Abstract
OBJECTIVE— Genetic and environmental factors modulate the susceptibility to diabetic nephropathy, as initiating and/or progression factors. The objective of the European Rational Approach for the Genetics of Diabetic Complications (EURAGEDIC) study is to identify nephropathy susceptibility genes. We report molecular genetic studies for 127 candidate genes for nephropathy.
RESEARCH DESIGN AND METHODS— Polymorphisms were identified through sequencing of promoter, exon, and flanking intron gene regions and a database search. A total of 344 nonredundant SNPs and nonsynonymous variants were tested for association with diabetic nephropathy (persistent albuminuria ≥300 mg/24 h) in a large type 1 diabetes case/control (1,176/1,323) study from three European populations.
RESULTS— Only one SNP, rs2281999, located in the UNC13B gene, was significantly associated with nephropathy after correction for multiple testing. Analyses of 21 additional markers fully characterizing the haplotypic variability of the UNC13B gene showed consistent association of SNP rs13293564 (G/T) located in intron 1 of the gene with nephropathy in the three populations. The odds ratio (OR) for nephropathy associated with the TT genotype was 1.68 (95% CI 1.29–2.19) (P = 1.0 × 10−4). This association was replicated in an independent population of 412 case subjects and 614 control subjects (combined OR of 1.63 [95% CI 1.30–2.05], P = 2.3 × 10−5).
CONCLUSIONS— We identified a polymorphism in the UNC13B gene associated with nephropathy. UNC13B mediates apopotosis in glomerular cells in the presence of hyperglycemia, an event occurring early in the development of nephropathy. We propose that this polymorphism could be a marker for the initiation of nephropathy. However, further studies are needed to clarify the role of UNC13B in nephropathy.
Diabetic nephropathy, characterized by persistent albuminuria, a relentless decline in glomerular filtration rate and raised arterial blood pressure, affects approximately one-third of patients with diabetes (1). Nephropathy accounts for 40% of end-stage renal disease and is associated with high cardiovascular morbidity and mortality (2). Epidemiological and familial studies suggest that genetic factors influence the risk of diabetic nephropathy in both type 1 and type 2 diabetic patients (3–6). Despite rapid research progress, robust predictors of this complication are still lacking.
Phenotypic characterization of nephropathy is more accurate in patients with type 1 diabetes than in those with type 2 diabetes, where the kidney failure may often be caused by nondiabetic factors, mainly hypertension. Using a concerted effort including 2,499 patients with type 1 diabetes from the Danish, Finnish, and French populations, the European Rational Approach for the Genetics of Diabetic Complications (EURAGEDIC) consortium has established a large study for association with diabetic nephropathy that includes 1,176 case subjects and 1,323 control subjects (7). Single nucleotide polymorphisms (SNPs) located in 127 candidate genes selected through assessment of linkage studies, knowledge of metabolic pathways, and animal models were sought for association with nephropathy.
RESEARCH DESIGN AND METHODS
Patient populations.
Three European centers, from Denmark, Finland, and France, contributed to the case/control study, with a total of 2,499 subjects with type 1 diabetes. Details for the recruitment of patients have previously been presented (7) and clinical characteristics of the patients are shown in online supplementary Table 1 (available in an online appendix at http://dx.doi.org/10.2337/dc08-0073). Type 1 diabetes was considered present if the age at onset of diabetes was ≤35 years and the time to definitive insulin therapy ≤1 year. Patients in the initial phase of type 1 diabetes, that is, duration of diabetes <5 years, were not included. Established diabetic nephropathy (case subjects) was defined by persistent albuminuria (≥300 mg/24 h or ≥200 μg/min or ≥200 mg/l) in two out of three consecutive measurements on sterile urine. Patients with clinical or laboratory suspicion of nondiabetic renal or urinary tract disease were excluded. Absence of diabetic nephropathy (control subjects) was defined as persistent normoalbuminuria (urinary albumin excretion rate: <30 mg/24 h or <20 μg/min or <20 mg/l) after at least 15 years of diabetes duration in patients not treated with ACE inhibitors or angiotensin II receptor blockers.
Accordingly, for the initial study, Denmark contributed 952 patients with type 1 diabetes including 489 case subjects and 463 control subjects for diabetic nephropathy, Finland contributed 856 patients including 387 case subjects and 469 control subjects, and France contributed 691 patients including 300 case subjects and 391 control subjects, adding up to a total of 2,499 patients including 1,176 case subjects and 1,323 control subjects for nephropathy.
Two independent datasets were used for replication, the first one being an additional case/control group from the FinnDiane study (8), including 412 case subjects and 614 control subjects who matched the criteria used in the initial study. The second set consisted of 674 patients with type 1 diabetes and microalbuminuria (urinary albumin excretion rate 30–300 mg/24 h or 20–200 μg/min or 20–200 mg/l) from the Danish (n = 60), Finnish (n = 421), and French (n = 193) populations. Clinical characteristics for the replication datasets are presented in online supplementary Table 2.
Molecular screening and SNP selection.
The study is a systematic investigation of 127 candidate genes selected through studies of susceptibility loci from linkage studies, metabolic pathways known to be affected in nephropathy, and data from animal models. A detailed list of genes and molecular analyses has been described elsewhere (7,9).
Single nucleotide polymorphisms (SNPs) in the genes selected for the study were identified through database searches and by direct SNP discovery. A total of 119 genes were screened by sequencing all exons, flanking intron sequences, 5′ and 3′ untranslated regions, and promoter regions in at least 32 DNA samples. The sample consisted of healthy French Caucasian subjects from the Epidemiological Study on the Genetics and Environment of Asthma (EGEA) (10). The sample size allowed us to detect SNPs with a minor allele frequency (MAF) of at least 5% with a probability of 96%. For 33 genes that were not initially included in the French National Genotyping Centre (CNG) resequencing effort of >15,000 human genes (http://www.cng.fr/en/teams/geneident/index.html), the screening was performed in 64 additional DNA samples from patients with type 1 diabetes. These included 24, 20, and 20 patients from Denmark, Finland, and France, respectively, half of them (n = 32) with nephropathy and the other half (n = 32) without. For sequencing, DNA samples of two individuals from the same population and with the same phenotype were pooled together. Accordingly, the screening was performed in 16 DNA pools for 86 genes and in 48 (16 + 32) DNA pools for 33 genes.
For each gene, primers were defined for PCR amplification of the exon and promoter regions. PCR was performed in a 15-μl reaction mixture containing 25 ng pooled genomic DNA. Primer sequences are available from the authors on request. Sequencing reactions were performed according to the dye terminator method using an ABI PRISM 3700 DNA Analyzer (Applied Biosystems, Foster City, CA). Alignment of experimental results, SNP detection, and genotype calling were performed using the Genalys software (11) that allows for genotype calls obtained from pooled DNA.
For each gene, the haplotype structure and frequencies were determined from the genotypic data obtained from the control group and population groups using the expectation maximization (EM) algorithm (12). A total of 350 variants were selected to account for all estimated haplotypes with frequencies >5%. These tagSNPs represented a median genetic variation (haplotype diversity) by gene of 87% (range 64–100%). All were retained for further genotyping in the case/control study. In addition, all nonsynonymous variants that were detected in at least one diseased population were systematically investigated (n = 19).
For two genes (RELA, TGFBR1) for which no polymorphisms were identified, SNPs were selected using the SNPBrowser software v.2 (Applera Corporation). For eight additional genes (CCR5, CNDP1, HNF4A, LTA, PON2, GCGR, INPPL1, PLA2G7), polymorphisms were selected according to reported associations with phenotypes relevant for diabetic nephropathy (13–20). We also examined 94 SNP markers (genomic control markers) in nongenic regions spaced throughout the genome to control for possible stratification within each population (21,22).
A total of 21 additional SNPs in the UNC13B gene (GeneID#10497; full name unc-13 homolog B [C. elegans]) were genotyped after the initial positive association results from the first step. These additional SNPs were selected from the Hapmap project (http://www.hapmap.org) so that >95% of the UNC13B haplotypic variability was characterized.
Genotyping.
Genomic DNA was isolated from human leukocytes using standard methods. SNP genotyping was performed at the French National Genotyping Center (CNG) using automated high-throughput methods including TaqMan, Amplifluor, MALDI-MS, and SNPlex methods. All liquid handling was performed robotically in 384-well plates with a BasePlate Robot (The Automation Partnership, Royston, U.K). For SNP genotyping by mass spectrometry, the GOOD assay was applied as previously described (23). TaqMan (assay-by-design) was carried out in a 5-μl volume according to the manufacturer's recommendations, with probes and mastermix from Applied Biosystems. For Amplifluor, primers were designed using “AssayArchitect” (http://www.assayarchitect.com). Primer sequences and conditions are available on request. End point fluorescence was detected for TaqMan and Amplifluor assays using an ABI7900HT reader (Applied Biosystems, Courtaboeuf, France), and genotypes were assigned with SDS 2.1 software. Genotyping with the SNPlex platform was performed according to the manufacturer's recommendations (Applied Biosystems, Courtaboeuf, France).
The genotyping success rate was >85% for all markers (<90% for 3% of the markers, between 90 and 95% for 17%, and >95% for 80% of the markers), and among 192 replicate samples genotyped blindly, no genotype differences were found. Hardy-Weinberg equilibrium was checked in case subjects and control subjects in all populations, and markers showing deviation from Hardy-Weinberg equilibrium at the 0.001 significance level were not considered in the case/control comparison.
Statistical analysis.
Allele frequencies were estimated by gene counting, and deviation from Hardy-Weinberg equilibrium was tested by use of a χ2 with 1 d.f. Difference in allele frequencies between case subjects and control subjects were tested by a χ2 test with 1 d.f. separately in each population, and associated P values were combined across populations using Fisher's method (24) to produce an overall test of significance. Adjustment for multiple testing was carried out by correcting for the effective number of independent tests (25) to take into account the linkage disequilibrium (LD) between SNPs. Logistic regression analyses were performed to estimate genetic ORs, adjusted for age, sex, smoking, diabetes duration, and A1C. LD matrices were obtained using Haploview software (26), and haplotype association analyses were carried out using THESIAS software (27). Homogeneity of ORs across populations was investigated using the Mantel-Haenszel statistic (28).
Expression studies
Cell culture.
Cell lines HepG2, MDCK I and II, MCF7, Cos7, HeLa, EAhy-926, SaOs-2, U2Os, SHSY5Y, and rat smooth muscle cells (rSMC) were maintained in Dulbecco's modified Eagle's medium (Sigma, Geissendorf, Germany) with 10% conditioned fetal calf serum (PAA, Cölbe, Germany), penicillin (100 units/ml), streptomycin (100 ng/ml), and l-glutamin (2 mmol · l−1 · ml−1). HEK293T cells received iron-supplemented fetal calf serum (Cell Concepts, Umkirch, Germany). Suspension cell lines THP1, U937, K562, HL60, and RAW264.7 were maintained in RPMI-1640 medium (Sigma) with the same additions plus 1 × minimal essential medium (MEM) minimal amino acids (PAA). Differentiation of THP-1 monocytes into macrophages induced by stimulation with 10−8 mol/l phorbol 12-myristate 13-acetate (PMA) and differentiation of SaOs-2 osteosarcoma cells was induced by stimulation with 100 mmol/l glycerol-1-phosphate and 10 mmol/l ascorbic acid.
Isolation of total RNA and generation of cDNA.
Total RNA from cells was isolated from 106 cells each with the RNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. RNA from human brain was extracted from the left frontal cortex of a 75-year-old male patient <24 h postmortem, and testis RNA was isolated 1 h after surgical operation from a 62-year-old patient who underwent orchidectomy for prostate cancer as described (29). RNA yield was controlled by TBE/agarose gel electrophoresis and adjusted nanophotometrically. For first-strand cDNA synthesis, 5 μg total RNA was used (Fermentas, St. Leon-Rot, Germany). Efficiency was routinely controlled by diagnostic PCR for ribosomal protein RP27. Podocyte cDNA was generated from an immortalized human podocyte cell line (30).
Diagnostic PCR.
Exon-spanning primers for nested diagnostic PCR were designed from UNC13B sequence NM_006377 (sense primers S1: GTGCACCACTCCTCATAACTT; S2: CAACCTACTGCTATGAGTGT; antisense primers A1: TGTGCAAGTCA GCAAAACTAAG, A2: AAGCCAAGGACAAAACAGGATC). PCR was conducted with GoTaqDNA-Polymerase (Promega) and 35 cycles of amplification. Integrity of the cDNA was controlled by diagnostic PCR for ribosomal protein 27 (rp27; sense primer: 5′-CCAGGATAAGGAAGGAATTCCTCCTG-3′, antisense primer: 5′-CCAGCACCACATTCATCAGAAGG-3′, not shown).
In silico analyses.
For the prediction of putative transcription factor binding sites, a sequence of 25 bases to either side of the SNP was submitted for each SNP individually to a net-based search tool (Alibaba2.1, Transfac7.0; http://www.gene-regulation.com). Settings for core and pair similarities, matrix conservation, and factor class levels were adjusted according to factors predicted.
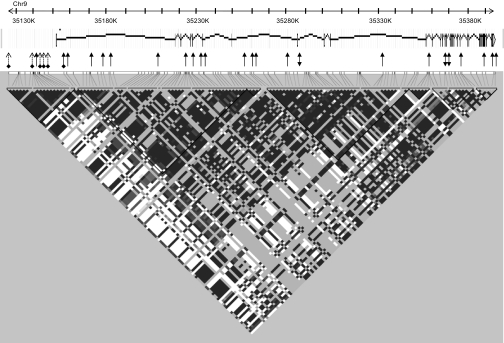
Five polymorphisms, rs10081672, rs10972356, rs13288912, rs12377498, and rs10972333, were in complete association with rs13293564 located in intron 1 of the UNC13B gene associated with nephropathy. They were detected on the NCBI B35 (http://www.ncbi.nlm.nih.gov) at the respective nucleotide positions 35145908, 35143435, 35143123, 35140841, and 35126146, with the beginning of the 5′-UTR within exon 1 residing at nucleotide position 35151989. This start site was confirmed in all reference sequences in the University of California, Santa Cruz genome browser, with no indication of alternative upstream exons or presence of alternative promoters. Hence, the variants rs10081672, rs10972356, rs13288912, rs12377498, and rs10972333 are located, respectively, −6,081, −8,554, −8,866, −11,148, and −25,843 bp upstream of the transcription start site of the human UNC13B gene. Sequence homology scans and chromosomal neighborhood analyses were performed using the University of California, Santa Cruz genome browser (http://genome.ucsc.edu) covering chromosomal region 9:35,101,909–35,160,332. Special emphasis was put on placental mammal conserved elements in a 28-way multiz alignment. Results were cross-checked using rVista 2.0 software (http://rvista.dcode.org). There was no noticeable sequence conservation this far upstream of UNC13B exon 1 in either species.
RESULTS
SNP discovery, selection, and genotyping.
A total of 119 genes were resequenced and 1,833 sequence variants were detected, including 1,673 SNPs and 160 insertion/deletion polymorphisms. A total of 773 (42.2%) of these variants were not present in the dbSNP (build 126) and therefore represent novel polymorphisms. All data have been cataloged in the dbSNP database and are available online at http://genecanvas.ecgene.net. They were located in the 5′-flanking region (n = 53), 5′UT (n = 31), intron (n = 1,166), nonsynonymous coding (n = 139), slice site (n =1), 3′UT (n = 221), and the 3′-flanking region (n = 40). The proportion of SNPs detected in exons was not different between the 773 newly discovered polymorphisms and the 1,060 variants in dbSNP build 126 (32.4 vs. 30.3%, χ2 test: P = 0.35). As expected, newly discovered SNPs were mainly rare, 66.1% with MAF <5% compared with 12.3% of SNPs in dbSNP (P < 10−4). The same held true for insertions/deletions (17.2% new vs. 2.5% in dbSNP, P < 10−4).
A total of 532 haplotypes with a frequency >5% in at least one population were determined. For these 119 genes, a total of 369 polymorphisms, including haplotype-tagging SNPs and nonsynonymous variants, were selected for genotyping. For two genes with no variant identified through resequencing (RELA, TGFBR1), four SNPs were selected with SNPbrowser. In addition, 15 SNPs were selected in eight genes from previously reported associations with phenotypes relevant for diabetic nephropathy. We were not able to obtain data for 28 markers due to the impossibility of obtaining a genotyping assay, and 16 markers were excluded because they showed significant deviation from the Hardy-Weinberg equilibrium in case subjects and control subjects from the three populations.
Association studies.
A total of 344 SNPs were investigated for association with nephropathy in the EURAGEDIC study. Allele frequencies in case and control groups from three populations are shown in supplementary Table 3 (in the online appendix). Nominally significant association across the three populations (P < 0.05) was observed for 33 SNPs of 344, with P values ranging from P = 1.79 × 10−5 to P = 0.050 (supplementary Table 3). Of the 15 polymorphisms in the eight genes selected from the literature, only one, rs1799987, located in the CCR5 gene, showed nominal significant association across the three populations (P = 0.025). However, this association did not remain significant after correction for multiple testing. For the 119 remaining genes, the number of independent tests was estimated to be Neff = 317, with a corresponding significance threshold of P = 1.58 × 10−4 (P = 0.05/317). Only one SNP, rs2281999, located in UNC13B, remained significantly associated with nephropathy (P = 1.79 × 10−5) after correction. This association was mainly observed in the Finnish sample, with a trend remaining in the Danish but not in the French samples (Table 1 and supplementary Table 3). Another UNC13B SNP (rs661712) showed nominal evidence for association with nephropathy (P = 4 × 10−4) but did not remain significant after correction for multiple testing. The association for the 94 genomic control markers was compatible with expectations under the null hypothesis of no association, indicating that stratification within one or more of the populations is an unlikely source of positive association results. Furthermore, correction of the association for the two significant eigenvectors identified by performing principal components analysis (using Eigenstrat) on the genomic control markers had no effect on the results.
TABLE 1.
Association analysis between UNC13B gene polymorphisms and diabetic nephropathy in the EURAGEDIC study
| Polymorphisms | Denmark |
Finland |
France |
Whole (P†) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allele frequency in control subjects | Allele frequency in case subjects | P* | Allele frequency in control subjects | Allele frequency in case subjects | P* | Allele frequency in control subjects | Allele frequency in case subjects | P* | ||
| rs13285401 (C/T) | 0.584/0.416 | 0.633/0.367 | 0.0282 | 0.599/0.401 | 0.624/0.376 | 0.3046 | 0.619/0.381 | 0.626/0.374 | 0.7864 | 0.1248 |
| rs13293564 (G/T) | 0.643/0.357 | 0.587/0.413 | 0.0126 | 0.579/0.421 | 0.514/0.486 | 0.0072 | 0.605/0.395 | 0.591/0.409 | 0.5974 | 0.0032 |
| rs10972365 (T/C) | 0.721/0.279 | 0.748/0.252 | 0.1711 | 0.740/0.260 | 0.797/0.203 | 0.0060 | 0.736/0.264 | 0.767/0.233 | 0.2113 | 0.0097 |
| rs4879877 (A/G) | 0.872/0.128 | 0.821/0.179 | 0.0020 | 0.822/0.178 | 0.799/0.201 | 0.2298 | 0.869/0.131 | 0.866/0.134 | 0.8739 | 0.0158 |
| rs4111859 (A/T) | 0.872/0.128 | 0.822/0.178 | 0.0026 | 0.821/0.179 | 0.796/0.204 | 0.1945 | 0.870/0.130 | 0.868/0.132 | 0.9012 | 0.0174 |
| rs3904435 (A/G) | 0.850/0.150 | 0.801/0.199 | 0.0055 | 0.726/0.274 | 0.734/0.266 | 0.7131 | 0.840/0.160 | 0.849/0.151 | 0.6603 | 0.0639 |
| rs12685290 (A/G) | 0.594/0.406 | 0.578/0.422 | 0.4892 | 0.518/0.482 | 0.448/0.552 | 0.0044 | 0.590/0.410 | 0.605/0.395 | 0.5779 | 0.0373 |
| rs17360668 (G/A) | 0.744/0.256 | 0.781/0.219 | 0.0591 | 0.758/0.242 | 0.821/0.179 | 0.0016 | 0.754/0.246 | 0.772/0.228 | 0.4483 | 0.0026 |
| rs10972396 (G/T) | 0.873/0.127 | 0.824/0.176 | 0.0038 | 0.821/0.179 | 0.814/0.186 | 0.7247 | 0.861/0.139 | 0.873/0.127 | 0.5063 | 0.0407 |
| rs10972397 (A/G) | 0.873/0.127 | 0.819/0.181 | 0.0012 | 0.827/0.173 | 0.812/0.188 | 0.4190 | 0.861/0.139 | 0.867/0.133 | 0.7500 | 0.0150 |
| rs7851161 (A/T) | 0.556/0.444 | 0.560/0.440 | 0.8530 | 0.595/0.405 | 0.531/0.469 | 0.0085 | 0.511/0.489 | 0.532/0.468 | 0.4566 | 0.0761 |
| rs10758301 (T/G) | 0.586/0.414 | 0.576/0.424 | 0.6467 | 0.520/0.480 | 0.450/0.550 | 0.0041 | 0.598/0.402 | 0.614/0.386 | 0.5392 | 0.0414 |
| rs10121009 (C/T) | 0.810/0.190 | 0.814/0.186 | 0.8417 | 0.711/0.289 | 0.784/0.216 | 0.0007 | 0.826/0.174 | 0.804/0.196 | 0.3110 | 0.0085 |
| rs10114937 (T/C) | 0.672/0.328 | 0.708/0.292 | 0.0915 | 0.641/0.359 | 0.734/0.266 | 0.0000 | 0.715/0.285 | 0.696/0.304 | 0.4601 | 0.0000 |
| rs10758303 (A/G) | 0.541/0.459 | 0.523/0.477 | 0.4154 | 0.541/0.459 | 0.461/0.539 | 0.0010 | 0.568/0.432 | 0.561/0.439 | 0.8021 | 0.0136 |
| rs661712 (C/T) | 0.671/0.329 | 0.709/0.291 | 0.0763 | 0.648/0.352 | 0.739/0.261 | 0.0001 | 0.713/0.287 | 0.700/0.300 | 0.5904 | 0.0004 |
| rs17296428 (C/G) | 0.822/0.178 | 0.833/0.167 | 0.5156 | 0.857/0.143 | 0.819/0.181 | 0.0323 | 0.826/0.174 | 0.851/0.149 | 0.2332 | 0.0852 |
| rs12684897 (T/C) | 0.821/0.179 | 0.830/0.170 | 0.6083 | 0.861/0.139 | 0.828/0.172 | 0.0608 | 0.790/0.210 | 0.819/0.181 | 0.1841 | 0.1255 |
| rs2282001 (G/C) | 0.928/0.072 | 0.933/0.067 | 0.6768 | 0.883/0.117 | 0.915/0.085 | 0.0347 | 0.952/0.048 | 0.927/0.073 | 0.0569 | 0.0394 |
| rs2281999 (C/T) | 0.660/0.340 | 0.693/0.307 | 0.1396 | 0.627/0.373 | 0.725/0.275 | 0.0000 | 0.707/0.293 | 0.704/0.296 | 0.8933 | 0.00002 |
| rs1927962 (T/C) | 0.781/0.219 | 0.792/0.208 | 0.5489 | 0.826/0.174 | 0.768/0.232 | 0.0031 | 0.773/0.227 | 0.802/0.198 | 0.2083 | 0.0143 |
| rs12339582 (G/T) | 1.000/0.000 | 1.000/0.000 | 1.0000 | 1.000/0.000 | 1.000/0.000 | 1.0000 | 1.000/0.000 | 0.997/0.003 | 0.1065 | 0.6121 |
| rs12726 (G/A) | 0.780/0.220 | 0.774/0.226 | 0.7467 | 0.782/0.218 | 0.757/0.243 | 0.2181 | 0.751/0.249 | 0.739/0.261 | 0.6007 | 0.5895 |
| rs10814234 (C/G) | 0.864/0.136 | 0.882/0.118 | 0.2294 | 0.923/0.077 | 0.950/0.050 | 0.0220 | 0.877/0.123 | 0.882/0.118 | 0.7525 | 0.0839 |
Difference in allele frequencies between case and control subjects was tested by a χ2 test with 1 d.f., separately in each population.
For each tested SNP, the P values of the association tests obtained in the three populations were combined by Fisher's method.
Our initial sequencing of the 39 exons of UNC13B has identified a total of 13 SNPs that could be tagged by four SNPs. These four SNPs, together with a nonsynonymous variant located in exon 28 (R1124Q), were genotyped in the whole EURAGEDIC sample. However, analysis of the available HapMap data revealed that these four SNPs were not sufficient to correctly characterize the haplotypic variability of the gene, which spans over ∼240 kb on chromosome 9p12-p11. Therefore, 21 additional tagging SNPs spanning the whole gene were further genotyped to clarify the observed association of UNC13B SNPs with nephropathy (Fig. 1). The results of association analyses of all UNC13B SNPs (apart from two rare variants shown in supplementary Table 3) with nephropathy are summarized in Table 1. While most of the SNPs were associated with nephropathy in the whole study, none showed significant allelic association in the three populations, and only one, rs13293564, showed nominal allelic association in two populations: Denmark and Finland. In these two populations, homozygous carriers of the T allele were more frequent in case subjects than in control subjects (0.18 vs. 0.12 and 0.25 vs. 0.17, respectively) and were then at higher risk of nephropathy (OR 1.60 [1.10–2.32], P = 0.013; OR 1.57 [1.11–2.22], P = 0.011, respectively) (supplementary Table 4). Interestingly, French homozygous carriers of this allele also tended to be more frequent in case subjects than in control subjects (0.18 vs. 0.15), but the association failed to reach nominal significance (OR 1.26 [0.83–1.92], P = 0.278). These three ORs were not statistically different from each other (P = 0.663) and were therefore combined, leading to an increased risk of nephropathy associated with the TT genotype of OR 1.49 (95% CI 1.20–1.85) (P = 0.0003). Further adjustment for smoking and A1C did not modify these associations (supplementary Table 4).
FIG. 1.
Schematic representation of the UNC13B gene. The structure of UNC13B gene on chromosome 9 is presented with the respective positions of the 39 exons and of the 24 SNPs genotyped (rsID are given in Table 1), as well as the HapMap haplotype blocks (in D′). Arrows at both ends: SNPs selected through sequencing; single arrow: haplotype tagging SNPs selected from Hapmap; arrow with bullet: position of rs13293564, the SNP associated with nephropathy; dashed arrow with bullet: SNPs in complete association with rs13293564 (not typed).
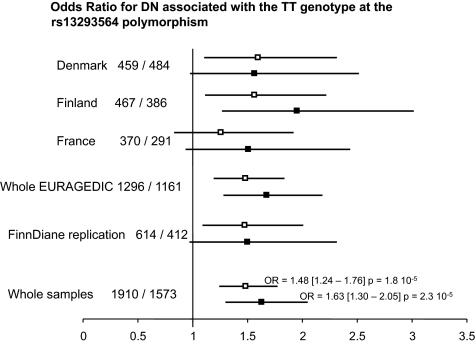
One feature of the French patients with diabetes is that 76% of them had proliferative retinopathy, whereas this percentage was 49 and 58 in Denmark and Finland, respectively (7, supplementary Table 1). Further adjustment for retinopathy status strengthened the observed association, in particular in France, where the OR associated with the TT genotype was then similar to that observed in Denmark (Fig. 2), leading to a common OR for nephropathy associated with the TT genotype of 1.68 (95% CI 1.29–2.19) (P = 0.0001). This was explained by the slightly more pronounced difference in TT genotype frequencies between case subjects and control subjects observed in patients without proliferative retinopathy (0.25 vs. 0.15) than in patients with proliferative retinopathy (0.19 vs. 0.13) (supplementary Table 5). However, no heterogeneity was detected according to the retinopathy status (P = 0.48).
FIG. 2.
ORs for diabetic nephropathy associated with the TT genotype at the rs13293564 polymorphism. Number of control subjects/number of case subjects is shown. □, OR adjusted for age and sex; ▪, OR adjusted for age, sex, smoking, A1C, and proliferative retinopathy.
A two-locus association analysis (Table 2) on the rs2281999 and rs13293564 SNPs showed that the difference in the genotype distribution between case subjects and control subjects mainly came from the rs13293564-TT genotype, suggesting that the initial association observed between the rs228199 and nephropathy was due to its LD with the rs13293564. All further LD, multilocus, and haplotype analyses converge to a unique recessive effect of the rs13293564 polymorphism (supplementary Tables 6–8, supplementary Fig. 1), an effect that occurs homogeneously in men and women (data not shown) and across the three EURAGEDIC populations.
TABLE 2.
Genotype distribution derived from rs2281999 and rs13293564 according to diabetic nephropathy status in the whole EURAGEDIC study
| Control subjects |
rs13293564 |
Case subjects |
rs13293564 |
||||
|---|---|---|---|---|---|---|---|
| rs2281999 | GG (36%) | GT (50%) | TT (14%) | rs2281999 | GG (32%) | GT (48%) | TT (20%) |
| CC (43%) | 112 (9%) | 261 (22%) | 147 (12%) | CC (50%) | 94 (9%) | 241 (22%) | 201 (19%) |
| CT (46%) | 219 (18%) | 323 (27%) | 17 (2%) | CT (42%) | 177 (17%) | 263 (24%) | 11 (1%) |
| TT (11%) | 115 (9%) | 15 (1%) | 1 | TT (8%) | 81 (7%) | 10 (1%) | 0 |
The rs13293564 SNP was further investigated in an independent Finnish sample from the FinnDiane study (8) including 412 case subjects with nephropathy and 614 control subjects. In this population, the TT genotype was also associated with an increased risk of nephropathy (OR 1.45 [1.06–1.98]) (P = 0.020) that was hardly modified by further adjustment for smoking, A1C, and proliferative retinopathy (OR 1.51 [0.97–2.36]; P = 0.070). Finally, in the combined sample from the EURAGEDIC and FinnDiane studies, the adjusted OR for nephropathy associated with the TT genotype was 1.63 (95% CI 1.30–2.05) (P = 2.3 × 10−5) (Fig. 2).
The rs13293564 SNP was further investigated in 674 patients with type 1 diabetes and microalbuminuria from the three populations. The frequency of the TT genotype in patients with microalbuminuria was significantly higher than the frequency of this genotype in patients with normoalbuminuria (0.22 vs. 0.15, P < 10 −4) (supplementary Table 9). The frequency of the TT genotype was similar, whatever the stage of diabetic nephropathy, incipient nephropathy (microalbuminuria) (0.22), macroalbuminuria (0.21), or end-stage renal disease (ESRD) (0.23) (supplementary Table 9).
Assuming a minor allele frequency of 0.39 at the rs13293564 locus in patients with type 1 diabetes and an increased risk of 1.6 in homozygous carriers of the T allele, the population attributable risk for rs13293564 would be 8.3%.
Expression studies.
A diagnostic PCR for UNC13B transcripts in various cell lines and tissues (supplementary Fig. 2) was performed. The strongest expression of UNC13B was detectable in human tissues from brain, testis, and podocytes, as well as the human immortalized podocyte cell line SHSy. Kidney cell lines COS7, and to a minor extent MDCK I and II, express UNC13B, but not embryonic kidney cell line HEK293T. Osteosarcoma cell lines (SaOs2, U2Os), liver (HepG2), and breast cancer (MCF7) show noticeable expression, whereas in monocytic cell lines, either differentiated or not, expression is strictly cell-line dependent. Choriocarcinoma cells HeLa do not express UNC13B.
In silico analyses.
There was no feature to suggest that rs13293564 located in intron 1 of the UNC13B is the functional variant. Analyses of the five polymorphisms in complete association with rs13293564 (rs10081672, rs10972356, rs13288912, rs12377498, and rs10972333) located in the putative promoter region showed that the rs10081672 and rs10972333 SNPs, located respectively at positions −6081 and −25843 bp upstream of the transcription start site of the human UNC13B gene, affect potential Sp1 and upstream stimulating factor binding sites, respectively.
DISCUSSION
We have shown for the first time that a common variation in the UNC13B gene was reproducibly associated with nephropathy. A recent study that assessed 115 candidate genes for nephropathy in 82 trios did not find significant results for any of the six UNC13B markers analyzed (31). This might be explained by the insufficient number of markers studied and a study of limited power.
The rs13293564 lies within the first intron of the UNC13B gene and is in strong LD with many other SNPs all along the gene (see supplementary data and HapMap data). However, it is in complete association with only five SNPs (rs10081672, rs10972356, rs13288912, rs12377498, and rs10972333) located in the putative promoter region. While there is no feature to suggest that rs13293564 is the functional variant, rs10081672 and rs10972333, located respectively at positions −6081 and −25843 bp upstream of the transcription start site of the human UNC13B gene, affect potential Sp1 and USF binding sites, respectively, suggesting that those SNPs could be the functional variants. It is possible that regulatory elements are located this far upstream of the proximal regulatory regions within the core promoter. Functional molecular analyses are needed to clarify their impact. It should also be stressed that an expressed repetitive element NM_001039797 of 3.032 kbp is located within UNC13B intron 1, 20 kb downstream of rs13293564, the function of which is unknown, and strong LD spans over this region. This opens up the possibility that genetic variation affects a regulatory element for UNC13B within the intronic region. Such mechanisms have recently been shown to be involved in susceptibility to different diseases (32–34).
The human UNC13B gene product (also called Hmunc13) has been cloned from the human kidney library (35) and is homologous to rat munc13 proteins, which are members of the protein kinase C (PKC) superfamily that lack a kinase domain. Human UNC13B has one C1 diacylglycerol (DAG) and three C2 (Ca2+) binding domains. It is both upregulated and activated in the presence of hyperglycemia in renal cortical tubular cells and in glomerular mesangial cells (36). Using RT-PCR to further assess the expression of UNC13B in human tissues, we found that UNC13B was also highly expressed in podocytes (supplemental Fig. 2). It has been shown that DAG activation of UNC13B-expressing cells induces apoptosis. As hyperglycemia increases intracellular DAG levels and is associated with apoptosis in various tissues, including human kidney, it is plausible that UNC13B plays a role in mediating renal complications of diabetes (36). Apoptosis of glomerular cells occurs quite early in the natural history of diabetic nephropathy, and it is now recognized that it could be an inciting event rather than a late consequence caused by increasing proteinuria (37–41). Interestingly, TT carriers of UNC13B_rs13293564 tended to be at slightly higher risk of nephropathy if they had not yet developed proliferative retinopathy, a clinical control for longstanding diabetes, emphasizing the role of UNC13B at the early stage of the disease. This is further strengthened by our observation that the frequency of the UNC13B_rs13293564 TT genotype in patients with incipient diabetic nephropathy, an early stage of the renal complication characterized by the presence of microalbuminuria, was significantly higher than the frequency of this genotype in control subjects. The similar distribution of the UNC13B_rs13293564 polymorphism genotype in three groups of patients with different stages of diabetic nephropathy also suggests that this polymorphism is implicated in the initiation rather than in the progression of the disease to more severe stages. However, because of the cross-sectional design of this study, we cannot exclude a survival bias in the ESRD group that would artificially modify the allele frequency patterns of susceptibility genes in this group.
The molecular analyses of the other 118 candidate genes identified no further variant associated with nephropathy. However, the SNPs genotyped in this study do not tag the haplotype architecture of these loci; rather, they tag a subset of the haplotypes across these genes based on the SNPs identified around exons, flanking intronic sequence, untranslated regions, and promoters. To fully characterize the haplotypic variability of these genes, as would tagging SNPs selected from the Hapmap project, the genotyping of ∼1,300 SNPs would be required, while 344 have been typed in this study. Therefore, we cannot exclude that these candidate genes contribute to the development of diabetic nephropathy.
Our strategy, based on the genetic assessment of many candidate genes in a large case/control study for diabetic nephropathy, including material from three different European populations, provided evidence of replicated association between UNC13B and nephropathy and allowed us to establish for the first time the involvement of UNC13B variants in nephropathy. To assess whether or not these variants are clinically relevant to predict the initiation of nephropathy or the progression to more advanced stages of nephropathy will require further investigation in cohorts of patients with follow-up data.
URLs.
HapMap: http://www.hapmap.org; UCSC genome browser: http://genome.ucsc.edu; NCBI: http://www.ncbi.nlm.nih.gov; Alibaba2.1, Transfac7.0: http://www.gene-regulation.com; and http://genecanvas.ecgene.net: genotypic data for all markers, phenotypic covariates from Danish and French samples, and a table describing all variations identified in the EURAGEDIC study.
Supplementary Material
Acknowledgments
The EURAGEDIC study was made possible through funding from the European Commission (contract number QLG2-CT-2001-01669). The FinnDiane study was supported by grants from the Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Liv och Hälsa Foundation, Sigrid Juselius Foundation, and the Finnish Medical Society (Finska Läkaresällskapet). D.G. holds a Wellcome Trust Senior Fellowship in Basic Biomedical Science (057733). This study was also supported by a grant from the EU-Project Network of Excellence, FP6-2005-LIFESCIHEALTH-6, Integrating Genomics, InGenious HyperCare proposal number 037093 to S.-M.B-H.
We thank the French Diabetes Association for its support (bourse Association Française des Diabétiques Recherche 2000). We acknowledge A. Boland, V. Dumaz, D. Lechner, A. Lemainque, L. Sobre, J.-A. Perrier, C. Plançon (CNG), and C. Mascarenhas (U525) for technical assistance as well as A. Thévard (U525) for data management. All patients with type 1 diabetes are sincerely acknowledged. We also thank the Danish Diabetes Association and the Sehested Hansen Foundation for their continued support of this study. We acknowledge all the physicians and nurses at each center participating in the collection of patients (online appendix supplemental material). Brain and testis tissue RNA was a gift of Dr. J. Kremerskothen, Münster, and podocyte cDNA was a gift from Dr. T. Weide, University Hospital, Münster.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T: Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 25:496–501, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Borch-Johnsen K, Kreiner S: Proteinuria value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. BMJ 294:1651–1654, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seaquist ER, Goetz FC, Rich S, Barbosa J: Familial clustering of diabetic kidney disease: evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 320:1161–1165, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Quinn M, Angelico MC, Warram JH, Krolewski AS: Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 39:940–945, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Earle K, Walker J, Hill C, Viberti GC: Familial clustering of cardiovascular disease in patients with insulin-dependent diabetes and nephropathy. N Engl J Med 326:673–677, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Control and Complications Trial Research Group: Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes 46:1829–1839, 1997 [PubMed] [Google Scholar]
- 7.Tarnow L, Groop PH, Hadjadj S, Kazeem G, Cambien F, Marre M, Forsblom C, Parving HH, Tregouet D, Thevard A, Farrall M, Gut I, Gauguier D, Cox R, Matsuda F, Lathrop M, Vionnet N, for the EURAGEDIC Consortium: European rational approach for the genetics of diabetic complications: EURAGEDIC: patient populations and strategy. Nephrol Dial Transplant 23:161–168, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Thorn L, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, Wadén J, Rönnback M, Rosengård-Bärlund M, af Björkesten C-G, Taskinen M-R, Groop P-H, on behalf of the FinnDiane Study Group: Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycaemic control (the FinnDiane Study). Diabetes Care 28:2019–2024, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Vionnet N, Tregouët D, Kazeem G, Gut I, Groop PH, Tarnow L, Parving HH, Hadjadj S, Forsblom C, Farrall M, Gauguier D, Cox R, Matsuda F, Heath S, Thévard A, Rousseau R, Cambien F, Marre M, Lathrop M: Analysis of 14 candidate genes for diabetic nephropathy on chromosome 3q in European populations: strongest evidence for association with a variant in the promoter region of the adiponectin gene. Diabetes 55:3166–3174, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dizier MH, Bouzigon E, Guilloud-Bataille M, Bétard C, Bousquet J, Charpin D, Gormand F, Hochez J, Just J, Lemainque A, Le Moual N, Matran R, Neukirch F, Oryszczyn MP, Paty E, Pin I, Vervloet D, Kauffmann F, Lathrop M, Demenais F, Annesi-Maesano I: Genome screen in the French EGEA study: detection of linked regions shared or not shared by allergic rhinitis and asthma. Genes Immun 6:95–102, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M, Matsuda F, Margetic N, Lathrop M: Automated identification of single nucleotide polymorphisms from sequencing data. J Bioinform Comput Biol 1:253–265, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Dempster AP, Laird NM, Rubin DB: Maximum likelihood from incomplete data via the EM algotrithm. J R Stat Soc B 39:1–38, 1977 [Google Scholar]
- 13.Nakajima K, Tanaka Y, Nomiyama T, Ogihara T, Piao L, Sakai K, Onuma T, Kawamori R: Chemokine receptor genotype is associated with diabetic nephropathy in Japanese with type 2 diabetes. Diabetes 51:238–242, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, Rychlik I, Cerna M, Romzova M, de Heer E, Baelde H, Bakker SJ, Zirie M, Rondeau E, Mathieson P, Saleem MA, Meyer J, Köppel H, Sauerhoefer S, Bartram CR, Nawroth P, Hammes HP, Yard BA, Zschocke J, van der Woude FJ: Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54:2320–2327, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Sale MM, Smith SG, Mychaleckyj JC, Keene KL, Langefeld CD, Leak TS, Hicks PJ, Bowden DW, Rich SS, Freedman BI: Variants of the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in an African-American population enriched for nephropathy. Diabetes 56:2638–2642, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kanková K, Stejskalová A, Pácal L, Tschoplová S, Hertlová M, Krusová D, Izakovicová-Hollá L, Beránek M, Vaskù A, Barral S, Ott J: Genetic risk factors for diabetic nephropathy on chromosomes 6p and 7q identified by the set-association approach. Diabetologia 50:990–999, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Pinizzotto M, Castillo E, Fiaux M, Temler E, Gaillard RC, Ruiz J: Paraoxonase2 polymorphisms are associated with nephropathy in type II diabetes. Diabetologia 44:104–107, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Hager J, Hansen L, Vaisse C, Vionnet N, Philippi A, Poller W, Velho G, Carcassi C, Contu L, Julier C, Cambien F, Passa P, Lathrop M, Kindsvogel W, Demenais F, Nishimura E, Forguel P: A missense mutation in the glucagon receptor gene is associated with non-insulin-dependent diabetes mellitus. Nat Genet 9:299–304, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Kaisaki PJ, Delépine M, Woon PY, Sebag-Montefiore L, Wilder SP, Menzel S, Vionnet N, Marion E, Riveline JP, Charpentier G, Schurmans S, Levy JC, Lathrop M, Farrall M, Gauguier D: Polymorphisms in type II SH2 domain-containing inositol 5-phosphatase (INPPL1, SHIP2) are associated with physiological abnormalities of the metabolic syndrome Diabetes 53:1900–1904, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ninio E, Tregouet D, Carrier JL, Stengel D, Bickel C, Perret C, Rupprecht HJ, Cambien F, Blankenberg S, Tiret L: Platelet-activating factor-acetylhydrolase and PAF-receptor gene haplotypes in relation to future cardiovascular event in patients with coronary artery disease. Hum Mol Genet 13:1341–1351, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Devlin B, Roeder K: Genomic control for association studies. Biometrics 55:997–1004, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D: Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38:904–909, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Sauer S, Lechner D, Berlin K, Plançon C, Heuermann A, Lehrach H, Gut IG: Full flexibility genotyping of single nucleotide polymorphisms by the GOOD assay. Nucleic Acid Res 28:E100, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher RA: Statistical Methods for Research Workers. 13th ed. London, Oliver & Loyd, 1925
- 25.Li J, Ji L: Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95:221–227, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Barrett JC, Fry B, Maller J, Daly MJ: Haploview: analysis and visualization of LD and haplotypes maps. Bioinformatics 21:263–265, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Tregouet DA, Garelle V: A new JAVA interface implementation of THESIAS: testing haplotype effects in association studies. Bioinformatics 23:1038–1039, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Paul SR, Donner A: A comparison of tests of homogeneity of odds ratio in k 2x2 tables. Stat Med 8:1455–1468, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Skryabin BV, Kremerskothen J, Vassilacopoulou D, Disotell TR, Kapitonov VV, Jurka J, Brosius J: The BC: 200 RNA gene and its neural expression are conserved in Anthropoidea (primates). J Mol Evol 47:677–685, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13:630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Ewens KG, George RA, Sharma K, Ziyadeh FN, Spielman RS: Assessment of 115 candidate genes for diabetic nephropathy by transmission/disequilibrium test. Diabetes 54:3305–3318, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Parle-McDermott A, Pangilinan F, Mills JL, Kirke PN, Gibney ER, Troendle J, O'Leary VB, Molloy AM, Conley M, Scott JM, Brody LC: The 19-bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR) may decrease rather than increase risk for spina bifida in the Irish population. Am J Med Genet A 143:1174–1180, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Greene E, Mahishi L, Entezam A, Kumari D, Usdin K: Repeat-induced epigenetic changes in intron 1 of the frataxin gene and its consequences in Friedreich ataxia. Nucleic Acid Res 35:3383–3390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu HQ, Montpetit A, Ge B, Hudson TJ, Polychronakos C: Toward further mapping of the association between the IL2RA locus and type 1 diabetes. Diabetes 56:1174–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Song Y, Ailenberg M, Silverman M: Cloning of a novel gene in the human kidney homologous to rat munc13s: its potential role in diabetic nephropathy. Kidney Int 53:1689–1695, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Song Y, Ailenberg M, Silverman M: Human munc13 is a diacylglycerol receptor that induces apoptosis and may contribute to renal cell injury in hyperglycemia. Mol Biol Cell 10:1609–1619, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shankland SJ: The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69:2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Susztak K, Raff AC, Schiffer M, Bottinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55:225–233, 2006 [PubMed] [Google Scholar]
- 39.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia 42:1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99:342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steffes MW, Schmidt D, McCrery R, Basgen JM, International Diabetic Nephropathy Study Group: Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59:2104–2113, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.