Abstract
OBJECTIVE—Pharmacogenomics is a key component of personalized medicine. The Israel Cardiovascular Events Reduction with Vitamin E Study, a prospective placebo-controlled study, recently demonstrated that vitamin E could dramatically reduce CVD in individuals with diabetes and the haptoglobin (Hp) 2-2 genotype (40% of diabetic individuals). However, because of the large number of clinical trials that failed to demonstrate benefit from vitamin E coupled with the lack of a mechanistic explanation for why vitamin E should be beneficial only in diabetic individuals with the Hp 2-2 genotype, enthusiasm for this pharmacogenomic paradigm has been limited. In this study, we sought to provide such a mechanistic explanation based on the hypothesis that the Hp 2-2 genotype and diabetes interact to promote HDL oxidative modification and dysfunction.
RESEARCH DESIGN AND METHODS—Hb and lipid peroxides were assessed in HDL isolated from diabetic individuals or mice with the Hp 1-1 or Hp 2-2 genotypes. HDL function was assessed based on its ability to promote cholesterol efflux from macrophages. A crossover placebo-controlled study in Hp 2-2 diabetic humans and in Hp 1-1 and Hp 2-2 diabetic mice assessed the ability of vitamin E to favorably modify these structural and functional parameters.
RESULTS—Hb and lipid peroxides associated with HDL were increased and HDL function was impaired in Hp 2-2 diabetic individuals and mice. Vitamin E decreased oxidative modification of HDL and improved HDL function in Hp 2-2 diabetes but had no effect in Hp 1-1 diabetes.
CONCLUSIONS—Vitamin E significantly improves the quality of HDL in Hp 2-2 diabetic individuals.
Pharmacogenomics is a key component of personalized medicine (1). Therapy targeted to a specific patient based on his or her genetically determined pathophysiology responsible for the disease offers the possibility of significantly improving patient care and reducing costs. However, despite the clear public health and economic benefits that would be attained by such an approach, this paradigm has not been successfully applied to a common disease.
Cardiovascular disease (CVD) is responsible for 75% of deaths among individuals with diabetes, and yearly expenditures for CVD in diabetes exceed $200 billion (2). Neither conventional risk factors nor the degree of glycemic control adequately predict which individuals with diabetes develop CVD, suggesting the existence of genetic susceptibility factors.
A polymorphism in the haptoglobin (Hp) gene may define which individuals with diabetes are at greatest risk of CVD. There exist two classes of alleles at the Hp locus denoted 1 and 2 with three possible Hp genotypes 1-1, 2-1, and 2-2 (3). In five independent longitudinal studies performed in ethnically and geographically diverse groups, individuals with the Hp 2-2 genotype and diabetes were found to have a two- to fivefold increased risk of CVD compared with diabetic individuals without the Hp 2-2 genotype (4–8). The prevalence of the Hp 2-2 genotype in the diabetic population in most Western countries is ∼40%, making this a common polymorphism.
The Hp polymorphism differs from nearly all polymorphisms being assessed in genome-wide association studies because it is a functional polymorphism (3). Understanding functional differences between the Hp 1 and Hp 2 allelic protein products, particularly in diabetes, may provide insight into why Hp 2-2 diabetic individuals have more CVD and how this increased burden of disease might be reduced. The most well-understood function of Hp is to bind Hb released from erythrocytes (3). Each day, >6 g Hb is released into the bloodstream due to turnover of erythrocytes, and heme iron in this Hb is a powerful oxidizing agent (9,10). Hp, which is present in a 400-fold molar excess to free Hb under normal conditions, binds Hb, reducing its ability to mediate oxidative modifications and directing its removal from the blood via the monocyte/macrophage CD163 Hp-Hb scavenger receptor (11).
More than 5 years ago, motivated by in vitro studies demonstrating that the Hp 2-2 protein provides inferior protection against Hb-mediated oxidative stress (9,10), coupled with the suggested importance of oxidative stress in diabetic atherosclerosis (12), we sought to determine whether antioxidant therapy might be particularly beneficial to the Hp 2-2 diabetic cohort. We first tested this hypothesis by examining stored samples from the Heart Outcomes Prevention Evaluation (HOPE) study, which had failed to demonstrate benefit from vitamin E (13). We found that myocardial infarction and CVD death were reduced by >40 and 50%, respectively, in Hp 2-2 diabetic HOPE participants who received vitamin E (14). To prospectively test the hypothesis, we initiated a double-blind randomized placebo-controlled study of vitamin E in 1,434 Hp 2-2 diabetic individuals (Israel Cardiovascular Events Reduction with Vitamin E [ICARE] Study). We found that vitamin E supplementation was associated with a >50% reduction in the combined primary outcome of stroke, myocardial infarction, and cardiovascular death in Hp 2-2 diabetes (7).
Enthusiasm for these findings, despite the considerable public health and economic benefits that they suggest, has been muted. Our study comes in the wake of numerous large clinical trials that failed to demonstrate that vitamin E provides any protection against CVD and may be harmful (13,15–20). Further hampering acceptance of this paradigm is the lack of a rational pathophysiological and pharmacogenomic mechanism to explain why Hp 2-2 diabetic individuals have an increased risk of CVD and how vitamin E mitigates this risk. In this study, we sought to provide a rationale for the pharmacogenomic application of the Hp genotype to prevent CVD in diabetes by elucidating the unique structural modifications and dysfunctional nature of HDL in Hp 2-2 diabetic individuals and how these structural and functional changes in HDL are rapidly reversed with vitamin E.
RESEARCH DESIGN AND METHODS
Ethical approval.
These studies were approved by the institutional review boards of the Rambam Medical Center and the Technion. All participants provided informed consent.
Human subjects.
All studies except where indicated were performed with type 2 diabetic individuals recruited from ICARE (7). The Hp type of participants was determined by gel electrophoresis, which has a 100% correspondence with the Hp genotype determined by PCR (21).
Animal studies.
The Hp 2 allele is present only in humans. All other species have only an Hp 1 allele, which is highly homologous with the human Hp 1 allele. We have previously described the construction of a murine Hp 2 allele and the targeting of its insertion by homologous recombination to the murine Hp genetic locus (22). Mice were fed normal chow. Diabetes was produced with streptozotocin at 2 months of age and studied after a diabetes duration of 1 month.
Measurement of the clearance rate of Hp-Hb in vivo.
Hp and Hb were labeled with 125I using chloramine T (23). 125I-labeled Hp-Hb was injected in the tail vein of mice (one million counts per minute [cpm] corresponding to 50 ng), and counts per minute [cpm] in serum was measured at defined intervals.
Purification of HDL.
Ultracentrifuge-purified HDL was prepared as previously described (24). Immunopurified HDL was prepared from human or murine serum using a rabbit anti-apoA1 antibody and protein A/G Sepharose.
HDL-associated lipid peroxides and HDL-associated redox active iron.
Total lipid peroxides (nanomoles) associated with HDL were assessed in 1 μg immunopurified HDL (25). For the assessment of redox active iron associated with HDL, the time-dependent oxidation of dihydrorhodamine by immunopurified HDL was assessed in the presence and absence of desferroxamine (25).
Assessment of the association of native Hp and Hb with HDL.
Hp and Hb were assessed in immunopurified HDL by Western blot with either rabbit anti-Hp or anti-Hb antiserum and alkaline phosphatase–coupled goat anti-rabbit antiserum for detection.
Cholesterol efflux.
Serum from mice or humans treated with placebo or vitamin E was assessed for its ability to promote the efflux of [3H]cholesterol from macrophages (26).
Study drugs.
For murine studies, vitamin E was administered in the drinking water at 40 mg · kg−1 · day−1 for 30 days beginning 1 month after onset of diabetes. For human studies, placebo or vitamin E (400 IU natural source d-α tocopherol/day) capsules were provided in a double-blinded format.
Human crossover study design.
The study (clinical trial reg. no. NCT00314379) was performed in 18 Hp 2-2 diabetic individuals who were not on antioxidant therapy at baseline (baseline characteristics provided in supplementary Table 1 available in an online appendix at http://dx.doi.org/10.2337/db08-0450). Blood was taken at baseline (test 1). Participants were randomly allocated to initially receive vitamin E or placebo for 2 months, after which another blood sample was taken (test 2), and this initial treatment was stopped. Two weeks later, the participants were crossed over to the other treatment, and a blood sample was taken after 2 months of treatment (test 3).
Statistical analysis.
All results are reported as means ± SE. Comparison between groups was performed using Student's t test or ANOVA and the Tukey-Kramer honestly significant difference method for comparisons of means test as appropriate, with a P value of ≤0.05 considered significant.
RESULTS
The half-life of the Hp 2-2–Hb complex is markedly increased in diabetes.
We sought to test the hypothesis that clearance of Hp-Hb from the plasmatic compartment is both Hp genotype and diabetes dependent. We tested this hypothesis by injecting 125I-Hp-Hb into Hp 1-1 or Hp 2-2 mice with or without diabetes. The half-life of Hp 1-1–Hb was ∼20 min with or without diabetes. The half-life of Hp 2-2–Hb was ∼50 min in mice without diabetes and >100 min in mice with diabetes (Table 1).
TABLE 1.
Half-life of the Hp 1-1–Hb and Hp 2-2–Hb complex in nondiabetic and diabetic mice and rats
| Animal strain | Diabetes | n | Hp-Hb complex | Half-life (min) |
|---|---|---|---|---|
| Hp 1 mice | − | 5 | Hp 1 | 20.4 ± 1.7 |
| Hp 1 mice | + | 4 | Hp 1 | 22.9 ± 2.1 |
| Hp 1 mice | − | 5 | Hp 2 | 57.8 ± 2.8 |
| Hp 1 mice | + | 4 | Hp 2 | 78.2 ± 4.1 |
| Hp 2 mice | − | 5 | Hp 1 | 24.5 ± 1.8 |
| Hp 2 mice | + | 6 | Hp 1 | 18.6 ± 1.8 |
| Hp 2 mice | − | 5 | Hp 2 | 53.8 ± 3.3 |
| Hp 2 mice | + | 6 | Hp 2 | 103 ± 3.9 |
| Rat | − | 4 | Hp 1 | 17.3 ± 2.1 |
| Rat | − | 4 | Hp 2 | 48.0 ± 3.8 |
Data are means ± SE. In the absence of diabetes, the half-life of the Hp 2-Hb complex was significantly increased compared with the Hp 1-Hb complex in all animals and strains studied (P < 0.0001). Diabetes had no effect on the half-life of the Hp 1-Hb complex. However, the half-life of the Hp 2-Hb complex was significantly increased in both Hp 1 and Hp 2 diabetic mice compared with that observed in Hp 1 mice or Hp 2 mice without diabetes (P < 0.015 comparing the Hp 2-Hb complex in Hp 1 nondiabetes vs. Hp 1 diabetes; and P < 0.0001 comparing the Hp 2-Hb complex in Hp 2 nondiabetes vs. Hp 2 diabetes). Moreover, the half-life of the Hp 2-Hb complex was increased to a greater degree in Hp 2 diabetic mice compared with Hp 1 diabetic mice (103 ± 3.9 vs. 78.2 ± 4.1 min, P < 0.005).
Hp is an HDL-associated protein in humans.
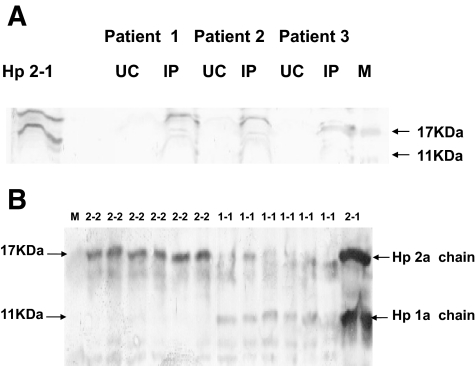
Hp has been shown by some but not all investigators to be an HDL-associated protein (24,27–29). Critical analysis of these prior studies suggested that the key difference in these studies was in the manner in which the HDL was prepared. We assessed the presence of Hp in human HDL prepared from serum by either ultracentrifugation or immunoabsorbtion (Fig. 1A). We found that Hp is present in the HDL when the HDL is prepared by immunoabsorbtion but not if the HDL is prepared by ultracentrifugation. Although we found that Hp is present in the HDL of all individuals, because the Hp 2-2 protein is made up of 3–10 disulfide-linked Hp monomers compared with the Hp 1-1 protein, which is made up of only 2 disulfide-linked Hp monomers (3), significantly more Hp was detected in the HDL of Hp 2-2 individuals (Fig. 1B).
FIG. 1.
Hp is an HDL-associated protein. A: Hp is an HDL-associated protein in humans. One microgram of HDL prepared from three different individuals with diabetes by ultracentrifugation (UC) or immunoprecipitation (IP) was subjected to Western blot analysis for Hp. Purified Hp 2-1 protein was run as a control to indicate the location of the Hp α-chains. M, MW marker. An immunoreactive band for Hp is seen only in HDL prepared by immunoprecipitation. To confirm equal loading of protein in all lanes, the same blot was subsequently developed with an anti-ApoA1 antibody. B: Increased amount of Hp 2-2 protein associated with human HDL. Hp was assessed in the HDL immunoprecipitate by Western blotting. Hp α-chains detected by Western blotting are shown. Purified Hp 2-1 protein (2-1) was run as control to indicate the location of Hp 2-α (17 kDa) and Hp 1-α chain (11 kDa). Samples denoted 2-2 or 1-1 represent the HDL immunoprecipitate from six different individuals with either the Hp 2-2 or Hp 1-1 genotype.
The amount of Hb associated with HDL is increased in Hp 2-2 diabetic individuals.
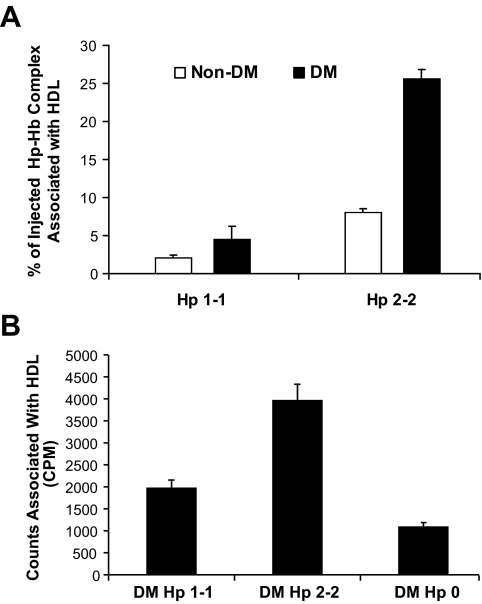
The binding of Hp to HDL and the high affinity of Hp for Hb suggested that Hp may tether Hb to HDL. Furthermore, the impaired clearance of Hp 2-2–Hb in diabetes would suggest that there might be more of the complex associated with HDL in Hp 2-2 diabetic mice or humans. We first investigated this possibility by assessing 125I-Hp-Hb in the HDL immunoprecipitate and found a dramatic increase, representing >25% of all injected cpm, in the amount of Hp 2-2–Hb associated with HDL in Hp 2-2 diabetic mice (Fig. 2A). However, in mice genetically deficient for Hp (Hp knockout), no 125I-Hb was found associated with HDL (zero cpm in HDL immunoprecipitate), demonstrating that Hp is critical for binding of Hb to HDL.
FIG. 2.
The association of 125Hp-Hb and 125Hb with HDL is Hp genotype- and diabetes-dependent. A: Increased association of injected Hp-Hb with HDL in Hp 2-2 diabetic mice. 125I-Hp-Hb complex (one million cpm) was injected in the tail vein. The percentage of the injected cpm that coimmunoprecipitated with HDL at all time points after the injection (1–180 min) is shown (n = 5 for Hp 1-1 and Hp 2-2 nondiabetes and n = 6 for Hp 1-1 and Hp 2-2 diabetes). There was a significant increase in cpm in the HDL immunoprecipitate of Hp 2-2 diabetes (P < 0.0001 compared with Hp 2-2 nondiabetes). There was no significant difference in cpm in the HDL immunoprecipitate of Hp 1-1 diabetes compared with Hp 1-1 nondiabetes (P = 0.24). B: The ability of 125I-Hb to bind to human HDL in vitro is increased in Hp 2-2 and decreased in Hp 0. 125I-Hb was incubated with serum from individuals with Hp 1-1, Hp 2-2, or Hp 0. 125I-Hb associating with HDL was assessed by immunoprecipitation, and the mean ± SE for 10 individuals from each of the three groups is shown. There was significantly more 125I-Hb associated with HDL using serum from Hp 2-2 individuals compared with Hp 1-1 individuals (P < 0.0001). The amount of 125I-Hb associating with HDL using Hp 0 serum was significantly less than that observed in Hp 1-1 serum (P < 0.002). Note that Hp 0 does not indicate that these individuals lack Hp, but rather that the level of Hp is below the level of detection by gel electrophoresis.
Parallel studies were performed in humans. First, we incubated serum from Hp 1-1, Hp 2-2, or Hp 0 (individuals in whom Hp was not detectable by gel electrophoresis) with 125I-Hb and assessed the amount of radioactive label in the HDL immunoprecipitate. We found significantly greater Hb associated with HDL in Hp 2-2 serum (Fig. 2B).
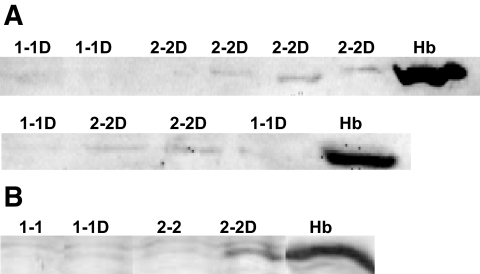
We then assessed the amount of endogenous Hb associated with HDL in Hp 1-1 and Hp 2-2 mice and humans with and without diabetes by Western blot. We detected substantial amounts of Hb associated with HDL in >90% of Hp 2-2 diabetic individuals but failed to find Hb associated with HDL in any Hp 1-1 diabetic individuals or in any individuals (Hp 1-1 or Hp 2-2) without diabetes (Fig. 3A). Similarly, we found a marked increase in the amount of endogenous Hb associated with HDL in Hp 2-2 diabetic mice (Fig. 3B).
FIG. 3.
Hb is an HDL-associated protein in Hp 2-2 diabetic humans and mice. A: The amount of Hb associated with HDL is increased in Hp 2-2 diabetic individuals. Western blot for Hb of HDL immunoprecipitate of serum of Hp 1-1 or Hp 2-2 diabetic individuals. Hb was identifiable in 14 of 15 diabetic individuals with the Hp 2-2 genotype and in 0 of 15 of the diabetic individuals with the Hp 1-1 genotype. Hb was not found associated with HDL from nondiabetic Hp 1-1 or Hp 2-2 individuals (not shown). Hb indicates purified Hb used as positive control. B: The amount of Hb associated with HDL is increased in Hp 2-2 diabetic mice. Western blot for Hb of HDL immunoprecipitate of serum of Hp 1-1 or Hp 2-2 mice with or without diabetes (D). Hb indicates purified Hb used as positive control.
The HDL of Hp 2-2 diabetic humans contains redox active iron and has increased lipid peroxides.
The increased association of the pro-oxidant Hb with HDL in Hp 2-2 diabetic individuals may result in the increased oxidative modification of HDL-associated lipid and proteins and may paradoxically make the HDL a pro-oxidant (30). We assessed oxidation of HDL-associated lipids in the HDL of Hp 1-1 and Hp 2-2 diabetic individuals and found a marked increase in the amount of lipid peroxides in the HDL of Hp 2-2 diabetes (1.8 ± 0.2 nmol/μg HDL vs. 1.2 ± 0.2 nmol/μg HDL, n = 20, P = 0.04). HDL from Hp 2-2 diabetic individuals was also associated with an increased amount of iron capable of mediating oxidation (4.4 ± 0.8 pmol redox active iron/μg HDL vs. 1.8 ± 0.5 pmol redox active iron/μg HDL, n = 20, P = 0.02).
The HDL in Hp 2-2 diabetes is dysfunctional.
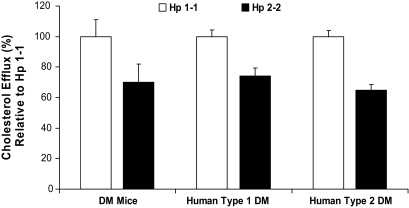
We assessed the ability of serum from Hp 1-1 or Hp 2-2 diabetic mice or humans with type 1 or type 2 diabetes to promote cholesterol efflux from macrophages in vitro. We found a significant 30–40% decrease in HDL function in Hp 2-2 diabetes compared with Hp 1-1 diabetes (Fig. 4). No differences were found between Hp 1-1 and Hp 2-2 in the absence of diabetes or between Hp 1-1 with and without diabetes (data not shown) (26).
FIG. 4.
HDL function is impaired in Hp 2-2 diabetic mice and humans. Cholesterol efflux from macrophages incubated with serum from Hp 2-2 diabetic mice and Hp 2-2 humans with type 1 diabetes and with type 2 diabetes is significantly decreased compared with Hp 1-1 diabetic mice and Hp 1-1 type 1 and type 2 diabetic humans (P = 0.0001, n = 10 comparing Hp 1-1 vs. Hp 2-2 diabetic mice; P < 0.0006, n = 15 comparing Hp 1-1 vs. Hp 2-2 type 1 diabetic individuals; P < 0.001, n = 30 comparing Hp 1-1 vs. Hp 2-2 type 2 diabetic individuals). Efflux is expressed as the percentage of that obtained for Hp 1-1 diabetic mice, type 1 diabetic, and type 2 diabetic individuals, respectively.
HDL oxidative modification and dysfunction can be corrected in Hp 2-2 diabetes with vitamin E.
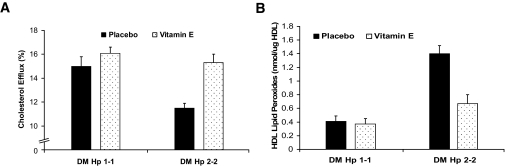
We assessed the ability of vitamin E to reduce HDL oxidative modification (HDL-associated lipid peroxides) and to improve HDL function in Hp 1-1 or Hp 2-2 diabetic mice. We found that vitamin E had no effect on HDL lipid peroxides or function in Hp 1-1 diabetic mice. However, vitamin E significantly improved HDL function and reduced HDL lipid peroxides in Hp 2-2 diabetic mice, restoring function and reducing lipid peroxides to levels similar to those found in Hp 1-1 diabetes (Fig. 5).
FIG. 5.
Vitamin E improves HDL function and reduces HDL oxidative modification in Hp 2-2 diabetic mice but not in Hp 1-1 diabetic mice. A: Vitamin E improves the ability of serum of Hp 2-2 diabetic mice, but not Hp 1-1 diabetic mice, to promote cholesterol efflux from macrophages. There was a significant difference in efflux elicited by serum from Hp 1-1 and Hp 2-2 diabetic mice (P = 0.002 comparing placebo groups). Vitamin E significantly improved cholesterol efflux in Hp 2-2 diabetic mice (P = 0.0006 comparing Hp 2-2 placebo vs. Hp 2-2 vitamin E). Efflux elicited by the serum of Hp 2-2 diabetic mice treated with vitamin E was not significantly different from that elicited by Hp 1-1 diabetic mice. Vitamin E had no effect on efflux in Hp 1-1 diabetic mice (P = 0.29). B: Vitamin E reduces HDL-associated lipid peroxides in Hp 2-2 diabetic mice but not in Hp 1-1 diabetic mice. There was a significant difference in HDL-associated lipid peroxides between Hp 1-1 and Hp 2-2 diabetic mice (P = 0.0001). Vitamin E significantly reduced lipid peroxides in Hp 2-2 diabetic mice (P = 0.001 comparing Hp 2-2 placebo vs. Hp 2-2 vitamin E) but had no effect on efflux in Hp 1-1 diabetic mice (P = 0.74 comparing Hp 1-1 placebo vs. Hp 1-1 vitamin E).
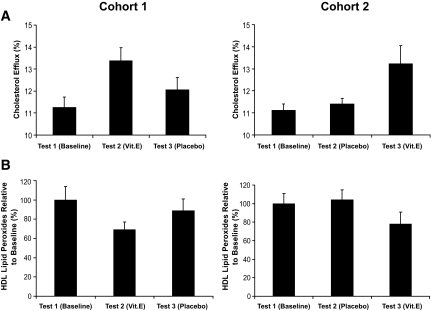
In humans, we assessed the ability of vitamin E to improve HDL function and reduce HDL-associated lipid peroxides in Hp 2-2 diabetes in a crossover study. We found that vitamin E significantly improved HDL function by 30–40% and reduced HDL lipid peroxides by 20–30%. Notably, in this crossover design we found that after vitamin E had restored HDL function and reduced lipid peroxides and the vitamin E was then withdrawn, HDL function deteriorated, and HDL-associated lipid peroxides increased to levels seen at baseline within 2 months after cessation of vitamin E supplementation (Fig. 6).
FIG. 6.
Vitamin E improves HDL function and reduces HDL oxidative modification in Hp 2-2 diabetic humans. Crossover-design, placebo-controlled, double-blind trial. Eighteen Hp 2-2 diabetic individuals divided into two cohorts were randomized to either vitamin E or placebo and treated for 2 months. After a 2-week washout, patients were crossed over to the other treatment and treated for an additional 2 months. Blood samples were taken at baseline (test 1), after 2 months of the initial treatment (test 2), and after 2 months with the second treatment (test 3). A: Improvement in cholesterol efflux stimulated by Hp 2-2 serum with vitamin E in humans. There was a significant improvement in efflux with vitamin E treatment (test 1-test 2 in cohort 1, P = 0.004; test 2-test 3 in cohort 2, P = 0.04) and no change with placebo treatment (test 1-test 2 in cohort 2, P = 0.33). Of note in cohort 1, test 3 is not significantly different from the baseline value, demonstrating that even though vitamin E improved HDL function (compare test 1-test 2), after a 2-month period without vitamin E, HDL function deteriorated to baseline levels (P = 0.13 comparing test 1-test 3 in cohort 1). B: Reduction in HDL-associated lipid peroxides with vitamin E. There was a significant reduction in lipid peroxides with vitamin E treatment (test 1-test 2 in cohort 1, P = 0.03; test 2-test 3 in cohort 2, P = 0.01) and no change with placebo treatment (test 1-test 2 in cohort 2, P = 0.35). Of note in cohort 1, test 3 was not significantly different from the baseline value, demonstrating that even though vitamin E reduced lipid peroxides (compare test 1-test 2), 2 months after the vitamin E was stopped, lipid peroxides returned to baseline levels (P = 0.31 comparing test 1-test 3 in cohort 1).
DISCUSSION
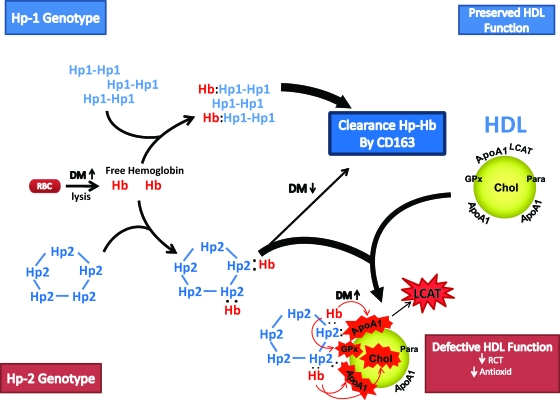
In this translational study, we have provided a pathophysiological and pharmacogenomic rationale as to why vitamin E may provide cardiovascular benefit to individuals with the Hp 2-2 genotype and diabetes (Fig. 7). The main reason why Hp 2-2 diabetic individuals appear to uniquely derive benefit from vitamin E is that there is substantially more Hb associated with the HDL of Hp 2-2 diabetic individuals. This key structural difference between HDL in Hp 1-1 and Hp 2-2 diabetic individuals is the result of an impairment in the CD163-mediated clearance of Hp-Hb in Hp 2-2 diabetes (23,31).
FIG. 7.
Hb released intravascularly from erythrocytes (RBC) is rapidly bound by Hp protein to form an Hp-Hb complex. In Hp 2-2 diabetic individuals, the complex is cleared more slowly than in Hp 1-1 diabetic individuals by the scavenger receptor CD163. The Hp-Hb complex can bind to Apo A1 in HDL, with increased binding of Hp 2-2–Hb occurring due its increased avidity for HDL and its increased plasma concentration. The Hp 2-2–Hb complex, but not the Hp 1-1–Hb complex, when bound to HDL can produce reactive oxygen species that can oxidize protein (i.e., ApoA1, glutathione peroxidase [GPx], and LCAT) and lipid components (cholesterol) of HDL and render the HDL dysfunctional (because of decreased reversed cholesterol transport [RCT] and antioxidant activity), proatherogenic, and prothrombotic.
The association of Hb with HDL results in the oxidative modification of HDL-associated proteins and lipids. The loss of function of HDL may be the direct result of its oxidative modification. Hb can oxidize ApoA1 (32), and oxidation of ApoA1 interferes with its ability to promote cholesterol efflux from macrophages (33). Oxidative modification of HDL-associated lipids can result in the inactivation of HDL-associated antioxidant enzymes such as glutathione peroxidase and paraoxonase (30).
A binding site for Hp on ApoA1 (amino acid residues 141–164) has been identified (27). Interestingly, lecithin acyl transferase (LCAT), whose activity is dependent on its binding to ApoA1, binds to ApoA1 residues 159–170 (34). Hazen and colleagues (34) have shown that nitration or oxidation of Tyr166 in ApoA1 results in an inhibition of the binding of LCAT to ApoA1. We have previously demonstrated a marked decrease in LCAT activity in Hp 2-2 diabetic individuals (26). We propose that binding of Hp-Hb to a site adjacent to the LCAT binding site may result in the nitration or oxidation (35) of Tyr166, resulting in an impairment in LCAT activity. An impairment in LCAT activity would be expected to impair the maturation of HDL and its ability to promote cholesterol efflux (36). We have found a very tight correlation between LCAT activity and cholesterol efflux in diabetic individuals (r = 0.81, P = 0.0002) (26).
The ability of Hb associated with HDL in Hp 2-2 diabetic individuals to sequester nitric oxide (NO) (37) may have a clinical significance that is of greater importance (38) than the effect of Hb on the function of HDL in reverse cholesterol transport. HDL in Hp 2-2 diabetes may actually be proatherogenic and prothrombotic by limiting NO bioavailability.
These mechanisms are also relevant to the atherosclerotic plaque. Plaque hemorrhage is recognized as an important determinant of plaque stability (39). The Hp genotype may determine the response to plaque hemorrhage (40). Impaired clearance of Hb in Hp 2-2 diabetic plaques may lead to oxidative modification of HDL within the plaque and an impairment of its ability to promote reverse cholesterol transport.
The current focus of the medical community toward HDL has been to increase its concentration. The hypothesis presented here may help to explain the dramatically increased CVD risk in patients with type 1 diabetes, despite a usually normal HDL and lipoprotein profile. Moreover, increasing the amount of HDL in individuals in whom the HDL is dysfunctional and potentially proatherogenic may actually be harmful (30). We believe that this is the first demonstration in humans that HDL function can be improved in a specific population with vitamin E. However, not all HDL dysfunction can be attributed to Hb-mediated oxidation, and consequently, not all individuals would be expected to improve the quality of their HDL with vitamin E, as we have demonstrated here with Hp 1-1 diabetes.
In conclusion, we believe that we have provided a pathophysiological and pharmacogenomic rationale as to why vitamin E may provide benefit to the Hp 2-2 diabetic cohort. The potential public health and economic benefits from application of this paradigm are enormous. We hope that these findings will encourage testing of this hypothesis in a large-scale clinical trial that could result in the establishment of treatment guidelines for individuals with diabetes.
Supplementary Material
Acknowledgments
A.P.L. has received support from the Kennedy Leigh Charitable Trust, the Israel Science Foundation, and the U.S. Israel Binational Science Foundation.
Published ahead of print at http://diabetes.diabetesjournals.org on 3 July 2008.
Clinical trial reg. no. NCT00314379, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Shastry BS: Pharmacogenetics and the concept of individualized medicine. Pharmacogenomics J 6: 16–21, 2006 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association: Economic costs of diabetes in the U.S. in 2007. Diabetes Care 31: 1–20, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bowman BH, Kurosky A: Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet 12: 189–261, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Levy AP, Hochberg I, Jablonski K, Resnick H, Best L, Lee ET, Howard BV: Haptoglobin phenotype and the risk of cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Card 40: 1984–1990, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP: Haptoglobin genotype is predictive of major adverse cardiac events in the one-year period after PTCA in individuals with diabetes. Diabetes Care 26: 2628–2631, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Suleiman M, Aronson D, Asleh R, Kapelovich MR, Roguin A, Meisel SR, Schochat M, Suleiman A, Reisner SA, Markiewicz W, Hammerman H, Lotan R, Levy NS, Levy AP: Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes 19: 2802–2806, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alsheik J, Bennett L, Kostenko M, Landau M, Keidar S, Levy Y, Khemlin A, Radan A, Levy AP: Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective, double-blinded clinical trial. Art Thromb Vasc Biol 28: 341–347, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Costacou T, Ferrell RE, Orchard TJ: Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes 57: 1702–1706, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N: Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry 43: 3899–3906, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Frank M, Lache O, Enav B, Szafranek T, Levy NS, Ricklis R, Levy AP: Structure/function analysis of the anti-oxidant properties of haptoglobin. Blood 98: 3693–3698, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK: Identification of the hemoglobin scavenger receptor. Nature 409: 198–201, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Giugliano D, Ceriello A, Paolisso G: Oxidative stress and diabetic vascular complications. Diabetes Care 19: 257–267, 1996 [DOI] [PubMed] [Google Scholar]
- 13.The Heart Outcomes Prevention Evaluation (HOPE) Study Investigators: Vitamin E supplementation and cardiovascular events in high risk patients. N Engl J Med 342: 154–160, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Levy AP, Gerstein H, Lotan R, Ratner R, McQueen M, Lonn E, Pogue J: The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes (Letter). Diabetes Care 27: 2767, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Heart Protection Study Collaborative Group: MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high risk individuals: a randomized placebo controlled trial. Lancet 360: 7–22, 2002. 12114036 [Google Scholar]
- 16.Collaborative Group of the Primary Prevention Project: Low dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice. Lancet 357: 89–95, 2001 [DOI] [PubMed] [Google Scholar]
- 17.GISSI-Prevenzione Investigators: Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 354: 447–455, 1999 [PubMed] [Google Scholar]
- 18.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE: Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women's Health Study: a randomized controlled trial. JAMA 294: 56–65, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Brown BG, Crowley J: Is there any hope for vitamin E? JAMA 293: 1387–1390, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Miller ER, Barriuso RP, Dalal D, Riemersma RA, Appel LJ, Guallar E: Meta-analysis: high dosage vitamin E supplementation may increase all cause mortality. Ann Intern Med 142: 37–46, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Koch W, Latz W, Eichinger M, Roguin A, Levy AP, Schomig A, Kastrati A: Genotyping of common haptoglobin polymorphism Hp1/2 based on the polymerase chain reaction. Clin Chem 48: 1377–1382, 2002 [PubMed] [Google Scholar]
- 22.Levy AP, Levy JE, Kalet-Litman, Miller-Lotan R, Levy NS, Asaf R, Guetta J, Yang C, Purushothaman KR, Fuster V, Moreno PR: Haptoglobin genotype is a determinant of iron, lipid peroxidation and macrophage accumulation in the atherosclerotic plaque. Arteriosclerosis Thromb Vasc Biol 27: 134–140, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Asleh R, Marsh S, Shiltruck M, Binah O, Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O, Cohen O, Levy NS, Levy AP: Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res 92: 1193–1200, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Vaiser T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P: Shotgun proteomics implicates protease inhibition and complement activation in the anti-inflammatory properties of HDL. J Clin Invest 117: 746–756, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP: Haptoglobin genotype and diabetes dependent differences in iron mediated oxidative stress in vitro and in vivo. Circ Res 96: 435–441, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Asleh R, Miller-Lotan R, Aviram M, Hayek T, Yulish M, Levy JE, Miller B, Blum S, Milman U, Shapira C, Levy AP: Haptoglobin genotype is a regulator of reverse cholesterol transport in diabetes in vitro and in vivo. Circ Res 99: 1419–1425, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Spagnuolo MS, Cigliano L, D'Andrea LD, Pedone C, Abrescia P: Assignment of the binding site for haptoglobin on apolipoprotein A-1. J Biol Chem 280: 1193–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kunitake ST, Carilli CT, Lau K, Protter AA, Naya-Vigne J, Kane JP: Identification of proteins associated with apolipoprotein A-1 containing lipoproteins purified by selected-affinity immunoabsorbtion. Biochemistry 33: 1988–1993, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Rezaee F, Casetta B, Levels JH, Speijer D, Meijers JCM: Proteomic analysis of high density lipoprotein. Proteomics 6: 721–730, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Navab M, Ananthaaramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM: Mechanisms of disease: proatherogenic HDL-an evolving field. Nat Clin Pract 2: 504–511, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Levy AP, Purushothaman KR, Levy NS, Purushothaman M, Strauss M, Asleh R, Marsh S, Cohen O, Moestrup SK, Moller HJ, Zias EA, Benhayon D, Fuster V, Moreno PR: Downregulation of the hemoglobin scavenger receptor in individuals with diabetes and the Hp 2-2 genotype: implications for the response to intraplaque hemorrhage and plaque vulnerability. Circ Res 101: 106–110, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Salvatore A, Cigliano L, Bucci EM, Corpillo D, Velasco S, Carlucci A, Pedone C, Abrescia P: Haptoglobin binding to apolipoprotein A-1 prevents damage from hydroxyl radicals on its stimulatory activity of the enzyme lecithin-cholesterol acyl-transferase. Biochemistry 46: 11158–11168, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Shao B, Oda MN, Vaiser T, Oram JF, Heinecke JW: Pathways for oxidation of high-density lipoprotein in human cardiovascular disease. Curr Opin Mol Ther 8: 198–205, 2006 [PubMed] [Google Scholar]
- 34.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, Smith JD, Gogonea V, Hazen SL: The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol 14: 861–868, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Grzelak A, Balcerczyk A, Mateja A, Bartosz G: Hemoglobin can nitrate itself and other proteins. Biochim Biophys Acta 1528: 97–100, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Rader DJ: Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest 116: 3090–3100, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azarov I, He X, Jeffers A, Basu S, Ucer B, Hantgan RR, Levy A, Kim-Shapiro DB: Rate of nitric oxide scavenging by hemoglobin bound to haptoglobin. Nitric Oxide 18: 296–302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rother RP, Bell L, Hillmen P, Gladwin MT: The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293: 1653–1662, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, KutysR, Narula J, Finn AV, Virmani R: Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 349: 2316–2325, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Levy AP, Moreno PR: Intraplaque hemorrhage. Curr Mol Med 6: 479–488, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.