Abstract
OBJECTIVE—Sulforaphane is an activator of transcription factor NF-E2–related factor-2 (nrf2) that regulates gene expression through the promoter antioxidant response element (ARE). Nrf2 regulates the transcription of a battery of protective and metabolic enzymes. The aim of this study was to assess whether activation of nrf2 by sulforaphane in human microvascular endothelial cells prevents metabolic dysfunction in hyperglycemia.
RESEARCH DESIGN AND METHODS—Human microvascular HMEC-1 endothelial cells were incubated in low and high glucose concentrations (5 and 30 mmol/l, respectively), and activation of nrf2 was assessed by nuclear translocation. The effects of sulforaphane on multiple pathways of biochemical dysfunction, increased reactive oxygen species (ROS) formation, hexosamine pathway, protein kinase C (PKC) pathway, and increased formation of methylglyoxal were assessed.
RESULTS—Activation of nrf2 by sulforaphane induced nuclear translocation of nrf2 and increased ARE-linked gene expression, for example, three- to fivefold increased expression of transketolase and glutathione reductase. Hyperglycemia increased the formation of ROS—an effect linked to mitochondrial dysfunction and prevented by sulforaphane. ROS formation was increased further by knockdown of nrf2 and transketolase expression. This also abolished the counteracting effect of sulforaphane, suggesting mediation by nrf2 and related increase of transketolase expression. Sulforaphane also prevented hyperglycemia-induced activation of the hexosamine and PKC pathways and prevented increased cellular accumulation and excretion of the glycating agent methylglyoxal.
CONCLUSIONS—We conclude that activation of nrf2 may prevent biochemical dysfunction and related functional responses of endothelial cells induced by hyperglycemia in which increased expression of transketolase has a pivotal role.
There is an increased risk of vascular disease in diabetes that is a major cause of patient morbidity and mortality. This gives rise to a characteristic spectrum of diabetic microvascular disease (retinopathy, nephropathy, and neuropathy) and macrovascular disease (heart disease and stroke) (1–4). Vascular disease in diabetes is associated with dysfunction of endothelial cells in hyperglycemia. Activation of multiple pathways of biochemical dysfunction induced in vascular endothelial cells by high glucose concentration is thought to underlie the link of hyperglycemia in diabetes to the development of vascular disease (5,6). A common feature of endothelial cell dysfunction in hyperglycemia is increased formation of reactive oxygen species (ROS) by mitochondria, oxidative stress with inactivation of glyceraldehyde-3-phosphate dehydrogenase, and accumulation of triosephosphates and fructose-6-phosphate (7–9). There is an associated activation of protein kinase C (PKC), hexosamine pathway O-linked protein glycosylation, and increased glycation by methylglyoxal and other dicarbonyls forming advanced glycation end products (10–12). This appears to be driven mainly by the accumulation of glycolytic intermediates. Recent research has indicated that activation of the reduced pentosephosphate pathway by high-dose thiamine and related prodrug benfotiamine may counter this metabolic dysfunction (9,13,14), but little is known of the endogenous coordinated stress response to decrease triosephosphate accumulation and its link to increased ROS formation and oxidative stress in hyperglycemia.
NF-E2–related factor-2 (nrf2) is a member of the cap ‘n’ collar subfamily of bZIP transcription factors. It is an essential transactivator of genes containing an antioxidant response element (ARE) in their promoter (rev. in 15,16). ARE-linked genes include a battery of protective and metabolic enzymes: γ-glutamylcysteine ligase, glutathione reductase (GSHRd), aldo-keto reductase (AKRd), glutathione transferases, quinone reductase (NQO1), nrf2 (17), and others (18). Nrf2-linked gene expression has a key role in the protection of cells against oxidative stress, carbonyl compounds, and electrophilic agents. Interestingly, the thiamine-dependent enzyme transketolase and transaldolase are also ARE-linked genes (18). Transketolase is considered to be the rate-controlling enzyme in the pentosephosphate pathway.
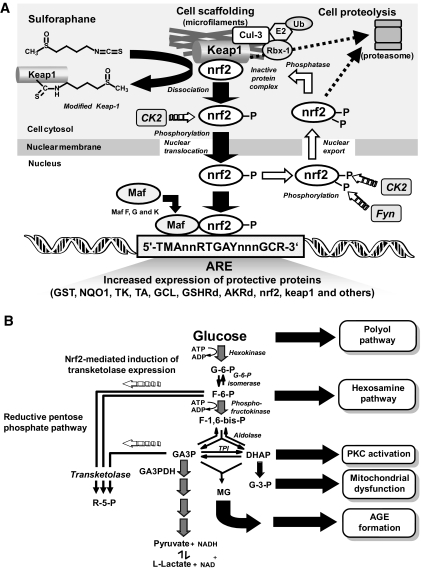
Under basal conditions, nrf2 is complexed with Kelch-like ECH-associated protein 1 (Keap1), a BTB-Kelch protein. Keap1 is a substrate adaptor protein for a Cul 3–dependent E3 ubiquitin ligase complex, directing nrf2 for proteasomal degradation (19). Oxidative stress, electrophiles, and sulforaphane-like inducers disrupt the Keap1-nrf2 complex: nrf2 translocates to the nucleus and, combining with small maf protein (20), induces ARE-linked gene expression (21). Sulforaphane releases nrf2 from Keap1 by modification of critical cysteine thiol residues (22). Keap1 has concurrent increased susceptibility to degradation but also has ARE-linked gene expression and may be induced by nrf2 activation, providing an autoregulatory feedback loop (23). Nrf2 also undergoes nuclear export, establishing cytoplasmic/nuclear dynamic shuttling (24). Recent research has suggested that the serine/threonine kinase CK2 has a role in nuclear import of nrf2 and that both CK2 and tyrosine kinase Fyn influence nuclear export and degradation of nrf2 (25,26) (Fig. 1A). There is an active nrf2–Keap-1 system in vascular endothelial cells (27).
FIG. 1.
Nrf2 activation and transketolase expression in human HMEC-1 endothelial cells in vitro. A: Schematic diagram showing activation of nrf2 and dynamic nuclear-cytoplasmic shuttling of nrf2 for ARE-linked expression. B: Multiple pathways of biochemical dysfunction induced by hyperglycemia in microvascular endothelial cells and effect of nrf2 activated, ARE-mediated induction of transketolase expression. Other mechanisms of biochemical dysfunction may be involved.
The role, if any, of nrf2-linked gene expression in countering endothelial dysfunction in hyperglycemia has not been disclosed. Disposal of glyceraldehyde-3-phosphate and fructose-6-phosphate by the reductive pentosephosphate pathway induced by activation of nrf2 and increased expression of transketolase suggested a possible mechanism of intervention. In this report, we show that activation of nrf2 by the dietary activator sulforaphane, limited to concentration ranges found in plasma after consumption of broccoli (28) for relevance to future clinical dietary intervention, increased the expression of protective enzymes under ARE-linked transcriptional control and prevented metabolic dysfunction in endothelial cells induced by hyperglycemia in which increased expression of transketolase has a critical role.
RESEARCH DESIGN AND METHODS
Reagents.
MCDB-131 medium, FCS, Alexa Fluor 488 rabbit anti-mouse IgG and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) were purchased from Invitrogen (Paisley, U.K.). Monoclonal (mouse) anti–O-linked N-acetylglucosamine (Clone RL2) was purchased from Abcam (Cambridge, U.K.). All other chemicals were obtained from Sigma-Aldrich (Poole, Dorset, U.K.), unless otherwise stated.
Cell culture.
Human microvascular HMEC-1 endothelial cells were cultured as described previously (29). HMEC-1 cells (4–6 × 106) were incubated in MCDB-131 medium with 10% serum, 10 mmol/l l-glutamine, 10 ng/ml epidermal growth factor, and 1 μg/ml hydrocortisone in 92-mm-diameter Petri dishes with low glucose (5 mmol/l) and high glucose (30 mmol/l) in the absence and presence of 4 μmol/l sulforaphane for 6–48 h, trypsinized, and analyzed as described below.
Small interfering RNA transfection.
HMEC-1 endothelial cells were incubated in MCDB-131 medium with 10% serum and then transfected with small interfering RNA (siRNA) for nrf2 (SI00657030; Qiagen) or transketolase (SI02653791) using the HiPerfect transfection reagent according to the manufacturer's instructions. The incubation was then continued with normal medium for 24 h. Cultures were then continued with low and high glucose concentrations in the absence and presence of 4 μmol/l sulforaphane for 6 or 24 h and/or other additives. Knockdown was confirmed by real-time RT-PCR for nrf2 and transketolase. RNA was extracted using the RNeasy minikit (Qiagen), reverse transcribed by SuperScript III First Strand Synthesis system (Invitrogen), and quantified by real-time RT-PCR using the TaqMan MGB probes, designed and supplied by Applied Biosystems. Knockdown was 60% for transketolase and >90% for nrf2.
Nuclear translocation of nrf2 by immunoblotting.
Nuclear and cytosolic proteins were isolated from HMEC-1 cells incubated with and without sulforaphane using a CelLytic NuCLEAR extraction kit (Sigma). Trypsinized cells were washed in PBS at 4°C, and the cell pellet was lysed with hypotonic lysate buffer containing dithiothreitol, a protease inhibitor cocktail, and IGEPAL CA-630 on ice for 15 min. The lysate was centrifuged (11,000 × g, 30 s, 4°C), and the supernatant was used for the cytosolic extract. The pellet (crude nuclear fraction) was treated with extraction buffer containing dithiothreitol and protease inhibitor cocktail at 4°C for 30 min, vortex mixed, and centrifuged (21,000 × g, 4°C, 5 min). The supernatant was collected for the nuclear extract. All protein extracts were frozen at −80°C immediately until further analysis. The protein concentration was determined with EZQ Protein Quantitation kit (Invitrogen).
Proteins in the cytosolic and nuclear fractions were separated by 10% SDS-PAGE and transferred electrophoretically onto polyvinylidene difluoride membrane, and the membrane was blocked with 5% nonfat milk in Tris-buffered saline–Tween buffer (10 mmol/l Tris-HCl, pH 7.5; 150 mmol/l NaCl; and 0.05% Tween-20). The membrane was probed with anti-nrf2 antibody (H-300; 1:1,500 dilution) overnight at 4°C. After washing, the membrane was incubated with horseradish peroxidase–conjugated second antibody (1:3,000 diluted; Sigma) for 1 h at room temperature, and immunoconjugate was detected with enhanced chemiluminescence. The membrane was then incubated with stripping buffer (100 mmol/l β-mercaptoethanol, 2% SDS, and 62.5 mmol/l Tris-HCl, pH 6.8), blocked with 5% nonfat milk in Tris-buffered saline–Tween buffer, and reprobed with antibodies to reference proteins β-actin and lamin A. Protein band intensities were quantified using ImageQuant TL software (Amersham Biosciences).
Characterization of biochemical dysfunction.
The intracellular formation of ROS was detected using the fluorogenic probe H2DCFDA. Cells (2 × 106) were incubated with and without sulforaphane and mitochondrial inhibitors for 24 h, washed with PBS, and then incubated further with 20 μmol/l H2DCFDA for 45 min, washed again, and analyzed by flow cytometry. Shorter incubations of 1-h preincubation in hyperglycemia with and without mitochondrial inhibitors were also performed by microplate fluorescence measurements, normalizing the fluorescence intensity to cell number. The effect of treatments on cell viability was assessed by Trypan blue exclusion.
Hexosamine pathway activity was assessed by a quantitative dot Western blot assay for O-linked N-acetylglucosamine modified protein. Cytosolic protein extracts (0.7 μg in 5 μl PBS) were immunoblotted with RL2 antibody (1:800 dilution in blocking buffer) for 3 h at room temperature, blocked with 10% BSA, and washed (30). The immunocomplexes were detected with Alexa Fluor 488 rabbit anti-mouse IgG (1:700 dilution) and quantified by microplate fluorimetry. The concentrations of methylglyoxal in HMEC-1 cells and medium of cultures with and without sulforaphane were determined by derivatization with 1,2-diaminobenzene and quantitation by liquid chromatography with tandem mass spectrometric detection and stable isotopic dilution analysis (12). PKC activity was assayed in membrane and particulate fractions of HMEC-1 cells with exogenous diacylglycerol (1,2-dioleoyl-sn-glycerol; DAG) activator and epidermal growth factor receptor peptide fragment VRKRTLRRL as substrate (9).
Statistical analyses.
All statistical analyses were performed using paired Student's t test, and results are expressed as means ± SD. A P value <0.05 was considered to be significant.
RESULTS
Activation of nrf2 and ARE-linked gene expression in endothelial cells by the dietary activator sulforaphane.
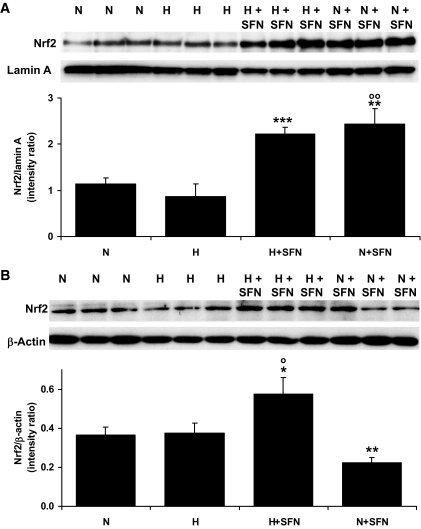
We investigated the activation status of nrf2 in human microvascular endothelial cells by assessing nuclear translocation of human nrf2 by immunoblotting in cytosolic and nuclear fractions and confocal microscopy of nrf2-GFP fusion protein. HMEC-1 endothelial cells incubated in model hyperglycemia (30 mmol/l glucose) showed no significant nuclear translocation of nrf2 with respect to normoglycemic control (5 mmol/l glucose) after incubation for 6 h. Addition of 4 μmol/l sulforaphane gave a twofold increase in nuclear nrf2 in both normoglycemic and hyperglycemic cultures. In the normoglycemic culture, the concentration of nrf2 in the cytosol was decreased concomitantly; whereas in the hyperglycemic culture, the concentration of nrf2 in the cytosol was increased. This suggests that the double insult of hyperglycemia and sulforaphane increased the cellular content of nrf2 protein (Fig. 2A and B). This concentration of sulforaphane did not induce significant cytotoxicity in HMEC-1 cells in incubations for up to 48 h, as assessed in previous studies (31). Transketolase activity of HMEC-1 cells was determined spectrophotometrically, as we have previously described (9).
FIG. 2.
Nuclear translocation of nrf2 in human HMEC-1 endothelial cells in vitro activated by sulforaphane. Nuclear fraction (A) and cytosolic fraction (B) immunoblotting for nrf2 (98-kDa band). Densitometric intensity ratios are means ± SD (n = 3). N, 5 mmol/l glucose; H, 30 mmol/l glucose; N+SFN, 5 mmol/l glucose + 4 μmol/l sulforaphane; and H+SFN, 30 mmol/l glucose + 4 μmol/l sulforaphane. *P < 0.05, **P < 0.01, and ***P < 0.001 with respect to N; ○P < 0.05 and ○○P < 0.01 with respect to H.
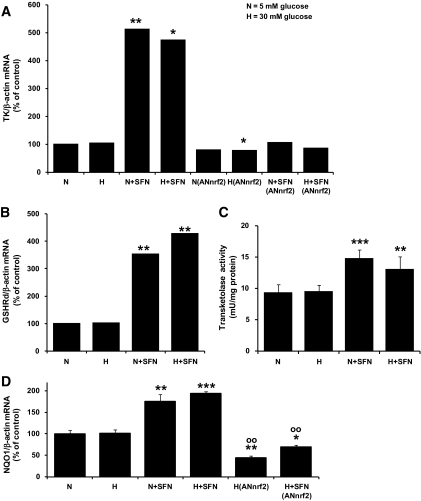
Real-time RT-PCR analysis of target ARE-linked gene expression revealed a marked fivefold induction of transketolase mRNA in cells stimulated with sulforaphane (Fig. 3A) and a lower, three- to fourfold increase in GSHRd mRNA (Fig. 3B). The cytosolic activity of transketolase was increased 40–60% by exposure to sulforaphane (Fig. 3C). The increase in expression of transketolase induced by sulforaphane in normoglycemic and hyperglycemic cultures was prevented by knockdown of nrf2 expression (Fig. 3A). To confirm that there is an ARE-linked induction of gene expression by sulforaphane in HMEC-1 cells, we studied the mRNA levels of the typical nrf2-linked, ARE-mediated gene, NQO1. NQO1 expression, normalized to culture in normoglycemia, was increased approximately twofold in HMEC-1 cells incubated with 4 μmol/l sulforaphane in normoglycemic and hyperglycemic conditions. Basal and sulforaphane-induced expression of NQO1 in hyperglycemic culture was decreased 56 and 31%, respectively, by knockdown of nrf2 with siRNA (Fig. 3D).
FIG. 3.
Effect of sulforaphane on ARE-linked gene expression in HMEC-1 endothelial cells in hyperglycemic culture in vitro. A: Effect of hyperglycemia and sulforaphane on expression of transketolase, with and without knockdown of nrf2. B: Effect of hyperglycemia and sulforaphane on expression of GSHRd. C: Transketolase activity of HMEC-1 cells; effect of hyperglycemia and sulforaphane. D: Effect of hyperglycemia and sulforaphane on expression of NQO1. Transketolase, GSHRd, and NQO1 mRNA were quantified by real-time RT-PCR; AN(nrf2), transfection for nrf2 knockdown. Data are means ± SD (n = 3, except n = 6 for transketolase activity). *P < 0.05, **P < 0.01, and ***P < 0.001 with respect to N; ○○P < 0.01 with respect to H.
Increased formation of ROS by endothelial cells in hyperglycemia, reversal by sulforaphane, and critical role of transketolase.
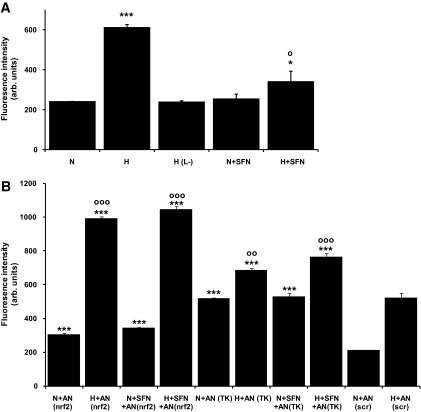
To examine the effect of ARE-linked gene expression on biochemical dysfunction in hyperglycemia, the cellular production of ROS was quantified. Hyperglycemic culture of endothelial cells produced a threefold increased formation of ROS (Fig. 4A). This was not induced by the addition of 25 mmol/l l-glucose (which does not permeate into endothelial cells) to the normoglycemic control. Incubation of endothelial cells with sulforaphane reversed the increase in ROS by 73%, suggesting that activation of ARE-linked gene expression prevented increased ROS formation. Increased ROS formation by HMEC-1 cells in hyperglycemic cultures was prevented by incubation for 60 min with mitochondrial inhibitors (ROS formation [percentage of normoglycemic control]: 10 μmol/l p-trifluoromethoxycarbonylcyanide phenylhydrazone, 94 ± 3%; 5 μmol/l rotenone, 104 ± 3%; and 2 μmol/l myxothiazole, 88 ± 3%). This suggests that dysfunction of mitochondria was a primary source of the increased ROS and electron flux, although complexes I and III contributed to this effect. Incubation of HMEC-1 cells with these inhibitors for 24 h decreased cell viability by 40–50% and masked the effect of rotenone.
FIG. 4.
Biochemical dysfunction in HMEC-1 endothelial cells in hyperglycemic culture and reversal by sulforaphane in vitro: ROS formation. A and B: Assessment of cellular ROS formation. H(L−), 5 mmol/l d(+)-glucose with 25 mmol/l l(−)-glucose; AN(nrf2), transfection with siRNA for nrf2 knockdown; AN(TK), transfection with siRNA for transketolase knockdown; and AN(scr), transfection with scrambled sequence siRNA. Data are means ± SD (n = 3). * and ○Significance with respect to normoglycemic (N) and hyperglycemic (H) control, respectively, with 1, 2, and 3 symbols representing P < 0.05, 0.01, and 0.001, respectively.
We next sought evidence of whether the prevention of increased formation of ROS was dependent on nrf2. We transfected endothelial cells with siRNA to knockdown expression of nrf2 and confirmed this by PCR and real-time RT-PCR (Fig. 4B). Decreasing the expression of nrf2 exacerbated ROS production in both normoglycemic and hyperglycemic cultures; ROS was increased 145% with 5 mmol/l glucose and 190% with 30 mmol/l glucose, with respect to cells with 5 and 30 mmol/l glucose transfected with scrambled siRNA. The increased formation of ROS with nrf2 knockdown was maintained and not reversed by sulforaphane, which is consistent with the prevention of increased ROS by sulforaphane being mediated by activation of nrf2 rather than a direct antioxidant effect of sulforaphane. Knockdown of transketolase by transfection of endothelial cells with siRNA also exacerbated ROS production in both normoglycemic and hyperglycemic cultures: ROS was increased 247 and 131%, respectively, with respect to cultures with 5 and 30 mmol/l glucose transfected with scrambled siRNA. The increased formation of ROS with transketolase siRNA knockdown was maintained and not reversed by sulforaphane, consistent with the prevention of increased ROS by sulforaphane being mediated by increased expression of transketolase (Fig. 4B).
Multiple pathways of biochemical dysfunction in endothelial cells in hyperglycemia and reversal by sulforaphane.
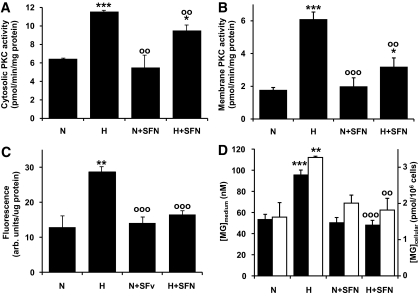
Reversal of increased mitochondrial ROS formation is expected to counter biochemical dysfunction in multiple pathways. Hyperglycemia increased the activity of PKC by 180% in the cytosolic fraction and by 347% in the membrane fraction of HMEC-1 endothelial cells. These increases were reversed partially in both cytosolic and membrane fractions by sulforaphane: The hyperglycemia-induced increase in PKC activity was reversed 40% in the cytosolic fraction and 67% in the membrane fraction (Fig. 5A and B). Similarly, hexosamine pathway–activated enzymatic O-linked glycosylation increased twofold in hyperglycemia and was reversed by sulforaphane (Fig. 5C). Finally, the concentrations of methylglyoxal in HMEC-1 cells and medium was increased twofold in hyperglycemia and reversed by sulforaphane (Fig. 5D).
FIG. 5.
Biochemical dysfunction in HMEC-1 endothelial cells in hyperglycemic culture and reversal by sulforaphane in vitro. A and B: Cytosolic and membrane PKC activity, respectively. C: O-linked protein glycosylation of cell protein extracts. D: Effect on methylglyoxal concentrations: concentration in culture medium (▪) and cellular content (□). Data are means ± SD (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001 with respect to N; ○○P < 0.01 and ○○○P < 0.001 with respect to H.
DISCUSSION
The reversal of biochemical dysfunction of endothelial cells in hyperglycemia by sulforaphane suggests that activation of nrf2 and related ARE-linked gene expression is a novel strategy to suppress endothelial cell dysfunction and possibly also the development of vascular disease in diabetes. For example, decreased cellular and extracellular secretion of methylglyoxal is expected to prevent dicarbonyl glycation of cellular and extracellular matrix proteins and to prevent hyperglycemia-induced endothelial cell detachment and anoikis (12). Similarly increased ARE-linked gene expression in human aortal endothelial cells engineered by adenovirus-mediated expression of Nrf2 protected human aortal endothelial cells from cytotoxicity induced by hydrogen peroxide and tumor necrosis factor-α–induced increased expression of monocyte chemoattractant protein-1 and vascular adhesion molecule-1. This suggests that increased activation of nrf2 may also confer antiatherogenic activity (32).
Activation of nrf2 by sulforaphane produced nuclear accumulation of the 98-kDa band of nrf2 protein. The 98-kDa band has ARE binding activity and is produced by kinase CK2-catalyzed phosphorylation. Nrf2 levels in cells are regulated by further phosphorylation, nuclear export, and degradation, which may be enhanced by ARE-linked expression of Keap1 (23,26,33). Nrf2 may also exhibit ARE-linked expression. Quantitative Western blotting for nrf2 revealed increased nrf2 in both the nucleus and cytosol in hyperglycemia with sulforaphane treatment, suggesting that total cellular nrf2 protein had increased but only with costimulation from both sulforaphane and hyperglycemia. The expression of nrf2 is ARE-regulated with nrf2 binding to its own promoter (17). Hyperglycemia may synergize with sulforaphane to increase nrf2 via ARE-induced expression by increasing endogenous activators of nrf2, such as 4-hydroxynonenal (34), increasing the activity of CK2 via Ca2+/calmodulin-dependent mechanisms (25,35) or other activatory mechanism. This may reflect an ARE-linked, antistress response to hyperglycemia in endothelial cells, albeit too weak to mount an adequate defense against the metabolic insult.
Although nrf2 activation leads to increased expression of enzymes linked to antioxidant functions countering oxidative stress, in this study, increased expression of transketolase made a critical and pivotal intervention. The increased reductive pentosephosphate pathway activity thereby achieved counters the accumulation of triosephosphates and fructose-6-phosphate driving metabolic dysfunction (9). The protective role of the reductive pentosephosphate pathway in ARE-linked gene expression has hitherto not been identified, although increased transketolase expression is associated with the dietary restriction model of healthy ageing (36). Enzymatic activity of transketolase in the pentosephosphate pathway produces indirectly the NADPH cofactor for other ARE-linked gene products, GSHRd, AKRd, NQO1, and thioredoxin reductase, that contribute to countering the effects of oxidative stress (37). Therefore, contributions of other ARE-linked gene expression in synergism with transketolase in reversal metabolic dysfunction in hyperglycemia cannot be excluded.
Multiple pathways of biochemical dysfunction in HMEC-1 cells induced by hyperglycemia were reversed by sulforaphane, except reversal of PKC activation was resistant to the response. This may be due to ARE-linked induced expression of 1-acylglycerol-3-phosphate O-acyltransferase 3 by sulforaphane (38). This enzyme converts lysophosphatidic acid to phosphatidic acid, a precursor of the PKC activator DAG in de novo synthesis stimulated in hyperglycemia. The increased expression of this ARE-linked gene may maintain increased levels of DAG, although the increased concentrations of glycolytic intermediates upstream driving de novo synthesis of DAG are expected to have been corrected. Prolonged exposure to increased levels of DAG has been suggested as a stimulus of increased PKC expression in hyperglycemia associated with diabetes that may underlie the increased specific activity of PKC in both membrane and cytosolic cell fractions found herein. Alternatively, when we studied activation of transketolase by high-dose thiamine supplements (7), we also found an incomplete reversal of hyperglycemia-induced increase in PKC activity. This may suggest that factors other than those influenced by transketolase contribute to PKC activation in hyperglycemia.
The prevention of nrf2 activation by sulforaphane by addition of GSH may be interpreted as an effect of GSH acting as an antioxidant. However, sulforaphane and other isothiocyanates bind reversibly to nonprotein thiols, such as GSH, decreasing the concentration of free sulforaphane available to modify Keap1. The residual low concentration of free sulforaphane is inadequate to produce a pharmacological response and undergoes slow inactivation by spontaneous hydrolysis (39). The inhibition of nrf2 activation by GSH may, therefore, also reflect interception of sulforaphane in the extracellular medium and prevention of it reaching the cellular receptors, such as Keap1.
Sulforaphane and related isothiocyanates are cytotoxic to endothelial cells and other cells at higher concentrations than used herein (typically 20–40 μmol/l) (31,40). The cytotoxicity is mediated through interactions with death receptors and apoptotic signaling (41), also involving inhibition of p38 mitogen-activated protein kinase (42), mitogen-activated protein kinase kinase kinase-1 (43), and protein phosphatase M3/6 (44), and activation of extracellular signal–related kinase 1/2 and Jun NH2-terminal kinase (45). This is independent of the disruption of the Keap1-nrf2 complex. Such concentrations of sulforaphane are higher than achieved by Brassica spp. vegetable consumption (28), and related cell signaling is expected to be of limited relevance to dietary exposures of sulforaphane.
There was increased formation of ROS by HMEC-1 cells in model hyperglycemia of 30 mmol/l d-glucose. The lack of similar effect induced by l-glucose indicated that glucose entry and metabolism into cells was required for increased ROS formation. Increased ROS formation was linked to mitochondrial dysfunction, consistent with previous reports (10), in which complexes I and III were involved—the former probably by reverse electron flow from complex II (46). Incubation of HMEC-1 cells with the mitochondrial inhibitors for 24 h produced significant cytotoxicity and masked the role of complex I in hyperglycemia-induced ROS formation. Incubation with inhibitors for 1 h did not induce cytotoxicity. Similar effects may have compromised the outcome of previous studies of this type (47). Other sources of increased ROS formation in microvascular endothelial cells in hyperglycemia have been identified: activation of vascular NADPH oxidase; inactivation and uncoupling of endothelial nitric oxide synthase (48,49); and related upstream signaling linked to poly(ADP-ribose) polymerase (PARP) (7), aldose reductase (50), and the xanthine/xanthine oxidase system (51). Mitochondrial metabolism appears to be a major site of ROS formation in the HMEC-1 cell culture model. There are interrelated mechanisms, however, that may explain how sulforaphane-induced ARE-linked gene expression could prevent activation of other pathways. Activation of PARP may be prevented by sulforaphane-induced increased expression of antioxidant enzymes, preventing oxidative damage to DNA. The activation of the polyol pathway may be prevented by sulforaphane decreasing the cellular concentration of methylglyoxal and thereby preventing methylglyoxal-induced expression of aldose reductase (52).
These findings provide the biochemical basis for the link of a vegetable-rich diet with decreased endothelial dysfunction (53), including that part of the Mediterranean diet (54), suggesting that dietary exposure to nrf2 activators derived from cruciferous vegetables may have be involved. The functional importance of this finding requires further evaluation. It is expected that the protective effect of nrf2 activators may be stratified by severity of exposure to hyperglycemia, with increasing effects in the nondiabetic, impaired glucose tolerance, and diabetic populations. Hyperglycemia alone did not induce ARE-linked gene expression in HMEC-1 endothelial cells, even when confronted with damaging insults of oxidative stress and accumulation of dicarbonyls. Physiological activators of nrf2 are thought to be the lipid peroxidation-derived aldehyde, 4-hydroxynonenal, and J3-isoprostanes (34,55). The metabolic insult of hyperglycemia is of nonlipidic origin. This may be why the nrf2 system does not respond strongly to it. There is now evidence that diabetes does induce a weak nrf2-mediated protective response, for example, in the high-fat–diet mouse model of type 2 diabetes (56). The weak response suggests that an effective protection to prevent vascular disease has not been mounted and, therefore, provides a clear future opportunity for pharmacological intervention. Cruciferous vegetable consumption and synthetic activators of nrf2 are expected to decrease the risk of vascular disease in diabetes.
Acknowledgments
This work has received support from the Juvenile Diabetes Research Foundation International (New York), the Wellcome Trust (U.K.), and the Biotechnology and Biosciences Research Council (U.K.).
Published ahead of print at http://diabetes.diabetesjournals.org on 15 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Stehouwer CDA, Gall MA, Twisk JWR, Knudsen E, Emeis JJ, Parving H-H: Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes. Diabetes 51:1157–1165, 2002 [DOI] [PubMed] [Google Scholar]
- 2.de Jager J, Dekker JM, Kooy A, Kostense PJ, Nijpels G, Heine RJ, Bouter LM, Stehouwer CDA: Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn study. Arterioscler Thromb Vasc Biol 26:1086–1093, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Coutinho M, Wang Y, Gerstein HC, Yusuf S: The relationship between glucose and incident cardiovascular events. Diabetes Care 22:233–240, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Meigs JB, O'Donnell CJ, Tofler GH, Benjamin EJ, Fox CS, Lipinska I, Nathan DM, Sullivan LM, D'Agostino RB, Wilson PWF: Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes 55:530–537, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemic with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du X, Matsumara T, Edelsttein D, Rosetti L, Zsengeller Z, Szabo C, Brownlee M: Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112:1049–1057, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyengaard JR, Ido Y, Kilo C, Williamson JR: Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes 53:2931–2938, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ: Prevention of incipient diabetic nephropathy by high dose thiamine and benfotiamine. Diabetes 52:2110–2120, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa T, Edelstein D, Liang Du X, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beede D, Oates PJ, Hammes H-P, Giardino I, Brownlee M: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemia damage. Nature 404:787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Du X-L, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M: Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 97:12222–12226, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobler D, Ahmed N, Song LJ, Eboigbodin KE, Thornalley PJ: Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes 55:1961–1969, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hammes H-P, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M: Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9:294–299, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Berrone E, Beltramo E, Solimine C, Ape AU, Porta M: Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem 281:9307–9313, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Yamamoto M: Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7:385–394, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Mathers J, Fraser JA, McMahon M, Saunders RDC, Hayes JD, McLellan LI: Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp 71:157–176, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kwak MK, Itoh K, Yamamoto M, Kensler TW: Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol 22:2883–2892, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S: Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide array. Cancer Res 62:5196–5203, 2002 [PubMed] [Google Scholar]
- 19.Kobayashi, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M: Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24:7130–7139, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motohashi H, Shavit JA, Igarashi K, Yamamoto M, Engel JD: The world according to Maf. Nucleic Acids Res 25:2953–2959, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang DD, Lo SC, Sun Z, Habib GM, Lieberman MW, Hannink M: Ubiquitination of Keap1, a BTB-kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J Biol Chem 280:30091–30099, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P: Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 99:11908–11913, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee OH, Jain AK, Papusha V, Jaiswal AK: An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem 282:36412–36420, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Jain AK, Bloom DA, Jaiswal AK: Nuclear import and export signals in control of Nrf2. J Biol Chem 280:29158–29168, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Pi JB, Bai YS, Reece JM, Williams J, Liu DX, Freeman ML, Fahl WE, Shugar D, Liu J, Qu W, Collins S, Waalkes MP: Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radic Biol Med 42:1797–1806, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain AK, Jaiswal AK: GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem 282:16502–16510, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C: Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. J Biol Chem 278:703–711, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Song LJ, Morrison JJ, Botting NP, Thornalley PJ: Analysis of glucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC-MS/MS. Anal Biochem 347:234–243, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ: HMEC-1: establishment of an immortalized human microvascular endothelial cell line. Invest Dermatol 99:683–690, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Snow CM, Senior A, Gerace L: Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol 104:1143–1156, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu K, Thornalley PJ: Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem Pharmacol 60:221–231, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C: Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol 290:H1862–H1870, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Andres E, Loukili NH, Noel E, Kaltenbach G, Abdelgheni MB, Perrin AE, Noblet-Dick M, Maloisel F, Schlienger JL, Blickle JF: Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 171:251–259, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE: Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res 94:609–616, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Tamareille S, Mignen O, Capiod T, Rucker-Martin C, Feuvray D: High glucose-induced apoptosis through store-operated calcium entry and calcineurin in human umbilical vein endothelial cells. Cell Calcium 39:47–55, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Lee C-K, Klopp RG, Weindruch R, Prolla TA: Gene expression profile of aging and its retardation by caloric restriction. Science 285:1390–1393, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA: Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 278:12029–12038, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW: Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway: identification of novel gene clusters for cell survival. J Biol Chem 278:8135–8145, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Xu K, Thornalley PJ: Involvement of GSH metabolism in the cytotoxicity of the phenethyl isothiocyanate and its cysteine conjugate to human leukaemia cells in vitro. Biochem Pharmacol 61:165–177, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Bertl E, Bartsch H, Gerhauser C: Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther 5:575–585, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Xu K, Thornalley PJ: Signal transduction activated by the cancer chemopreventive isothiocyanates: cleavage of BID protein, tyrosine phosphorylation and activation of JNK. Br J Cancer 84:670–673, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keum YS, Yu S, Chang PPJ, Yuan X, Kim JH, Xu C, Han J, Agarwal A, Kong ANT: Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res 66:8804–8813, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Cross J, Foss F, Rady J, Macdonald T, Templeton D: The isothiocyanate class of bioactive nutrients covalently inhibit the MEKK1 protein kinase. BMC Cancer 7:183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y-R, Han J, Kori R, Kong ANT, Tan T-H: Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J Biol Chem 277:39334–39342, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Yeh CT, Yen GC: Effect of sulforaphane on metallothionein expression and induction of apoptosis in human hepatoma HepG2 cells. Carcinogenesis 26:2138–2148, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Zhang DX, Gutterman DD: Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292:H2023–H2031, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Quagliaro L, Piconi L, Assaloni R, Da Ros R, Szabo C, Ceriello A: Primary role of superoxide anion generation in the cascade of events leading to endothelial dysfunction and damage in high glucose treated HUVEC. Nutr Metab Cardiovasc Dis 17:257–267, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RAK, Warnholtz A, Meinertz T, Griendling K, Harison DG, Fostermann U, Munzel T: Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88:14–22, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM: Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105:1656–1662, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Frisch SM, Screaton RA: Anoikis mechanisms. Curr Opin Cell Biol 13:555–562, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J: Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 51:1118–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Chang KC, Paek KS, Kim HJ, Lee YS, Yabe-Nishimura C, Seo HG: Substrate-induced up-regulation of aldose reductase by methylglyoxal, a reactive oxoaldehyde elevated in diabetes. Mol Pharmacol 61:1184–1191, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB: Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 80:1029–1035, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D: Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 292:1440–1446, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Gao L, Wang JK, Sekhar KR, Yin HY, Yared NF, Schneider SN, Sasi S, Dalton TP, Anderson ME, Chan JY, Morrow JD, Freeman ML: Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J Biol Chem 282:2529–2537, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD: NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice Fed a high-fat diet. J Pharmacol Exp Ther 325:655–664, 2008 [DOI] [PubMed] [Google Scholar]