Abstract
Oxidative stress has been implicated in allergic responses. SHP-1 is a target of oxidants and has been reported as a negative regulator in a mouse model of asthma. We investigated the effect of oxidative stress on the development of allergic airway inflammation in heterozygous viable motheaten (mev/+) mice deficient of SHP-1. Wild-type (WT) and mev/+ mice were compared in this study. Human alveolar epithelial cells (A549) transfected with mutant SHP-1 gene were used to evaluate the role of SHP-1 in lung epithelial cells. Hydrogen peroxide (H2O2) and Paraquat were used in vitro and in vivo, respectively. We also investigated whether mev/+ mice can break immune tolerance when exposed to aeroallergen intranasally. Compared with WT mice, bronchoalveolar lavage (BAL) cells and splenocytes from mev/+ mice showed a different response to oxidant stress. This includes a significant enhancement of intracellular reactive oxygen species and STAT6 phosphorylation in vitro and increased CCL20, decreased IL-10, and increased number of dendritic cells in BAL fluid in vivo. Mutant SHP-1-transfected epithelial cells secreted higher levels of CCL20 and RANTES after exposure to oxidative stress. Furthermore, break of immune tolerance, as development of allergic airway inflammation, was observed in mev/+ mice after allergen exposure, which was suppressed by antioxidant N-acetylcystein. These data suggest that SHP-1 plays an important role in regulating oxidative stress. Thus, increased intracellular oxidative stress and lack of SHP-1 in the presence of T helper cell type 2–prone cellular activation may lead to the development of allergic airway inflammation.
Keywords: SHP-1, oxidative stress, allergen, asthma, immune tolerance
CLINICAL RELEVANCE
Phosphatase SHP-1 plays an important role in regulating oxidative stress. Thus, increased intracellular oxidative stress and lack of SHP-1 in the presence of T helper cell type 2–prone cellular activation may lead to development of allergic airway inflammation.
Studies have shown increased oxidants in the asthmatic airway, and it is believed that oxidative stress plays a role in the pathogenesis of allergic asthma (1–4). It has been suggested that, as an important underlying mechanism, oxidative stress activates various intracellular signaling pathways leading to secretion of a variety of pro-inflammatory cytokines and chemokines (5).
However, it is not clear whether oxidative stress is a direct contributing factor in the pathogenesis of asthma. The increased oxidative stress observed in asthmatic airways may be a consequence of inflammation. Furthermore, the mechanism by which increased oxidative stress elicits a polarized immune response is unclear considering its nonspecific effects, although some observations have suggested that oxidative stress imposed on immune cells can be closely associated with break of immune tolerance (6–8). Also it has been shown that oxidative stress generated by pollen NADPH oxidases augments allergic airway inflammation induced by pollen antigen in a mouse model of asthma (9). Intrinsic defects of some intracellular molecules, which interact with not only reactive oxygen species (ROS) but also signaling pathways involving certain polarized immune responses, may be a possible molecular mechanism explaining the crucial role of oxidative stress in the pathogenesis of specific inflammatory immune responses.
SHP-1, a protein tyrosine phosphatase (PTP), has been recognized as a critical negative regulator in intracellular signaling and is expressed predominantly in hematopoietic cells and epithelial cells (10–13). The function and importance of SHP-1 was recognized upon the discovery of two strains of SHP-1–deficient mutant mice, the motheaten (me/me) and viable motheaten (mev/mev) mice (14, 15). Recently, it was reported that SHP-1 has an inhibitory role in the development of allergic airway inflammation in an allergen-induced asthma model using heterozygous me (me/+) mice (16). It was demonstrated that me/+ mice showed significantly increased STAT6 phosphorylation and production of T helper cell type 2 (Th2) cytokines after antigen stimulation (16). Furthermore, we observed that SHP-1–deficient homozygous mev/mev mice develop a spontaneous allergic inflammation in the lung without known allergen stimulation (S. Y. Oh and Z. Zhu, unpublished observations).
Meanwhile, intracellular hydrogen peroxide (H2O2), generated endogenously upon stimulation and exogenously from environmental oxidants (17), has been known to be involved in the process of intracellular signaling through inhibiting enzymatic activities of various PTPs (7, 8, 18). In general, low levels of oxidative stress imposed on cells result in transient and well-controlled cellular activation with prompt activation of intracellular antioxidant systems without eliciting any abnormal events. In contrast, higher levels of oxidative stress can cause exaggerated inflammatory response and break of immune tolerance (6, 19). Increased intracellular ROS can inhibit the function of SHP-1 (18). In addition, it is also well known that suppressed function of SHP-1 results in increased intracellular ROS levels by permitting the exaggerated and prolonged activation of intrinsic intracellular ROS-generating mechanisms (20–22).
Thus, it is reasonable to speculate that SHP-1 deficiency can render the cells or host more susceptible to oxidative stress, which may cause exaggerated cellular activation followed by a Th2 immune response in the presence of low levels of oxidant. We deemed that this hypothesis might provide one explanation for direct participation of oxidative stress in the pathogenesis of asthma. To address these questions, we investigated the effect of oxidative stress on the development of allergic inflammation using SHP-1–deficient heterozygous mev mice.
MATERIALS AND METHODS
Animals
Heterozygous mev (mev/+) mice and wild-type (WT, or +/+) mice on C57BL/6 genetic background were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen–free conditions and were used at 6 to 8 weeks of age. Genotyping was performed with PCR amplification of tail DNA as described before (23). All animal experiments were approved by the IACUC of the Johns Hopkins University.
SHP-1 Protein Phosphatase Activity in the Lung
The SHP-1 protein phosphatase activity in the lung tissues was determined by immunoprecipitation of SHP-1 followed by a colorimetric assay using the SensoLyte pNPP protein phosphatase assay kit (AnaSpec, San Jose, CA). Lungs were obtained from WT, mev/+, and mev/mev mice as described below and homogenized in the lysis buffer provided in the kit. SHP-1 protein was immunoprecipitated from 1 mg of protein from each lung sample using anti–SHP-1 (sc-287) and protein A/G Plus-Agarose (Santa Cruz Biotechnology, Santa Cruz, CA) following their protocol and resuspended in 200 μl of assay buffer. The phosphatase activity in the SHP-1 solution (50 μl) was measured with pNPP as a substrate using the SensoLyte kit as instructed. The optical density (O.D.) readings were calibrated on the negative control wells containing no protein.
In Vitro Cellular Responses after Exposure to H2O2
The tracheas of WT and mev/+ mice were cannulated under anesthesia, and bronchoalveolar lavage (BAL) was performed using 2 ml of PBS. The recovered fluid was centrifuged at 400 × g for 5 minutes to pellet the cells. The supernatant was saved for analysis and the cell pellets were resuspended and incubated in PBS with or without 100 μM of H2O2 (Sigma Aldrich Chemical Co., St. Louis, MO) at 37°C for 30 minutes. Next, the cells were loaded with 20 μM 2′7′-dichlorofluorescin diacetate (DCFH-DA) (Molecular Probes, Eugene, OR) at 37°C for 20 minutes. After washing and incubation at 37°C for 1 hour, the cells were subjected to flow cytometry for DCF emission.
Similarly, splenocytes were prepared and resuspended in PBS with or without H2O2 at 37°C for 1 hour. In some experiments, anti-mouse IL-4 antibody (eBioscience, San Diego, CA) was added (10 ng/ml). Proteins were extracted from cytoplasmic and nuclear compartments using commercial kits (Pierce, Rockford, IL). Total proteins were extracted using protein lysis buffer containing 20 μl/ml of protease inhibitor cocktail (BD PharMingen, San Diego, CA) and 5 μl/ml of Na3VO4. For determination of nuclear and cytoplasmic Nrf2, total STAT6, phosphorylated STAT6, I-κBα, and phosphorylated I-κBα, Western blot was performed using primary antibodies to Nrf2, I-κBα, phosphorylated I-κBα (Santa Cruz Biotechnology, Santa Cruz, CA), STAT6, or phosphorylated STAT6 (Cell Signaling Technology, Danvers, MA).
In Vivo Responses to Oxidative Stress in the Lung
To generate oxidative stress in vivo, Paraquat (Sigma) in PBS was administered intraperitoneally twice a week for 2 weeks at 2.5 μg/g body weight—10 times less than the usual dose used in the acute lung injury model (24). In one set of experiments, total lung cells were analyzed. After pulmonary perfusion, single-cell suspensions were prepared by digesting the minced lung tissues for 1 hour with disaggregation buffer containing 300 unit/ml collagenase IV (Gibco, Grand Island, NY), followed by gently pushing the tissues through a 100-μm nylon cell strainer (BD Falcon, Franklin Lakes, NJ). Single-cell suspensions were analyzed by flow cytometry after staining with fluorescein isothiocyanate–anti-CD11b, APC–anti-CD11c, and PE–anti-B220 antibodies (BD PharMingen).
In another set of experiments, total BAL cell counts and differential were determined. The levels of C-C chemokine ligand (CCL) 20/macrophage inflammatory protein-3α (MIP-3α), CCL5/RANTES, eotaxin, and IL-10 in BAL were determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, MN).
Effect of Mutant SHP-1 on Epithelial Cell Responses to Oxidative Stress
A549 human alveolar epithelial cells (ATCC, Manassas, VA) were cultured in Ham's F12K medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum and transfected with the pEF-flag plasmid vector containing either wild-type human SHP-1 gene (pEF-flag-SHP-1-wt) or mutant SHP-1 (pEF-flag-SHP-1-C/S) (25) (kindly provided by Dr. Hidekara Yakura, Tokyo Metropolitan Institute for Neuroscience, Tokyo, Japan) using Lipofectamine 2000. H2O2 (100 μM) was added to the culture medium 36 hours after transfection and overnight serum starvation. The supernatant was collected at 1 and 6 hours after H2O2 treatment, and the levels of CCL20 and RANTES were determined.
Pulmonary Responses to Intranasal Ovalbumin Challenges and the Effect of Antioxidant on Inflammation
To investigate the effect of specific antigen on SHP-1–deficient mev/+ mice, ovalbumin (OVA) allergen was introduced intranasally 100 μg in 50 μl PBS once a day for 5 consecutive days. Antioxidant NAC was given intraperitoneally at 200 μg/g body weight right before administration of OVA. At Day 7, the mice were anesthetized and killed by cardiac puncture. Blood was obtained and serum was collected. Serum OVA-specific IgE was determined by ELISA (BD Bioscience, San Jose, CA).
BAL was performed and total cell counts and differential were determined. In the meantime, splenocytes were prepared and cultured (1×106 cells/ml) in RPMI 1640 medium for 24 hours with or without increasing concentrations of OVA (2.5, 25, and 250 μg/ml). The MTT cell proliferation assay was performed as instructed (ATCC).
For histopathologic assessment, lungs were removed, fixed in Streck tissue fixative solution (Streck Laboratories, La Vista, NE), embedded in paraffin, sliced, and then stained with hematoxylin and eosin and Alcian Blue.
Statistical Analysis
For comparison between groups, Student's t test was used, data were expressed as mean ± SE, and statistical significance was defined as P < 0.05.
RESULTS
SHP-1 Deficiency in mev/+ Mice
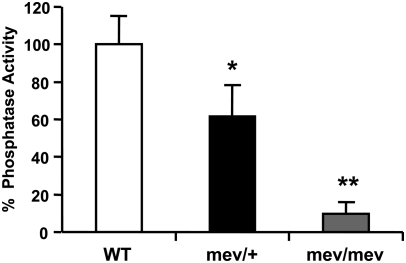
Using Western blot we found comparable levels of SHP-1 in the lung homogenates of WT, mev/+, and mev/mev mice (data not shown). This is consistent with previous reports that SHP-1 mRNA transcripts and full-length protein were present in hematopoietic cells of mev/+ and mev/mev mice, in contrast to me/+ and me/me mice (23, 26). However, the mutant SHP-1 protein is not functional because there is either a deletion or insertion in the enzyme catalytic region that abrogates its phosphatase activity (26). Using immunoprecipitation and a phosphatase assay we determined the SHP-1 phosphatase activity in the lung of WT, mev/+, and mev/mev mice. As shown in Figure 1, SHP-1 activity was present in the lung tissues of WT mice. However, mev/+ and mev/mev mice had only approximately 60% and 10%, respectively, residual SHP-1 activity in the lung tissues.
Figure 1.
SHP-1 protein phosphatase activity in lung tissues. SHP-1 protein was immunoprecipitated from lung homogenates of wild-type (WT), heterozygous viable motheaten (mev/+), and viable motheaten (mev/mev) mice and the phosphatase activity was determined using substrate pNPP in a colorimetric assay. The data are expressed as percent of SHP-1 phosphatase activity relative to that of WT mice. Shown is a representative of two experiments with similar results (WT, n = 3; mev/+, n = 4; mev/mev, n = 4; *P < 0.05 and **P < 0.01).
Effect of SHP-1 Deficiency on Cellular Response to H2O2
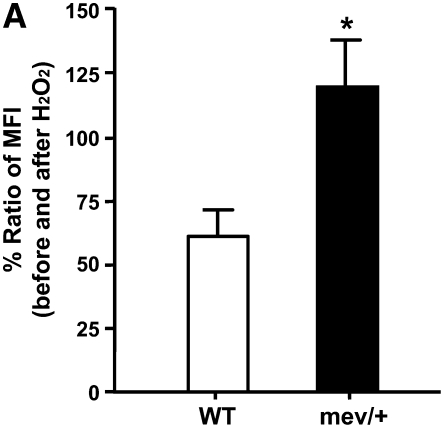
First, we examined the effect of exogenous ROS on cells. After exposure to H2O2, BAL cells from mev/+ mice showed significantly increased intracellular ROS compared with WT mice (Figure 2A), suggesting that SHP-1 deficiency is associated with decreased cellular tolerance to oxidative stress.
Figure 2.
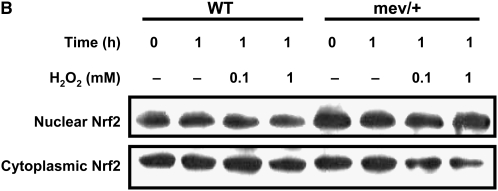
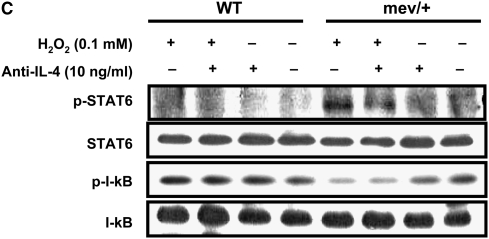
In vitro cellular responses to oxidative stress. (A) Ratio of mean fluorescent intensity (MFI) for intracellular reactive oxygen species in H2O2-treated (100 μM) bronchoalveolar lavage (BAL) cells over basal conditions. Data were collected from 5,000 events (n = 3, *P < 0.05). The data are the mean ± SEM. (B) Nrf2 in nucleus and cytoplasm of splenocytes from WT and mev/+ mice 1 hour after H2O2 treatment. (C) Total and phosphorylated STAT6 and I-κBα in the splenocytes from WT and mev/+ mice. Data are representative of three experiments.
Next, we investigated the activation of signaling pathways related to oxidative stress after H2O2 stimulation. The amount of Nrf2, a known key transcription factor regulating antioxidant defense, was determined by Western blot in the nucleus and cytoplasm compartments of splenocytes, respectively, 1 hour after H2O2 treatment. Interestingly, basal nuclear Nrf2 of mev/+ mice without H2O2 was significantly higher compared with that of WT mice (Figure 2B). However, there was no further increase in Nrf2 after exposure to H2O2 (Figure 2B). These results suggest that even in resting state, mev/+ mice may already suffer from substantial levels of oxidative stress. Whether Nrf2 activation is time dependent in these cells remains to be determined. We investigated the phosphorylation status of STAT6, since it is important in Th2-type immune response and can be regulated by SHP-1. Phosphorylated STAT6 was observed in the mev/+ mice treated with H2O2, while no band was found in the WT mice or mev/+ mice without H2O2 treatment. Blocking IL-4 pathway by anti–IL-4 antibody did not show any effect on the activation of STAT6, suggesting an intracellular event (Figure 2C). NF-κB is known to be a pivotal transcription factor related to oxidative stress. We examined the phosphorylation of I-κBα to determine the activation of NF-κB. I-κBα phosphorylation was lower in the splenocytes of mev/+ mice relative to that in the splenocytes of WT mice when exposed to H2O2 (Figure 2C).
Effects of SHP-1 Deficiency on Pulmonary Response to Oxidative Stress
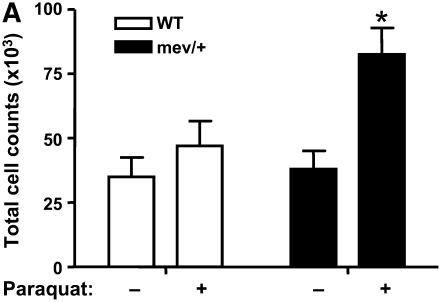
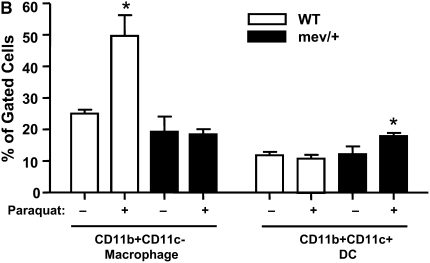
We evaluated the in vivo effect of oxidative stress imposed on SHP-1–deficient mev/+ mice. A significant increase in the total number of BAL cells was found in the mev/+ mice exposed to Paraquat (Figure 3A). Differential cell count showed that most of the cells were monocytes or macrophages. The subtypes of macrophages and dendritic cells (DCs) were determined by flow cytometry using total lung cells. Interestingly, the portion of CD11b+CD11c+ DC was significantly increased in the lung of mev/+ mice, while CD11b+CD11c- macrophages were remarkably higher in WT mice when exposed to oxidative stress (Figure 3B, and Figure E1 in the online supplement).
Figure 3.
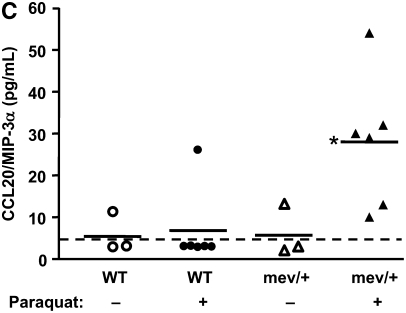
In vivo responses to oxidative stress. (A) Total cell counts of BAL cells from mev/+ and WT mice with or without Paraquat. (B) Percentage of CD11c+CD11b+ dendritic cells and CD11b+CD11c− macrophages in the lung after exposure to Paraquat. (C) CCL20 in BAL fluids. Dotted line represents detection limit (n = 3–6, *P < 0.05 compared with other groups). The data are the mean ± SEM.
The BAL levels of cytokines and chemokines were measured to see if the microenvironment in the lung was altered after oxidative stress exposure in SHP-1–deficient mice, which may be able to explain the different profiles of recruited inflammatory cells. The level of CCL20, which is recently reported to play an important role in the recruitment of immature DCs, was found to be significantly enhanced in the BAL fluid of mev/+ mice exposed to oxidative stress (Figure 3C). The level of CCL5/RANTES, which is also one of the important monocyte/DC chemoattractant factors, was evaluated, but no significant difference was found. The levels of CCL2/monocyte chemoattractant protein-1 (MCP-1) and eotaxin were below detection limit. The level of IL-10 showed a decrease in the BAL fluids of mev/+ mice treated with oxidative stress (Figure E2), while no significant difference was observed in the transforming growth factor-β level (data not shown).
SHP-1 and Lung Epithelial Cell Response to H2O2
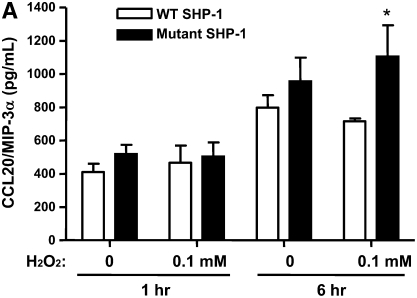
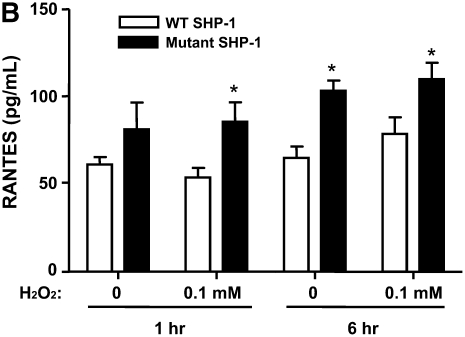
We evaluated the effect of SHP-1 deficiency on the release of certain chemokines by alveolar epithelial cells when exposed to H2O2, since lung epithelial cells are an important source of several critical chemokines, including CCL20 and RANTES. A549 human alveolar epithelial cells transfected with a plasmid vector containing a mutant SHP-1 gene (C/S) lacking catalytic activity showed increased production of CCL20 and RANTES compared with the cells transfected with vector with WT SHP-1 gene, regardless of exposure to H2O2 (Figures 4A and 4B). Treatment with H2O2 tended to stimulate higher levels of CCL20 and RANTES by A549 cells. The production of CCL2 and IL-10, however, revealed no difference regardless of SHP-1 deficiency (data not shown).
Figure 4.
Levels of (A) CCL20 and (B) RANTES produced by human lung epithelial cells transfected with plasmid vector containing WT or mutant human SHP-1 gene after H2O2 exposure. The data are the mean ± SEM of triplicate samples. *P < 0.05 compared with groups transfected with plasmid vectors containing WT SHP-1 gene in each pair of experiments.
OVA Induced Airway Allergic Inflammation and the Effect of Antioxidant
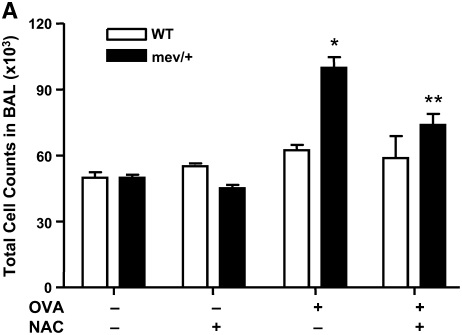
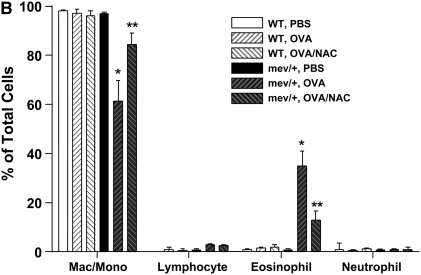
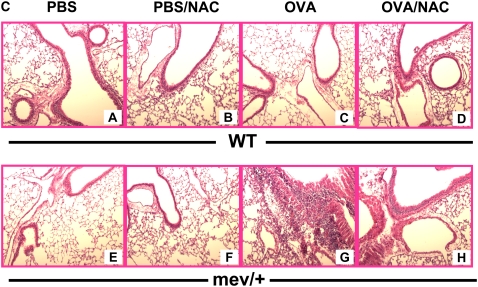
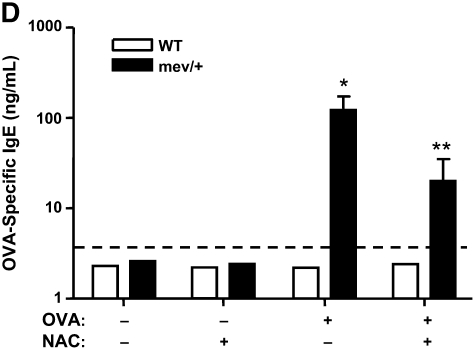
First, we demonstrated that a single intranasal administration of OVA could induce significantly enhanced levels of intracellular ROS in the BAL cells of mev/+ mice compared with those of WT mice (Figure E3). We then performed intranasal OVA challenges without adjuvant (Figure E4). Using this protocol, no evidence of airway inflammation was found in the WT mice. However, in mev/+ mice with OVA challenge, substantial airway inflammation was observed evidenced by increased infiltrating cells, particularly eosinophils, in the BAL and in lung tissue (Figures 5A–5C). Alcian Blue stain was performed to detect mucin-producing cells in the airways. A few positive-stained cells were found in the large airways but not medium or small airways of WT mice challenged with PBS or OVA and mev/+ mice with PBS. In contrast, increased Alcian Blue positive-stained cells were seen in the medium and small airways of mev/+ mice with OVA (Figure E6). These changes were accompanied by increased levels of serum OVA-specific IgE (Figure 5D) and enhanced cellular proliferation in the presence of OVA (Figure E5). Furthermore, treatment with antioxidant NAC significantly suppressed all aspects of airway inflammation (Figures 5A–5D), although it did not eliminated the mucin-producing cells in the airways (Figure E6).
Figure 5.
Allergic inflammatory responses to ovalbumin (OVA) challenge. (A and B) Total and differential cell counts of BAL cells. (C) Lung histopathology of mev/+ mice and WT mice after intranasal OVA stimulation in the presence or absence of antioxidant NAC (hematoxylin and eosin, ×100). (D) Serum OVA-specific IgE. Dotted line represents detection limit. (n = 4–6, *P < 0.05 and **P < 0.05 compared with groups without NAC). The data are the mean ± SEM.
DISCUSSION
Epidemiologic studies have demonstrated an association between increased oxidative stress and adverse effects on asthma and allergic inflammation (1, 2, 4). However, it is still unclear if increased oxidative stress plays a causative role in the development of asthma. The study presented herein demonstrated that oxidative stress contributes to the development of inflammation in the lungs of SHP-1–deficient mice through break of immune tolerance to aeroallergen.
SHP-1 is a negative regulator in various signaling pathways (13, 27). Recently, SHP-1 was reported to be able to suppress intrinsic generation of oxidative stress through counteracting NADH-oxidase and iNOS (20, 21). It is also believed that reversible redox regulation of cysteine-based PTPs plays a dominant role in setting the level of tyrosine phosphorylation in cells and is likely to be an important mechanism for maintaining appropriate cellular responses (18, 22). ROS, one of the critical intracellular second messengers, can modulate intracellular protein phosphorylation by reversible oxidation of the active site cystein residues of PTPs, leading to transient inactivation of these enzymes (8, 10, 18). With two cysteinyl residues in its catalytic site, SHP-1 is vulnerable to oxidation (10).
Thus, it is conceivable that functional deficiency in SHP-1 may be related to increased susceptibility to oxidative stress that may cause improper activation of cells. To test this we used the SHP-1–deficient mev mice. The genetic mutations, the expression and function of SHP-1 in me and mev mice, have been well characterized. Severely diminished SHP-1 phosphatase activity was found in hematopoietic cells of homozygous me/me and mev/mev mice that correlated with their spontaneous inflammatory phenotypes (23, 26). However, it is unclear what levels of SHP-1 activity are present in the heterozygous mev/+ mice, which show no abnormalities under normal conditions. The SHP-1–specific phosphatase activity in the lung tissues was determined. Compared with WT mice, mev/+ mice have approximately 60%, and mev/mev mice 10%, SHP-1 activity left in the lung (Figure 1), despite the presence of comparable levels of full-length SHP-1 protein (data not shown). Our study found that homozygous mev/mev mice develop a spontaneous Th2-dominated pulmonary inflammation (S. Y. Oh and Z. Zhu, unpublished data). Thus SHP-1–deficient heterozygous mev/+ mice are more suitable for studying cellular and immune responses under stress.
Our study revealed enhanced intracellular ROS in BAL cells after exposure to H2O2 and activation of Nrf2 even in resting state in mev/+ mice. These findings strongly suggest that SHP-1–deficient mice even without any stimulation may already have substantially increased burden of oxidative stress, although no phenotypical abnormalities could be observed under normal conditions.
Increased intracellular ROS reaching certain threshold might be a critical common pathway to induce immunity regardless of initial stimuli. In a recent report, it was found that effective T cell responses can occur with adjuvants regardless of the kinds of stimulation through a universal mechanism such as recruitment of DCs (28). Those observations further suggest that various adjuvants might cause a common intracellular event such as increased ROS in the development of immune responses. Enhanced ROS may be closely involved in mounting an immune response through recruitment and activation of DCs. In this study, enhanced ROS in BAL cells and increased recruitment of DCs into the lung were found in mev/+ mice after exposure to H2O2 or OVA antigen. Substantial airway inflammation was also induced in these mice by intranasal OVA challenges without adjuvant, a regimen unable to elicit any immune response in WT mice under normal conditions (29, 30). Furthermore, treatment with an antioxidant effectively suppressed the airway inflammation, suggesting a pivotal role of oxidative stress in the development of inflammation in mev/+ mice.
Airway DCs are essential for the development and maintenance of allergic asthma (31–35). The airway DC system has evolved into a fine-tuned, highly sensitive sentinel network capable of both igniting and shutting down immune responses as appropriate (32, 36). Even in the absence of overt inflammation, DCs are constantly recruited from the blood into the lung to maintain the steady-state deployment of the airway DC network (32). Not surprisingly, various stimuli in the airway have a profound impact on this steady-state dynamics (36–38). Rapid recruitment of DCs into the airway indicates that it is a critical part of the early phase of innate immune response, with the potential to progress toward an adaptive immune response (39). Selective depletion of DCs during allergen challenge abolished all cardinal features of asthma (40). In our study, increased recruitment of DCs into the airway occurred only in the mev/+ mice exposed to oxidative stress. Considering that significantly increased intracellular ROS was also observed only in the H2O2-treated BAL cells from mev/+ mice, enhanced oxidative stress imposed on those mice may be a principal mechanism to recruit airway DCs. Our findings also strongly support the idea that recruited DCs at the site of antigen exposure would play a crucial role in the development of immunity instead of immune tolerance in SHP-1–deficient mice.
At the cellular and molecular level, airway DCs were shown to be attracted by a whole array of stimuli including chemokines, complement cleavage products, and bacterial peptides (41). Lung epithelial cells are a front line defense and have been known to actively contribute to the pathogenesis of asthma (42). In this study, we hypothesized that lung epithelial cells would be a major contributor to the recruitment of DCs into the airway through enhanced production of chemokines capable of attracting DCs. It is reasonable to speculate that increased oxidative stress could make SHP-1–deficient epithelial cells produce more chemokines that in turn attract DCs into the airways.
Indeed, after exposure to oxidative stress, increased production of CCL20 and RANTES by lung epithelial cells transfected with a dominant-negative mutant SHP-1 was observed in parallel with enhanced recruitment of DCs in mev/+ mice in vivo. CCL20, a pivotal chemoattractant for CCR6-positive immature DCs, has been reported to be secreted by lung epithelial cells upon stimulation with ultrafine particulate matter (43). However, the mechanism by which the SHP-1–deficient state recruited more DCs into the airway, and induced the immunity instead of immune tolerance in association with enhanced intracellular ROS, has not yet been precisely defined.
In the meantime, airway inflammation observed in the mev/+ mice is characterized by airway eosinophilia, increased mucin-producing cells, and increased production of OVA-specific IgE, which are features of Th2 immune responses. Although the function of SHP-1 in the development of a specific type of immune response is still largely unknown, it is recently reported that exaggerated allergic inflammation was induced in SHP-1–deficient motheaten mice (16). In addition, SHP-1 deficiency is known to cause enhanced IL-4–dependent STAT6 activation through suppression of dephosphorylation of phosphorylated STAT6 (44–46). Thus SHP-1 appears to act as a negative regulator of Th2 signaling pathway. Consistent with these observations, our results also showed that when exposed to ROS, enhanced STAT6 phosphorylation was observed in the cells from mev/+ mice. Interestingly, decreased activation of NF-κB in the ROS-exposed SHP-1–deficient cells was also observed. This might be caused by enhanced activation of STAT6, which is known to have a strong inhibitory effect on JNK and NF-κB pathways (44). Further studies, therefore, are needed to clarify the exact mechanism underlying intracellular signaling of SHP-1. Taken together, these results indicate that break of immune tolerance followed by Th2-dominant airway inflammation can occur when the function of SHP-1 is reduced.
The prevalence of allergic diseases including asthma has increased dramatically over the last half decade (47). Various environmental factors eliciting increased oxidative stress such as air pollution and decreased intake of antioxidant-containing food have been suggested as contributing factors (48, 49). However, it is still not understood by which mechanism the immune disorders could develop through an increased oxidative stress burden that has substantial effect on the host's immune system but without eliciting any clinically apparent host response.
Interestingly, our study revealed that neither phenotypical nor immunological abnormalities could be found in the heterozygous mev mice in resting state until mucosa exposure to allergens, which is usually tolerated. Therefore, a defect in certain regulatory molecules could result in altered host microenvironment when exposed to even subtle increase of oxidative stress, such as suboptimal amount of antigens or inert antigens, leading to clinically apparent immune disorders. The effects of oxidative stress on immune response are complex. SHP-1 is probably one of many molecules that participate in the regulation of allergic inflammation through changes in intracellular redox state.
In conclusion, our findings suggest that SHP-1 plays an important role in regulating oxidative stress, and that SHP-1 deficiency plus increased intracellular oxidative stress in the presence of Th2-prone activation may lead to the development of allergic airway inflammation. In addition, this study expands our understanding of molecular mechanisms required to maintain the critical oxidative stress levels in the lung under various conditions and establishes a novel link between oxidative stress, immune response, and lung inflammation.
This work was supported by National Institutes of Health grants RO1HL074095 and RO1HL079349 (to Z.Z.), and Korean Research Foundation grant M01–2005–000–10143–0 (to Y.S.C.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0229OC on April 25, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet 1999;354:482–483. [DOI] [PubMed] [Google Scholar]
- 2.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol 2003;111:72–78. [DOI] [PubMed] [Google Scholar]
- 3.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996;154:1055–1060. [DOI] [PubMed] [Google Scholar]
- 4.Ercan H, Birben E, Dizdar EA, Keskin O, Karaaslan C, Soyer OU, Dut R, Sackesen C, Besler T, Kalayci O. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol 2006;118:1097–1104. [DOI] [PubMed] [Google Scholar]
- 5.Rahman I, Yang SR, Biswas SK. Current concepts of redox signaling in the lungs. Antioxid Redox Signal 2006;8:681–689. [DOI] [PubMed] [Google Scholar]
- 6.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 2000;16:534–554. [DOI] [PubMed] [Google Scholar]
- 7.Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, Oeda J, Masayasu H, Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol 2003;171:3010–3018. [DOI] [PubMed] [Google Scholar]
- 8.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol 2002;3:1129–1134. [DOI] [PubMed] [Google Scholar]
- 9.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest 2005;115:2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell 2004;117:699–711. [DOI] [PubMed] [Google Scholar]
- 11.Matthews RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol 1992;12:2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol 2000;12:361–378. [DOI] [PubMed] [Google Scholar]
- 13.Tsui FW, Martin A, Wang J, Tsui HW. Investigations into the regulation and function of the SH2 domain-containing protein-tyrosine phosphatase, SHP-1. Immunol Res 2006;35:127–136. [DOI] [PubMed] [Google Scholar]
- 14.Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse: I. Genetics and pathology. J Hered 1975;66:250–258. [DOI] [PubMed] [Google Scholar]
- 15.Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL. “Viable motheaten,” a new allele at the motheaten locus: I. Pathology. Am J Pathol 1984;116:179–192. [PMC free article] [PubMed] [Google Scholar]
- 16.Kamata T, Yamashita M, Kimura M, Murata K, Inami M, Shimizu C, Sugaya K, Wang CR, Taniguchi M, Nakayama T. src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin Invest 2003;111:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol 2002;110:349–356. [DOI] [PubMed] [Google Scholar]
- 18.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 2002;9:387–399. [DOI] [PubMed] [Google Scholar]
- 19.Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal 2006;8:88–98. [DOI] [PubMed] [Google Scholar]
- 20.Krotz F, Engelbrecht B, Buerkle MA, Bassermann F, Bridell H, Gloe T, Duyster J, Pohl U, Sohn HY. The tyrosine phosphatase, SHP-1, is a negative regulator of endothelial superoxide formation. J Am Coll Cardiol 2005;45:1700–1706. [DOI] [PubMed] [Google Scholar]
- 21.Hardin AO, Meals EA, Yi T, Knapp KM, English BK. SHP-1 inhibits LPS-mediated TNF and iNOS production in murine macrophages. Biochem Biophys Res Commun 2006;342:547–555. [DOI] [PubMed] [Google Scholar]
- 22.Heneberg P, Draber P. Regulation of cys-based protein tyrosine phosphatases via reactive oxygen and nitrogen species in mast cells and basophils. Curr Med Chem 2005;12:1859–1871. [DOI] [PubMed] [Google Scholar]
- 23.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 1993;73:1445–1454. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem 2003;278:25179–25190. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno K, Tagawa Y, Mitomo K, Arimura Y, Hatano N, Katagiri T, Ogimoto M, Yakura H. Src homology region 2 (SH2) domain-containing phosphatase-1 dephosphorylates B cell linker protein/SH2 domain leukocyte protein of 65 kDa and selectively regulates c-Jun NH2-terminal kinase activation in B cells. J Immunol 2000;165:1344–1351. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski M, Mlinaric-Rascan I, Feng GS, Shen R, Pawson T, Siminovitch KA. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med 1993;178:2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K, Esselman WJ. Inhibition of PTPs by H(2)O(2) regulates the activation of distinct MAPK pathways. Free Radic Biol Med 2002;33:1121–1132. [DOI] [PubMed] [Google Scholar]
- 28.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Ait-Yahia S, Vicari A, Kaiserlian D, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 2006;24:191–201. [DOI] [PubMed] [Google Scholar]
- 29.Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J Immunol 2002;169:3499–3506. [DOI] [PubMed] [Google Scholar]
- 30.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004;200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Heer HJ, Hammad H, Kool M, Lambrecht BN. Dendritic cell subsets and immune regulation in the lung. Semin Immunol 2005;17:295–303. [DOI] [PubMed] [Google Scholar]
- 32.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 2005;172:530–551. [DOI] [PubMed] [Google Scholar]
- 33.Koya T, Kodama T, Takeda K, Miyahara N, Yang ES, Taube C, Joetham A, Park JW, Dakhama A, Gelfand EW. Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am J Respir Crit Care Med 2006;173:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest 2006;116:783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huh JC, Strickland DH, Jahnsen FL, Turner DJ, Thomas JA, Napoli S, Tobagus I, Stumbles PA, Sly PD, Holt PG. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J Exp Med 2003;198:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stumbles PA, Strickland DH, Pimm CL, Proksch SF, Marsh AM, McWilliam AS, Bosco A, Tobagus I, Thomas JA, Napoli S, et al. Regulation of dendritic cell recruitment into resting and inflamed airway epithelium: use of alternative chemokine receptors as a function of inducing stimulus. J Immunol 2001;167:228–234. [DOI] [PubMed] [Google Scholar]
- 37.Pichavant M, Charbonnier AS, Taront S, Brichet A, Wallaert B, Pestel J, Tonnel AB, Gosset P. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J Allergy Clin Immunol 2005;115:771–778. [DOI] [PubMed] [Google Scholar]
- 38.Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev 2005;16:581–592. [DOI] [PubMed] [Google Scholar]
- 39.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med 1994;179:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 2005;201:981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, Stumbles PA, Wells TN, Holt PG. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med 1996;184:2429–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies DE, Holgate ST. Asthma: the importance of epithelial mesenchymal communication in pathogenesis: inflammation and the airway epithelium in asthma. Int J Biochem Cell Biol 2002;34:1520–1526. [DOI] [PubMed] [Google Scholar]
- 43.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol 2003;28:648–654. [DOI] [PubMed] [Google Scholar]
- 44.Hirayama T, Dai S, Abbas S, Yamanaka Y, Abu-Amer Y. Inhibition of inflammatory bone erosion by constitutively active STAT-6 through blockade of JNK and NF-kappaB activation. Arthritis Rheum 2005;52:2719–2729. [DOI] [PubMed] [Google Scholar]
- 45.Abu-Amer Y. IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-kappaB. J Clin Invest 2001;107:1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Z, Coleman JM, Su Y, Mann M, Ryan J, Shultz LD, Huang H. SHP-1 regulates STAT6 phosphorylation and IL-4-mediated function in a cell type-specific manner. Cytokine 2005;29:118–124. [DOI] [PubMed] [Google Scholar]
- 47.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–920. [DOI] [PubMed] [Google Scholar]
- 48.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Tellez-Rojo MM, Moreno-Macias H, Reyes-Ruiz NI, del Rio-Navarro BE, Ruiz-Navarro MX, Hatch G, Slade R, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med 2002;166:703–709. [DOI] [PubMed] [Google Scholar]
- 49.Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch Environ Health 2001;56:242–249. [DOI] [PubMed] [Google Scholar]