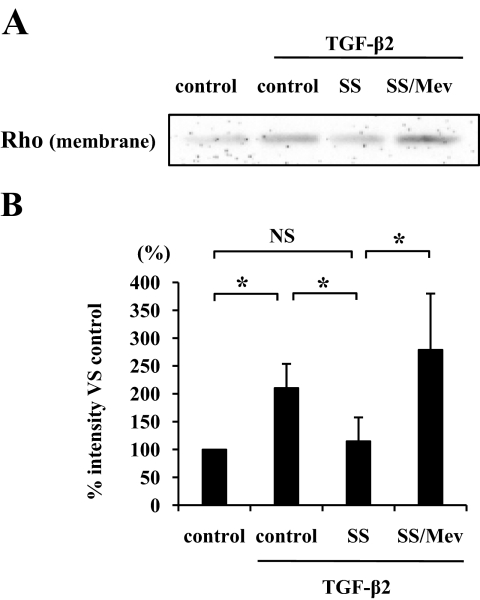
TGF-β2–dependent Rho translocation to the plasma membrane and its inhibition by simvastatin.
TGF-β2 significantly enhanced the Rho translocation to the plasma membrane (P < 0.05), whereas simvastatin suppressed the translocation (Fig. 4A and B). However, the effect of simvastatin was reversed in the presence of 400 μmol/l mevalonate, a component of the mevalonate pathway. These findings suggest that simvastatin inhibits Rho/Rho kinase pathway by preventing Rho from translocating to the plasma membrane via inhibition of the mevalonate pathway.
FIG. 4.
Inhibitory effect of simvastatin on the Rho translocation to the plasma membrane. A: Hyalocytes were pretreated with 5 μmol/l simvastatin with or without mevalonate (Mev) and then treated with 3 ng/ml TGF-β2 for 24 h. Plasma membrane proteins were isolated and subjected to Western blot analysis with an antibody against Rho. B: Signal intensities were expressed as percentage of control intensity. *P < 0.05.
Comparison of the effects of various statins on TGF-β2–dependent MLC phosphorylation and collagen gel contraction.
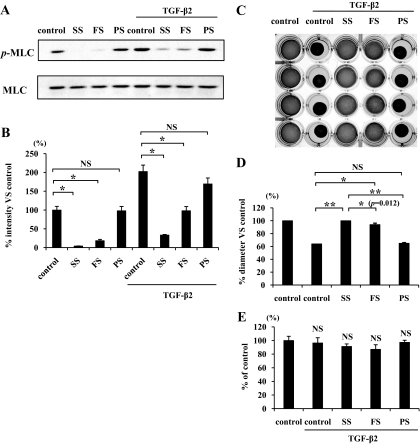
Simvastatin and fluvastatin significantly suppressed TGF-β2–dependent MLC phosphorylation by hyalocytes, whereas pravastatin did not show an effect (Fig. 5A and B). In addition, simvastatin significantly suppressed MLC phosphorylation compared with fluvastatin (P < 0.05). Next, we compared the inhibitory effect of statins on the contraction of hyalocyte-containing collagen gels. Simvastatin and fluvastatin significantly suppressed TGF-β2–dependent contraction of collagen gel, whereas pravastatin did not show an effect (Fig. 5C and D). In addition, the inhibitory effect of simvastatin (100% vs. control) was significantly higher than that by fluvastatin (94% vs. control) (P < 0.05). To test whether cytokine and/or statin treatment affected the growth and/or viability of the cells in our experiments, we treated the collagen gels with collagenase and counted the number of viable cells. Both TGF-β2 and statins showed no significant effects on cell number in the three-dimensional collagen gels (Fig. 5E).
FIG. 5.
Comparison of inhibitory effects of simvastatin, fluvastatin, and pravastatin on TGF-β2–dependent MLC phosphorylation and collagen gel contraction. A: Starved hyalocytes were pretreated with vehicle, 5 μmol/l simvastatin, fluvastatin, or pravastatin for 30 min and subsequently treated with or without 3 ng/ml TGF-β2 for 24 h. Total cell lysates were subjected to Western blot analysis with an antibody against p-MLC. Lane loading differences were normalized by reblotting the membranes with an antibody against MLC. B: Signal intensity ratios (p-MLC to MLC) were expressed as percentage of intensity ratio of vehicle alone. *P < 0.05. C: Hyalocytes were embedded in type I collagen gels (n = 4). After starvation and pretreatment with vehicle, 5 μmol/l simvastatin, fluvastatin, or pravastatin for 24 h, the collagen gels were stimulated with 3 ng/ml TGF-β2. Five days after the stimulation, the gels were photographed. D: The diameter of the collagen gels was measured and expressed as percentage of the average diameter of control group. **P < 0.01, *P < 0.05; NS, not significant. E: The viable cell number in the collagen gels was counted to exclude the effect of cell growth or cytotoxicity on the collagen gel contraction or its inhibition. Five days after the treatment, the collagen gels were dissolved, and the cell suspension was collected. The viable cell number was counted with hemocytometer after trypan blue staining (n = 4; NS, not significant compared with control).
Impact of simvastatin on MLC phosphorylation and collagen gel contraction induced by vitreous samples from patients with PVD.
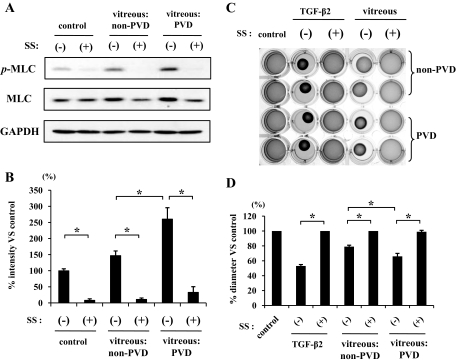
Vitreous samples from patients with PVD caused a significantly larger enhancement of MLC phosphorylation than those from patients with non-PVD (Fig. 6A and B). Simvastatin (5 μmol/l) strongly attenuated the vitreous-induced MLC phosphorylation. Expression of GAPDH did not change after the treatment with simvastatin or stimulation with vitreous samples. However, total MLC increased in cells stimulated with the vitreous samples compared with cells without vitreous treatment, and the increase was larger in cells stimulated with vitreous samples from patients with PVD than those from patients without PVD. These increases in an amount of total MLC were suppressed by 5 μmol/l simvastatin.
FIG. 6.
Inhibitory effects of simvastatin on vitreous-induced MLC phosphorylation and contraction of hyalocyte-containing collagen gels. A: Starved hyalocytes were pretreated with 5 μmol/l simvastatin for 30 min and subsequently treated with or without 400 μl vitreous samples of non-PVD (macular hole) or PVD (PDR and PVR) for 24 h. Total cell lysates were subjected to Western blot analysis with an antibody against p-MLC. Lane loading differences were normalized by reblotting the membranes with an antibody against MLC and GAPDH. B: Signal intensity ratios (p-MLC to GAPDH) were expressed as percentage of control intensity ratio. C: Hyalocytes were embedded in type I collagen gels (n = 4). After starvation and pretreatment with 5 μmol/l simvastatin for 24 h, the collagen gels were stimulated with 400 μl vitreous samples of non-PVD or PVD. Five days after the stimulation, the gels were photographed. D: The diameter of the collagen gels was measured and expressed as percentage of the average diameter of the control group. *P < 0.05.
The contraction of hyalocyte-containing collagen gels stimulated with the vitreous samples from patients with PVD was significantly larger compared with the contraction of the gels stimulated with vitreous samples from patients without PVD (P < 0.05) (Fig. 6C and D). We further examined the inhibitory effect of simvastatin on vitreous-dependent contraction of collagen gels and found that 5 μmol/l simvastatin suppressed the collagen gel contraction induced by vitreous samples even from patients with PVD.
Simvastatin inhibition of PVR development in vivo.
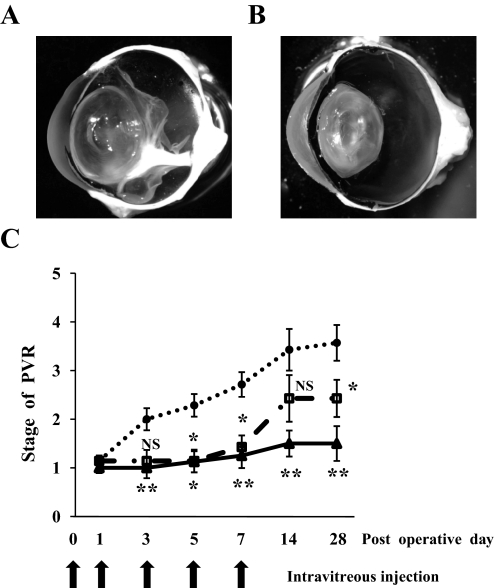
The control eyes of rabbits injected with vehicle developed PVR and were accompanied by proliferative membrane formation and cicatricial contraction, resulting in tractional retinal detachment (Fig. 7A). In comparison, 5 and 15 μmol/l simvastatin (final intravitreal concentrations) significantly prevented PVR development (Fig. 7C). Simvastatin inhibited the contraction of the proliferative membrane, and the membranes in eyes treated with simvastatin were much thinner than those in vehicle-treated eyes (Fig. 7B). After simvastatin injections were stopped at day 7, the eyes treated with 5 μmol/l simvastatin developed a mild but significant PVR, whereas no significant PVR development was observed in the eyes treated with 15 μmol/l simvastatin.
FIG. 7.
Inhibitory effects of simvastatin on experimental PVR in rabbit eyes. Rabbits underwent vitrectomy and intravitreal injection of fibroblasts with and without simvastatin on day 0. The eyes were injected with same contents of simvastatin on days 1, 3, 5, and 7 and examined using indirect ophthalmoscope as long as 28 days after the surgery. A: A representative vehicle-injected eye with PVR in stage 5, 28 days after the surgery. Proliferative membranes were observed in the vitreous cavity, causing a traction to the retina and retinal detachment. B: A representative simvastatin-injected eye (15 μmol/l) with PVR in stage 1. A thin proliferative membrane was observed, however, there was no evidence of tractional retinal detachment. C: Clinical observations were categorized according to the PVR classification of Fastenberg et al. (35). Stage 1, intravitreal membrane; stage 2, focal traction, localized vascular changes, hyperemia, engorgement, dilation, and blood vessel elevation; stage 3, localized detachment of medullary ray; stage 4, extensive retinal detachment, total medullary ray detachment, and peripapillary retinal detachment; and stage 5, total retinal detachment. •, vehicle; □, 5 μmol/l simvastatin; ▴, 15 μmol/l simvastatin. **P < 0.01, *P < 0.05; NS, not significant compared with vehicle.
Absence of apparent adverse effects of simvastatin in the retina.
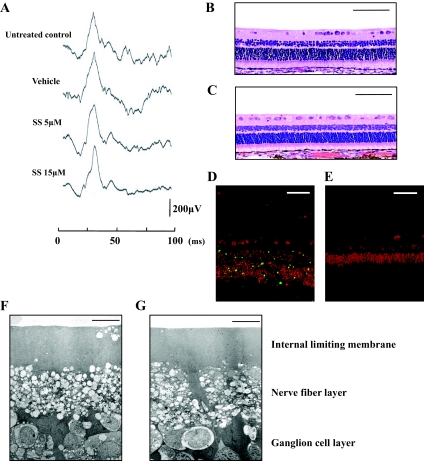
To evaluate the retinal function after intravitreal application of simvastatin, we performed electroretinography. The mean amplitude and latency of the 2Hz b wave in the eyes treated with 5 or 15 μmol/l simvastatin were 203.4 μV and 28.1 ms and 201.7 μV and 28.4 ms, respectively, and were not significantly different from those without any treatment or those of the vehicle-treated eyes (204.8 μV and 28.1 ms), suggesting that at these concentrations, simvastatin may not impede retinal function (Fig. 8A).
FIG. 8.
Histological and physiological examinations of eyes injected with simvastatin. Rabbits received intravitreal injections of 0.1 ml vehicle or vehicle with simvastatin (final concentration of 5 or 15 μmol/l simvastatin) on days 0, 1, 3, 5, and 7. A: Electroretinograms on day 28. A flash stimulus of intensity 1.30 log cd.s/m2 was superimposed on a background luminance of 1.15 log cd/m2. Light microscopy of the rabbit eye without any treatment (B) and that of the eye injected with 15 μmol/l simvastatin (C). Scale bar = 100 μm. Apoptotic and potentially necrotic cell death detected by TUNEL in the section from positive control (rabbit PVR model on day 7 after its onset) (D) and in the section from the 15 μmol/l simvastatin-treated eye (E). Scale bar = 50 μm. Transmission electron microscopy of the rabbit eye without treatment (F) and that of the eye injected with 15 μmol/l simvastatin (G). Scale bar = 10 μm. (Please see http://dx.doi.org/10.2337/db08-0302 for a high-quality digital representation of this image.)
The histological structure of the retina in the eyes injected with simvastatin appeared normal when observed on day 28 (Fig. 8B, C).
In the retina of an experimental PVR used as a positive-control, apoptotic cells, which present TUNEL-positive staining (green), were detected in the inner nuclear layer and the outer nuclear layer (Fig. 8D). The eyes injected with simvastatin had no apparent TUNEL-positive staining in the retina when observed on day 28 (Fig. 8E).
The eyes injected with simvastatin had no apparent ultrastructual changes in internal limiting membrane, nerve fiber layer, and ganglion cell layer (Fig. 8G) compared with the untreated control eyes (Fig. 8F). In other parts of the retina, such as inner and outer nuclear layers, photoreceptors, and retinal pigment epitheliums, there were also no apparent changes.
DISCUSSION
Blindness is a devastating consequence of PVDs such as PDR and PVR. Currently, the progression of these diseases cannot be effectively prevented, and the treatment options are limited to vitreoretinal surgery. An effective pharmacological treatment is thus urgently needed to complement or potentially replace the surgical intervention. In the current study, we show statins’ novel function in inhibiting the Rho/Rho-kinase pathway, the contraction of collagen gel, and the progression of experimental PVR, suggesting the therapeutic potential of these compounds for the treatment of PVDs.
Various cytokines, such as TGF-β, connective tissue growth factor, interleukin-6, and platelet-derived growth factor (PDGF), are overexpressed in the vitreous and membranes associated with PDR and PVR, and they contribute to the pathogenesis of these diseases (15,37–40). Among these cytokines, TGF-β2 induces the transformation of retinal pigment epithelial cells or hyalocytes to myofibroblastic cells (18,41) and plays a key role in the formation and contraction of proliferative membranes. In this study, we confirmed the overexpression of TGF-β2 in the vitreous from patients with PVD (PDR and PVR) and showed that TGF-β2 inhibition strongly suppressed the vitreous-induced contraction of the collagen gels. This indicates the possibility that TGF-β2 is the dominant contributor to the contraction of proliferative membrane in the vitreous cavity. Thus, we focused on the role of TGF-β2 to investigate the mechanisms of membrane contraction. TGF-β2 enhanced MLC phosphorylation in hyalocytes that was responsible for the contraction of the hyalocyte-containing collagen gels. We showed that simvastatin suppressed TGF-β2–induced MLC phosphorylation and collagen gel contraction in a dose-dependent fashion by inhibiting GGPP-mediated translocation of Rho to the plasma membrane, whereas no signs of cytotoxicity were apparent.
Differences in the structural characteristics of statin cause different levels of lipophilicity and possibly of efficacies and also cytotoxicity. Pravastatin is strongly hydrophilic, and simvastatin is much more lipophilic than pravastatin (42). Fluvastatin is also more lipophilic than pravastatin; however, it is less lipophilic than simvastatin (43). Although our comparison of the inhibitory effects of various statins, simvastatin, fluvastatin, and pravastatin, revealed that simvastatin was more effective in reducing MLC phosphorylation than the other statins under the chosen experimental conditions, the results might vary at other time points or concentrations. Further examination is necessary to determine the order. Additionally, because cerivastatin is more lipophilic than simvastatin (44), we studied the effects of cerivastatin and simvastatin. Many hyalocytes treated with 5 μmol/l cerivastatin for 24 h shrank and detached from the culture plates, whereas the cells treated with 5 μmol/l simvastatin remained morphologically unchanged. However, at higher concentrations (>20 μmol/l), some hyalocyte shrinkage was observed even with simvastatin (data not shown). Cerivastatin is known to be cytotoxic and induce apoptosis (45). The morphological changes we observed in cerivastatin-treated hyalocytes in our study suggest that this drug may also be toxic to hyalocytes and promote their apoptosis. Because the lipophilicity of lovastatin is similar to that of simvastatin (42), we compared their effect and found the inhibitory effect of simvastatin on MLC phosphorylation and collagen gel contraction to be greater than that of 5 μmol/l lovastatin for 24 h (data not shown).
Vitreous samples from patients with PDR and PVR induced MLC phosphorylation and contraction of hyalocyte-containing collagen gels, which were effectively inhibited by simvastatin. It may appear surprising that not only vitreous samples from patients with PVD but also those from non-PVD patients induced MLC phosphorylation and collagen gel contraction. However, this might be explained by the fact that TGF-β2 is expressed in vitreous samples of patients with macular hole at high enough concentrations to induce the observed phenomena. The concentrations of TGF-β2 in vitreous samples from patients with PVD (PDR and PVR) were higher than in those from macular hole patients. Therefore, in a concentration-dependent manner, the inductions might be greater in the vitreous samples from patients with PVD than in those from patients without PVD. The concentrations of TGF-β2 in some vitreous samples from patients with macular hole were higher than those with PDR and PVR. However, the pathology of macular hole does not generally involve proliferative membrane formation and tractional retinal detachment. Occasionally, epiretinal membranes and retinal folds accompany macular hole. Retinal folds are considered to be caused by the contraction of the epiretinal membranes. TGF-β2 concentrations in vitreous samples from patients with macular hole may be high enough to induce contraction of proliferative membranes, however, because the epiretinal membrane in these patients does not extend into the vitreous and is very thin; the expression of TGF-β2 remains inconsequential.
MLC phosphorylation depends on the concentration of TGF-β2 (18); thus, the eyes with PVR having lower TGF-β2 concentrations might represent a less severe pathology, or remission, compared with those with higher TGF-β2 concentrations. The occurrence of tractional retinal detachment might depend on the presence or absence of proliferative membranes and the concentration of TGF-β2.
Although TGF-β2–stimulated hyalocytes showed no significant change in MLC expression, those stimulated with vitreous samples showed elevated MLC expression that was inhibited by simvastatin treatment. The vitreous includes various cytokines, released from retinal pigment epithelial cells, glial cells, macrophages, and other intravitreal cells (46). Thus, some cytokines other than TGF-β2 might elevate the expression of MLC. Among the cytokines found in the vitreous, insulin-like growth factor-I (IGF-I), PDGF, and members of the endothelin family have been shown to stimulate extracellular matrix contraction (18,47,48). This study reveals a key role for TGF-β2 in the pathology of PVDs; however, it is possible that other cytokines found in the vitreous might also be involved in MLC phosphorylation and contraction of hyalocyte-containing collagen gels. Because simvastatin almost completely inhibited the phenomena induced by vitreous samples, it might also inhibit the effect of the other cytokines, such as IGF-I, PDGF, and members of the endothelin family and unknowns, which might be exerted through the Rho/Rho-kinase pathway.
Simvastatin also prevented the development of PVR in vivo. Proliferative membranes in simvastatin-injected eyes, even if present, were very thin, whereas the membranes in vehicle injected eyes with PVR in stage 4 or 5 were thick. Thus, simvastatin might have also an inhibitory effect on the formation and growth of proliferative membranes in addition to the cicatricial contraction of proliferative membranes. However, after the end of the simvastatin injections, even in the groups treated with higher concentration of simvastatin (15 μmol/l) the development of PVR was not completely inhibited. This may be due to a short biological half-life time of the compound in the vitreous cavity. Therefore, to sustain a constant level of intravitreal simvastatin concentration, frequent injections or a slow release drug delivery system might be necessary.
Recent findings suggest that statins might have a number of beneficial effects in the eye. Although the applicability of statins on age-related macular degeneration is still under investigation (49), statins are shown to have protective effects on primary open-angle glaucoma, a major cause of blindness (50). The regulation of the intraocular pressure (IOP) within a physiological range is of great clinical importance. Statins are reported to downregulate the IOP by increasing aqueous humor outflow via the inhibition of Rho/Rho-kinase pathway in the trabecular meshwork and the ciliary body (50).
In conclusion, simvastatin potently inhibits the Rho/Rho-kinase pathway and thus might have therapeutic potential in the prevention of cicatricial contraction of proliferative membranes in vivo. This is the first report demonstrating the beneficial effects of simvastatin in inhibiting the development of PVR. Statins might provide a new strategy for the treatment and prevention of the development of PVDs in humans.
Acknowledgments
S.N. has received a Research Fellowship Award from Bausch & Lomb and a Fellowship Award from the Japan Eye Bank Association. A.H.-M. has received National Institutes of Health Grants AI050775 and HL086933. This study has received Grants-in-Aid for Scientific Research 19592026, 18791283, and 18591925 from the Ministry of Education, Science, Sports and Culture, Japan; National Eye Institute Core Grant EY14104; and support from the Massachusetts Lions Eye Research Fund, the Marion W. and Edward F. Knight Age-Related and Macular Degeneration Fund, and Research to Prevent Blindness.
We acknowledge David Goodman (Pharmascience), Waichiro Katsuda, Tomoko Saeki, and Noriyuki Yoshida (Aqumen Biopharmaceuticals) for their excellent help.
Published ahead of print at http://diabetes.diabetesjournals.org on 3 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Pastor JC, de la Rua ER, Martin F: Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res 21: 127–144, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Pastor JC: Proliferative vitreoretinopathy: an overview. Surv Ophthalmol 43: 3–18, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Friedlander M: Fibrosis and diseases of the eye. J Clin Invest 117: 576–586, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campochiaro PA: Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol 115: 237–241, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Jerdan JA, Pepose JS, Michels RG, Hayashi H, de Bustros S, Sebag M, Glaser BM: Proliferative vitreoretinopathy membranes: an immunohistochemical study. Ophthalmology 96: 801–810, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Vinores SA, Campochiaro PA, Conway BP: Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci 31: 14–28, 1990 [PubMed] [Google Scholar]
- 7.Machemer R, Laqua H: Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation). Am J Ophthalmol 80: 1–23, 1975 [DOI] [PubMed] [Google Scholar]
- 8.Lazarus HS, Hageman GS: In situ characterization of the human hyalocyte. Arch Ophthalmol 112: 1356–1362, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Grabner G, Boltz G, Forster O: Macrophage-like properties of human hyalocytes. Invest Ophthalmol Vis Sci 19: 333–340, 1980 [PubMed] [Google Scholar]
- 10.Mitchell CA, Risau W, Drexler HC: Regression of vessels in the tunica vasculosa lentis is initiated by coordinated endothelial apoptosis: a role for vascular endothelial growth factor as a survival factor for endothelium. Dev Dyn 213: 322–333, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Kampik A, Kenyon KR, Michels RG, Green WR, de la Cruz ZC: Epiretinal and vitreous membranes: comparative study of 56 cases. Arch Ophthalmol 99: 1445–1454, 1981 [DOI] [PubMed] [Google Scholar]
- 12.Faulborn J, Dunker S, Bowald S: Diabetic vitreopathy: findings using the celloidin embedding technique. Ophthalmologica 212: 369–376, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer BA, Flanders KC, Guerin CJ, Danielpour D, Anderson DH: Transforming growth factor beta 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp Eye Res 59: 323–333, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Connor TB Jr, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, de Bustros S, Enger C, Kato H, Lansing M: Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest 83: 1661–1666, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kita T, Hata Y, Kano K, Miura M, Nakao S, Noda Y, Shimokawa H, Ishibashi T: Transforming growth factor-β2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes 56: 231–238, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ando A, Ueda M, Uyama M, Masu Y, Ito S: Enhancement of dedifferentiation and myoid differentiation of retinal pigment epithelial cells by platelet derived growth factor. Br J Ophthalmol 84: 1306–1311, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinores SA, Henderer JD, Mahlow J, Chiu C, Derevjanik NL, Larochelle W, Csaky C, Campochiaro PA: Isoforms of platelet-derived growth factor and its receptors in epiretinal membranes: immunolocalization to retinal pigmented epithelial cells. Exp Eye Res 60: 607–619, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Hirayama K, Hata Y, Noda Y, Miura M, Yamanaka I, Shimokawa H, Ishibashi T: The involvement of the rho-kinase pathway and its regulation in cytokine-induced collagen gel contraction by hyalocytes. Invest Ophthalmol Vis Sci 45: 3896–3903, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Jester JV, Ho-Chang J: Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res 77: 581–592, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Molgaard J, von Schenck H, Olsson AG: Effects of simvastatin on plasma lipid, lipoprotein and apolipoprotein concentrations in hypercholesterolaemia. Eur Heart J 9: 541–551, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ: Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev 30: 609–641, 2004 [DOI] [PubMed] [Google Scholar]
- 22.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360: 7–22, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Coons JC: Hydroxymethylglutaryl-coenzyme A reductase inhibitors in osteoporosis management. Ann Pharmacother 36: 326–330, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, Baehner F, Kumar AS, Adduci K, Marx C, Petricoin EF, Liotta LA, Winters M, Benz S, Benz CC: Breast cancer growth prevention by statins. Cancer Res 66: 8707–8714, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Matar P, Rozados VR, Binda MM, Roggero EA, Bonfil RD, Scharovsky OG: Inhibitory effect of Lovastatin on spontaneous metastases derived from a rat lymphoma. Clin Exp Metastasis 17: 19–25, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Van Aelst L, D'Souza-Schorey C: Rho GTPases and signaling networks. Genes Dev 11: 2295–2322, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Laufs U, Liao JK: Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 273: 24266–24271, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Hall A: Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Paterson HF, Self AJ, Garrett MD, Just I, Aktories K, Hall A: Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol 111: 1001–1007, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y: Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene 9: 273–279, 1994 [PubMed] [Google Scholar]
- 31.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M: Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 272: 12257–12260, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K: Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275: 1308–1311, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Noda Y, Hata Y, Hisatomi T, Nakamura Y, Hirayama K, Miura M, Nakao S, Fujisawa K, Sakamoto T, Ishibashi T: Functional properties of hyalocytes under PDGF-rich conditions. Invest Ophthalmol Vis Sci 45: 2107–2114, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Dong X, Chen N, Xie L, Wang S: Prevention of experimental proliferative vitreoretinopathy with a biodegradable intravitreal drug delivery system of all-trans retinoic acid. Retina 26: 210–213, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Fastenberg DM, Diddie KR, Dorey K, Ryan SJ: The role of cellular proliferation in an experimental model of massive periretinal proliferation. Am J Ophthalmol 93: 565–572, 1982 [DOI] [PubMed] [Google Scholar]
- 36.Ito S, Sakamoto T, Tahara Y, Goto Y, Akazawa K, Ishibashi T, Inomata H: The effect of tranilast on experimental proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol 237: 691–696, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Robbins SG, Mixon RN, Wilson DJ, Hart CE, Robertson JE, Westra I, Planck SR, Rosenbaum JT: Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Invest Ophthalmol Vis Sci 35: 3649–3663, 1994 [PubMed] [Google Scholar]
- 38.Hinton DR, Spee C, He S, Weitz S, Usinger W, LaBree L, Oliver N, Lim JI: Accumulation of NH2-terminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care 27: 758–764, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Hinton DR, He S, Jin ML, Barron E, Ryan SJ: Novel growth factors involved in the pathogenesis of proliferative vitreoretinopathy. Eye 16: 422–428, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Kauffmann DJ, van Meurs JC, Mertens DA, Peperkamp E, Master C, Gerritsen ME: Cytokines in vitreous humor: interleukin-6 is elevated in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 35: 900–906, 1994 [PubMed] [Google Scholar]
- 41.Gamulescu MA, Chen Y, He S, Spee C, Jin M, Ryan SJ, Hinton DR: Transforming growth factor beta2-induced myofibroblastic differentiation of human retinal pigment epithelial cells: regulation by extracellular matrix proteins and hepatocyte growth factor. Exp Eye Res 83: 212–222, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Serajuddin AT, Ranadive SA, Mahoney EM: Relative lipophilicities, solubilities, and structure-pharmacological considerations of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors pravastatin, lovastatin, mevastatin, and simvastatin. J Pharm Sci 80: 830–834, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Hamelin BA, Turgeon J: Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci 19: 26–37, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Chauhan NB, Siegel GJ, Feinstein DL: Effects of lovastatin and pravastatin on amyloid processing and inflammatory response in TgCRND8 brain. Neurochem Res 29: 1897–1911, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi M, Kaido F, Kagawa T, Itagaki S, Hirano T, Iseki K: Preventive effects of bicarbonate on cerivastatin-induced apoptosis. Int J Pharm 341: 181–188, 2007 [DOI] [PubMed] [Google Scholar]
- 46.El-Ghrably IA, Dua HS, Orr GM, Fischer D, Tighe PJ: Intravitreal invading cells contribute to vitreal cytokine milieu in proliferative vitreoretinopathy. Br J Ophthalmol 85: 461–470, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guidry C, Hook M: Endothelins produced by endothelial cells promote collagen gel contraction by fibroblasts. J Cell Biol 115: 873–880, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guidry C: Tractional force generation by porcine Muller cells: development and differential stimulation by growth factors. Invest Ophthalmol Vis Sci 38: 456–468, 1997 [PubMed] [Google Scholar]
- 49.Wilson HL, Schwartz DM, Bhatt HR, McCulloch CE, Duncan JL: Statin and aspirin therapy are associated with decreased rates of choroidal neovascularization among patients with age-related macular degeneration. Am J Ophthalmol 137: 615–624, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Song J, Deng PF, Stinnett SS, Epstein DL, Rao PV: Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci 46: 2424–2432, 2005 [DOI] [PubMed] [Google Scholar]