Abstract
Vascular endothelial growth factor (VEGF) is known to have a pivotal role in lung development and in a variety of pathologic conditions in the adult lung. Our earlier studies have shown that NO is a critical mediator of VEGF-induced vascular and extravascular effects in the adult murine lung. As significant differences have been reported in the cytokine responses in the adult versus the neonatal lung, we hypothesized that there may be significant differences in VEGF-induced alterations in the developing as opposed to the mature lung. Furthermore, nitric oxide (NO) mediation of these VEGF-induced effects may be developmentally regulated. Using a novel externally regulatable lung-targeted transgenic murine model, we found that VEGF-induced pulmonary hemorrhage was mediated by NO-dependent mechanisms in adults and newborns. VEGF enhanced surfactant production in adults as well as increased surfactant and lung development in newborns, via an NO-independent mechanism. While the enhanced survival in hyperoxia in the adult was partly NO-dependent, there was enhanced hyperoxia-induced lung injury in the newborn. In addition, human amniotic fluid VEGF levels correlated positively with surfactant phospholipids. Tracheal aspirate VEGF levels had an initial spike, followed by a decline, and then a subsequent rise, in human neonates with an outcome of bronchopulmonary dysplasia or death. Our data show that VEGF can have injurious as well as potentially beneficial developmental effects, of which some are NO dependent, others NO independent. This opens up the possibility of selective manipulation of any VEGF-based intervention using NO inhibitors for maximal potential clinical benefit.
Keywords: vascular endothelial growth factor, nitric oxide, lung, surfactant
CLINICAL RELEVANCE
This article provides improved understanding of the regulation of vascular endothelial growth factor–induced lung maturation and injury in the murine lung with supportive human data.
Vascular endothelial growth factor (VEGF) is an important cytokine that regulates angiogenesis in many physiologic and pathologic conditions. For the former, VEGF plays a critical role during embryogenesis, skeletal development, and reproduction (1–3). Pathologic states that involve VEGF-induced neovascularization include cancer, cardiovascular disease, obesity, retinopathies, and neurological disorders (1, 4–7). In addition to these vascular effects, recent studies have demonstrated that VEGF has other prominent effects on tissues (8–11).
Nitric oxide (NO) is a diffusible gas that is produced from L-arginine in a large number of tissues by the NO synthase (NOS) family of enzymes. There are three isoforms of NOS: nNOS (type I), iNOS (type II), and eNOS (type III). There is mounting evidence demonstrating an interaction between NO and VEGF. These include studies showing that VEGF stimulates the proliferation of endothelial cells via an NO-dependent mechanism (12), NO is a downstream imperative of VEGF-induced angiogeneis (13), and VEGF is produced by lung epithelium and up-regulates eNOS (14). In accord with the above, we have recently reported that NO mediates VEGF-induced pulmonary angiogenesis, edema, mucus metaplasia, airway hyperresponsiveness, T- and dendritic cell numbers, but not dendritic cell activation in the adult murine lung (15).
VEGF plays a pivotal role in lung development and has been implicated in a variety of disorders in the adult lung (8). This is nicely illustrated in the adult lung, where studies from our laboratory demonstrated that VEGF induces an asthma-like phenotype (9) as well as impressive cytoprotective effects in oxidant-induced lung injury (10, 11). Significant differences have been noted in the cytokine responses of the developing versus the adult lung, for example, in ventilator-induced injury (16) or on exposure to hyperoxia (17, 18). This led us to hypothesize that there may be significant differences in VEGF-induced alterations in the developing as opposed to the mature lung. Furthermore, NO mediation of these VEGF-induced effects may be developmentally regulated.
In the present article, we show that VEGF induced NO-dependent pulmonary hemosiderosis but NO-independent surfactant production in both the newborn (NB) and adult lung, as well as alveolar development in the NB lung. We also noted positive correlations with human amniotic fluid (AF) VEGF levels and surfactant phospholipids. While the enhanced survival in hyperoxia in the adult was partially NO dependent, there was enhanced hyperoxia-induced acute lung injury (HALI) in the NB. Furthermore, tracheal aspirate (TA) levels of VEGF were significantly increased early on (first 12 h of life) in premature neonates with respiratory distress syndrome (RDS) who had an adverse outcome (bronchopulmonary dysplasia [BPD]/death). In addition, the TA VEGF levels followed a pattern in which there was an initial spike, followed by a decline, and then a subsequent rise, by Days 21 to 28, in those neonates with an adverse outcome. Our data show, for the first time, that there are significant developmental differences in the NO-mediated effects of VEGF, some of which are NO dependent, others NO independent. In addition, we also show the human relevance of the NB murine lung data.
MATERIALS AND METHODS
Animals
Transgenic (TG) mice (VEGF165) were generated and used in these studies, as described previously (9). They were generated using CBA×C57BL/6 zygotes and bred onto a C57BL/6 genetic background. Unless otherwise indicated, wild-type (WT) littermates were used as negative controls. Four- to six-week-old VEGF TG and WT littermate controls were randomized to receive normal water or water containing doxycycline (dox) (0.5 mg/ml) and evaluated at intervals thereafter. l-NAME was given as daily intraperitoneal injections (10 mg/kg) or put in the drinking water (0.5 mg/ml) for 2 weeks. For the newborns, VEGF induction was accomplished by transplacental (in utero) or transmammary (ex utero) administration of dox. NOS-inhibition was accomplished by transmammary administration of l-NAME for 7 days.
All animal work was approved by the Institutional Animal Care and Use Committee at the Yale University School of Medicine.
Bronchoalveolar Lavage
Mice were killed, the trachea was isolated by blunt dissection, and a small-caliber tube was inserted into the airway and secured. Two volumes of 1 ml of PBS with 0.1% bovine serum albumin were instilled and gently aspirated and pooled (bronchoalveolar lavage [BAL] fluid). Samples were then centrifuged at 1,250 × g for 5 minutes to recover cells, and the supernatants were collected and stored at −70°C for further analysis. Cell pellets were resuspended in PBS and total cell counts determined using a hemocytometer. Aliquots were cytospun onto microscope slides and stained for cellular differentials. Human VEGF levels were measured as per manufacturer's instructions using the ELISA kit from R&D Systems (Minneapolis, MN).
Analysis of mRNA
Mice were anesthetized, and the lungs were rapidly removed and frozen on liquid nitrogen. RNA was isolated from frozen lungs using TRIzol Reagent (Life Technologies Inc., Grand Island, NY) according to the manufacturer's instructions. RNA samples were then DNase treated and subjected to semiquantitative RT-PCR. Primers used: surfactant protein (SP)-A, 5′-TCTTGACTGTTGTTGCTGGC-3′, 5′-AGAAGCCCCATCCAGGTAGT-3′; SP-B, 5′-GACCTGTGCCAAGAGTGTGA-3′, 5′-GGCATAGCCTGTTCACTGGT-3′; SP-C, 5′-GCAAAGAGGTCCTGATGGAG-3′, 5′-GCCCGTAGGAGAGACACCTT-3′; SP-D, 5′-CTCTCGCAGAGATCAGTACC-3′, 5′-GGAAAGCAGCCTTGTTGTGG-3′; A1, 5′-CAGGGAAGATGGCTGAGTCT-3′, 5′-TTCTGCCGTATCCATTCTCC-3′; iNOS, 5′-GGTATGCTGTGTTTGGCCTT-3′, 5′-GGCTGGACTTTTCACTCTGC-3′; eNOS, 5′-GCAAGACCTCCTGAGGACAG-3′, 5′-TGCAAAGAAAAGCTCTGGGT-3′; nNOS, 5-CCTTAGAGAGTAAGGAAGGGGGCGGG-3′, 5′-GGGCCGATCATTGACGGCGAGAATGATG-3′; β-actin, 5′-GTGGGCCGCTCTAGGCACCA-3′, 5′-TGGCCTTAGGGTTCAGGGGG-3′.
Data were confirmed by real-time RT-PCR, as described previously (19). Primers used for real-time RT-PCR are as follows: iNOS, 5′-GGTATGCTGTGTTTGGCCTT-3′, 5′-GGCTGGACTTTTCACTCTGC-3′; eNOS, 5′-GCAAGACCTCCTGAGGACAG-3′, 5′-TGCAAAGAAAAGCTCTGGGT-3′; nNOS, 5′-AGTCTCCCAGGCTAATGGTGT-3′, 5′-AGGTCTCTGTCCACCTGGATT-3′; SP-B, 5′-CTACTTCCAGAGCCAGATTAAC-3′, 5′-TGTCCAGCAGAGGGTTTG-3′; SP-C, 5′-ACTGAGATGGTCCTTGAGATG-3′, 5′-CGCTGGTAGTCATACACAAC-3′.
Histology
Tissues were fixed overnight in 10% buffered formalin. After washing in fresh PBS, fixed tissues were dehydrated, cleared, and embedded in paraffin by routine methods. Sections (5 μm) were collected on Superfrost Plus positively charged microscope slides (Fisher Scientific Co., Houston, TX), deparaffinized, and stained with hemotoxylin and eosin.
Wet/Dry Lung Weight Ratio
Lactating dams were kept on regular or dox water for 1 week, which allowed for the transmammary activation of VEGF (in the latter instance) in the NB VEGF TG mice. l-NAME was given as daily intraperitoneal injections (10 mg/kg) to the lactating dam for 7 days, which resulted in NOS inhibition by the transmammary route. The NB mice were killed, their lungs removed, and placed in pre-weighed petri dishes and the “wet” weight ascertained. After being placed in an oven kept at a constant temperature of 55°C for 72 hours, the petri dishes were removed and the “dry” weight of the lungs measured. The wet/dry lung weight ratio was calculated.
Oxygen Exposure
Four- to six-week-old mice were placed in cages in an airtight Plexiglas chamber (55 × 40 × 50 cm3). Throughout the experiment, they were given free access to food and water. Oxygen levels were constantly monitored by an oxygen sensor that was connected to a relay switch incorporated into the oxygen supply circuit. The inside of the chamber was kept at atmospheric pressure, and mice were exposed to a 12-hour light-dark cycle. For the newborn experiments, two lactating dams were used. They were alternated in hyperoxia and room air every 24 hours. The litter size was kept limited to 12 pups to control for the effects of litter size on nutrition and growth.
Western Blotting
We detected SP-B (45 kD) and C (21 kD) protein from lung lysates using Western analysis undertaken with antibodies that reacted selectively with SP-B and SP-C (Abcam Inc., Cambridge, MA) and with β-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA) as control, as previously described (20, 21).
Immunohistochemistry
Staining was performed on a DAKO autostainer (DAKO Corp., Carpinteria, CA). Paraffin-embedded tissues were cut, exposed to three changes of xylene, rehydrated in a series of graded alcohols, rinsed, blocked with avidin and biotin (Biotin Blocking Kit; DAKO Corp.), and endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide for 5 minutes. The slides were incubated for 1 hour at room temperature with either a 1:200 dilution of a murine IgG2 mAb that recognized SP-B/SP-C or a 1:200 dilution of a nonspecific anti-mouse IgG2 control antibody (R&D Systems). To prevent nonspecific binding to mouse tissue, the antibodies were previously biotinylated and blocked with nonspecific mouse serum using a commercially available kit (Animal Research Kit; DAKO Corp.). After incubation with antibody, the slides were incubated with a streptavidin–peroxidase enzyme conjugate (DAKO Corp.) for 15 minutes followed by 3, 3′-diaminobenzidine-tetrahydrochloride (DAB; DAKO Corp.) for 7 minutes. The slides were counterstained with hematoxylin, dehydrated in a series of graded alcohols, and cleared with xylene.
Lung Morphometry
Alveolar size was estimated from the mean cord length (Lm) of the airspace by two different methods. This measurement is a standard measure of airspace size, but has the advantage that it is independent of alveolar septal thickness. The first one was done as described previously by our laboratory (22). In addition, Lm was also determined by a second technique of counting intersections with an array of lines, and recording the numbers of alveoli in rectangles of defined areas, as described (23). Six random ×100 fields were evaluated per section, with two independent investigators masked to the study group, each using a separate technique.
Human AF
Measurement of lecithin/sphingomyelin (L/S) ratios and the presence/absence of phosphatidylglycerol (PG) were done using the Helena Fetal-Tek 200 kit (Helena Laboratories, Beaumont, TX), as per the manufacturer's instructions.
Human TA
The TA samples were collected from neonates admitted to the Yale-New Haven Children's Hospital NewBorn Special Care Unit. TA samples were only collected if the infant had a clinically indicated endotracheal tube in place. There were no statistically significant differences between the no BPD (n = 7) versus BPD/death (n = 9; 4 deaths) groups in gestational age (mean ± SEM, 25.6 ± 0.7 versus 26.1 ± 0.5 wk) or birth weight (781 ± 44 versus 783 ± 48 g). All infants had RDS and were intubated, administered at least one dose of natural surfactant, and ventilated for treatment, as per standard nursery guidelines. TA samples were collected in the first 12 hours of life and subsequently on Days 3 to 5, 7 to 14, and 21 to 28. BPD was defined as the need for supplemental oxygen at 36 weeks after menstrual age, along with characteristic radiographic features (24).
Human VEGF levels were measured as per manufacturer's instructions using the ELISA kit from R&D Systems.
All human work was approved by the Human Investigational Committee at the Yale University School of Medicine.
Statistical Analyses
Values are expressed as means ± SEM. As appropriate, groups were compared with the Student's two-tailed unpaired t test, Mann-Whitney test, or the logrank test, using GraphPad Prism 3.0 (GraphPad Software, Inc., San Diego, CA). A P ≤ 0.05 was considered statistically significant.
RESULTS
Effect of VEGF on NOS Isoforms in the NB Lung
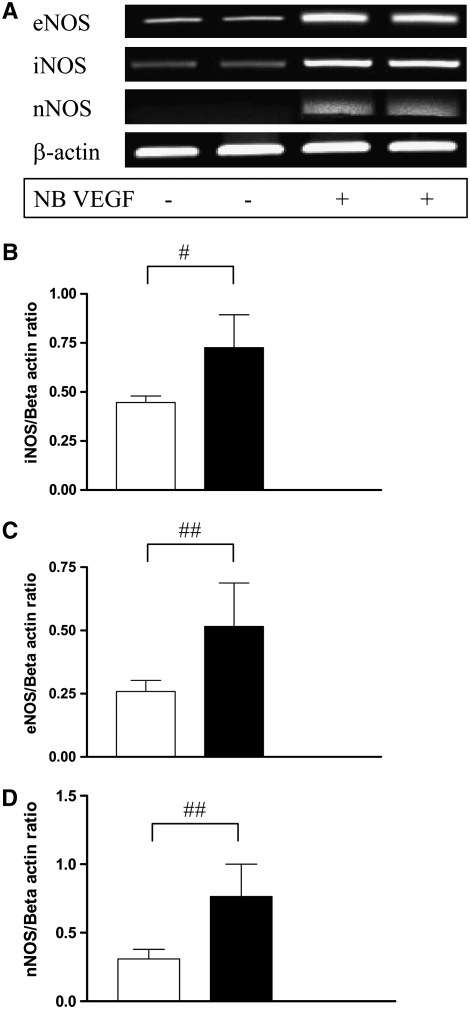
In our earlier report, we have noted that VEGF induces iNOS and eNOS in the adult murine lung, but not nNOS (15). To address the roles of NO and NOS in the pathogenesis of VEGF-induced pulmonary alterations, we evaluated the levels of mRNA encoding the three NOS isoforms in WT and VEGF TG NB mice. In contrast to the observation in the adult, we note that by postnatal (PN) Day 2 of VEGF induction, there was an impressive increase in the mRNA of all three NOS isoforms in the developing murine lung (Figure 1A). These data were confirmed by real-time RT-PCR (Figures 1B–1D). The levels of mRNA encoding all three NOS isoforms in lungs from WT or TG mice on normal water were near or below the limits of detection our assay (data not shown). Thus, VEGF is a potent and selective stimulator of iNOS, eNOS, and nNOS in the developing murine lung.
Figure 1.
Effect of vascular endothelial growth factor (VEGF) on nitric oxide (NO) synthase (NOS) isoforms in the newborn (NB) lung. Expression of mRNA for NOS enzymes (iNOS, eNOS, nNOS) in NB VEGF transgenic (TG) mice lungs (A). These were confirmed with real-time RT-PCR (B–D). Mice were on dox water for 2 days. The figure is representative of assessments in a minimum of four mice. Open bars, NB WT; solid bars, NB VEGF. #P = 0.02; ##P < 0.05.
Role of NO in VEGF-Induced Pulmonary Hemosiderosis in the Adult and NB Lungs
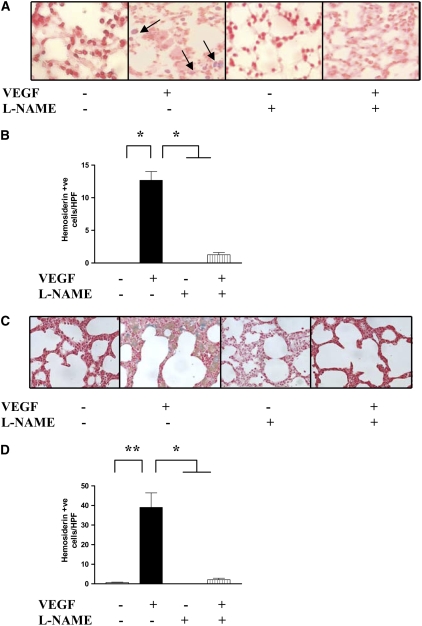
We compared the vascular effects in WT and VEGF TG mice treated with the nonspecific NOS inhibitor, l-NAME. Pulmonary hemorrhage was not readily appreciated in lungs from WT mice on normal or dox water and lungs from TG mice on normal water (Figure 2 and data not shown). In contrast, activation of the VEGF transgene caused a significant increase in pleural and parenchymal hemorrhage and the accumulation of hemosiderin-laden macrophages (Figure 2 and data not shown) in both the NB and adult lungs. In all cases, NO played a significant role in these responses because VEGF-induced hemorrhage was decreased with l-NAME treatment (Figure 2). These studies demonstrate that VEGF induces a hemorrhagic response in the murine adult and NB lungs via a mechanism that is, at least in part, NO dependent.
Figure 2.
Role of NO in VEGF-induced pulmonary hemosiderosis in adult and NB lungs. NO-inhibition (with l-NAME) led to decreased pulmonary hemosiderosis (arrows point to hemosiderin-laden macrophages stained blue) in the adult (A and B) and NB (PN7) (C and D) lungs. The noted values represent assessments in a minimum of four mice. Mice were on dox water and/or l-NAME as described in Materials and Methods. *P ≤ 0.0001, **P ≤ 0.01.
Role of NO in VEGF-Induced Pulmonary Endothelial Permeability in the NB Lung
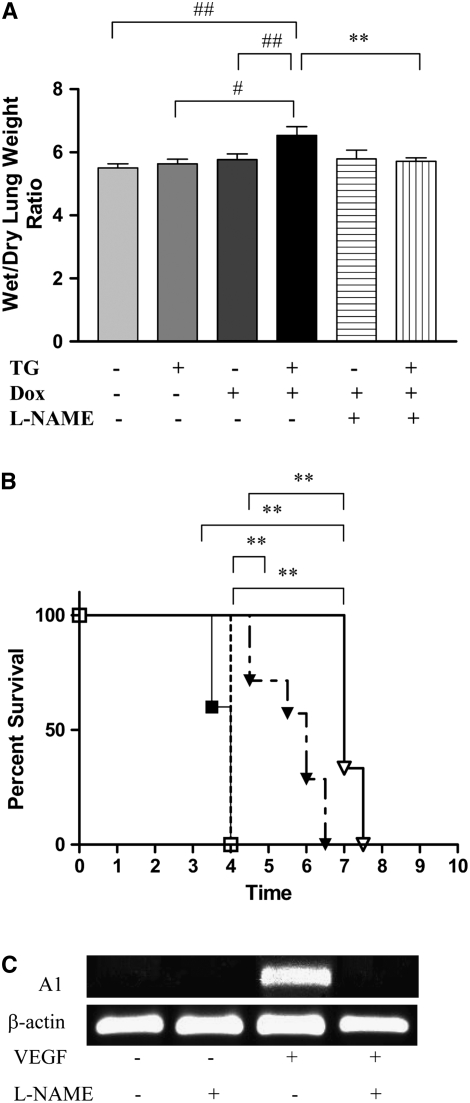
Pulmonary endothelial permeability can be assessed by wet/dry lung weight ratios. As noted (Figure 3A), transgenic VEGF caused a significant increase in NB lung wet/dry ratios. NO played a significant role in this response because VEGF-induced increased NB lung wet/dry ratios were decreased with l-NAME treatment (Figure 3A). Thus, VEGF increases vascular permeability in the newborn murine lung via a mechanism(s) that is, at least partially, NOS dependent.
Figure 3.
Role of NO in VEGF-induced pulmonary endothelial permeability in NB mice and survival in hyperoxia in adult mice. (A) NO inhibition (with l-NAME) abrogated the increased VEGF-induced lung wet/dry weight ratios in NB VEGF TG mice. (B) NO inhibition (with l-NAME) abrogated the increased VEGF-induced survival in hyperoxia in adult VEGF TG mice. NO-inhibition (with l-NAME) was associated with abrogation of VEGF-induced A1 mRNA expression (C). NB mice were exposed to dox water and/or l-NAME for 1 week via the transmammary route. Adult mice were on dox water and/or l-NAME for 2 weeks. The data are representative of experiments conducted in a minimum of four mice. Open squares, WT + DOX; solid squares, WT + DOX + l-NAME; open triangles, VEGF + DOX; solid triangles, VEGF + DOX + l-NAME. #P < 0.03, ##P < 0.05, **P ≤ 0.01.
Role of NO in VEGF-Induced Survival in Hyperoxia in the Adult
Previous studies from our laboratory have demonstrated that VEGF TG mice have increased survival in hyperoxia (10, 11). To define the importance of NO in the pathogenesis of this response, we compared the survival of mice treated with l-NAME or its vehicle control in 100% O2. Under these conditions, WT mice on dox and normal water and TG mice on normal water died after 3 to 4 days of 100% O2 exposure (Figure 3B and data not shown). In contrast, TG mice on dox lived for an extended interval, dying after 6.5 to 7.5 days of 100% O2 exposure. This enhanced survival was at least partially NOS mediated because it was significantly decreased after l-NAME treatment (Figure 3B). Comparable alterations were not induced by the l-NAME vehicle control (Figure 3B). Thus, VEGF enhances survival in the setting of oxidant-induced lung injury via a mechanism that is, at least in part, NO mediated. Since VEGF-induced protection in HALI was, in part, mediated by a member of the Bcl-2 family, specifically A1 (11), we studied the effect of NOS inhibition on A1, in the presence of VEGF. As shown in Figure 3C, NOS inhibition resulted in abrogation of VEGF-induced increase in A1. Taken together, this shows that NO-mediated VEGF-induced enhanced survival in hyperoxia is associated with changes in A1 expression.
Effect of VEGF on Survival in Hyperoxia and HALI in NB Mice
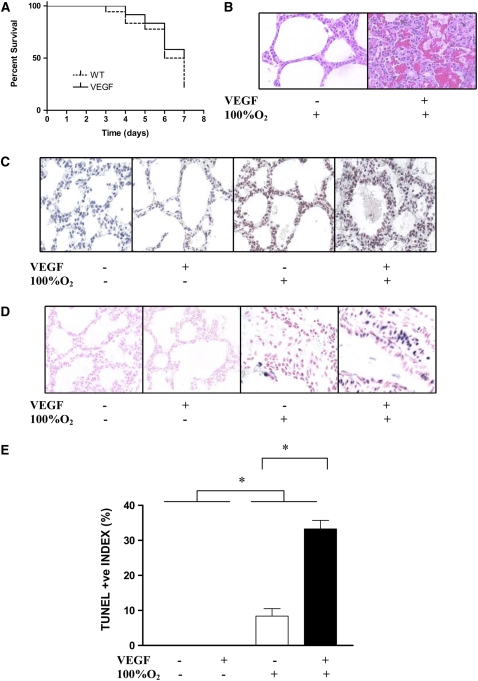
In contrast, there was no beneficial effect of turning on VEGF in the presence of hyperoxia in the NB (Figure 4A). Furthermore, there was evidence of increased HALI in the NB lung after 7 days, as noted on histopathologic examination (Figure 4B). This was accompanied by a significant increase in 8-OhDG (Figure 4C) and TUNEL-positive staining (Figures 4D and 4E). Thus, it is obvious that there is a significant developmental regulation of the response to hyperoxia, in the presence of VEGF induction, in the adult versus the NB lung.
Figure 4.
Effect of VEGF on survival in hyperoxia and hyperoxia-induced acute lung injury (HALI) in NB mice. In contrast to the adult, the NB had no benefit to survival in hyperoxia (A), but had evidence of increased HALI by histopathology (B), 8-OhDG (C), and TUNEL staining (D and E) compared with littermate controls (all at PN7). Mice were on dox water for 1 week. The data are representative of experiments conducted in a minimum of four mice. **P ≤ 0.01.
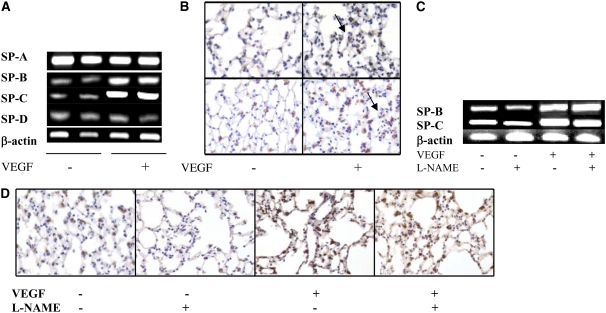
Role of NO in VEGF-Induced Surfactant Proteins in the Adult Lung
VEGF has recently been shown to stimulate lung development and maturation (25–27). To define the role of NO in the maturational response, we compared the levels of surfactant proteins in mice with normal NOS function and mice treated with l-NAME. The levels of SP-B and SP-C mRNA and protein were increased in lungs from TG mice on dox water when assessed via RT-PCR (Figure 5A) and immunohistochemistry (Figure 5B), respectively. These inductive events appeared to be NO independent, since neither was significantly altered by l-NAME (Figures 5C and 5D).
Figure 5.
Role of NO in VEGF-induced surfactant proteins in the adult lung. VEGF-induced increased expression of surfactant protein (SP)-B and SP-C mRNA (A) or proteins (B) (arrows point to positive staining in the Type II cells; upper panel: SP-B, lower panel: SP-C) were not altered with NO inhibition (with l-NAME) (mRNA for [C] SP-B and -C and [D] SP-B protein). Mice were on dox water and/or l-NAME for 2 weeks. The data are representative of experiments conducted in a minimum of four mice.
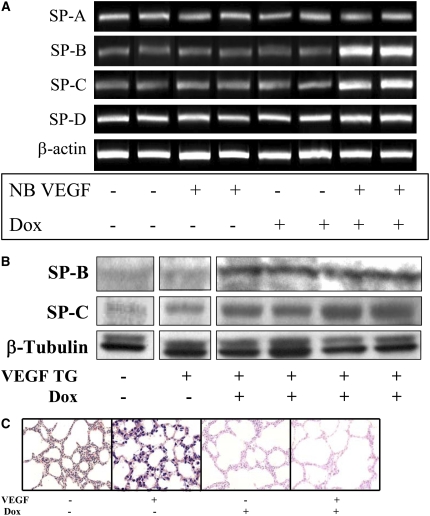
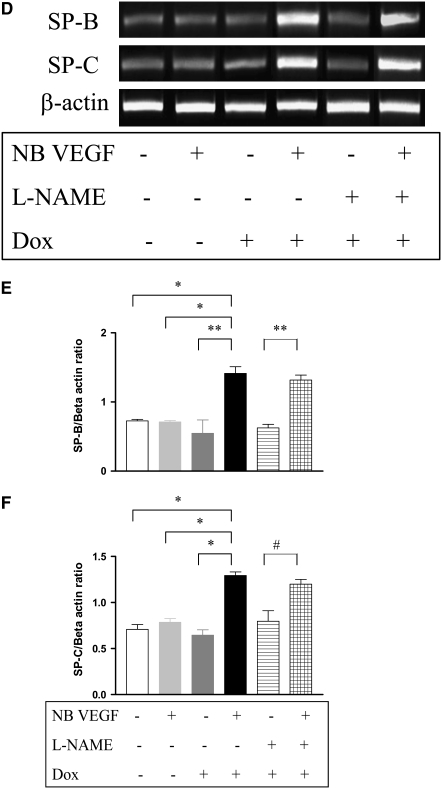
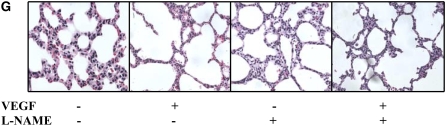
Role of NO in VEGF-Induced Maturation in the NB Lung
Similar to the VEGF-induced response in the adult lung, in the NB there was increased SP-B and -C mRNA and protein noted as early as after 2 days of VEGF-induction (data not shown). After 7 days, in addition to the increase in SP-B and C mRNA and protein (Figures 6A and 6B), there was evidence of lung maturation as noted by larger alveoli and thinning intervening mesenchyme (Figure 6C). This was confirmed by lung morphometry (Table 1). NOS inhibition by l-NAME did not lead to any alteration of the SP-B and -C (Figure 6D, confirmed with real time RT-PCR, as shown in Figures 6E and 6F), similar to that in the adult, or the maturational response (Figure 6G).
Figure 6.
Role of NO in VEGF-induced maturation in the NB lung (PN7). VEGF induced the increased expression of SP-B and SP-C (A) mRNA and (B) protein. (C) VEGF-induced lungs had thinner alveolar walls, increased alveolar space, and decreased intervening mesenchyme. The maturational effects on SP-B and SP-C mRNA expression (D) were not altered with l-NAME treatment in the presence of VEGF, and these were confirmed with real-time RT-PCR (E and F). Similarly, the effects on the lung parenchyma (G) with l-NAME treatment in the presence of VEGF were unchanged. Mice were on dox water and/or l-NAME for 1 week. The data are representative of experiments conducted in a minimum of four mice. *P < 0.0001; **P < 0.01, #P < 0.02.
TABLE 1.
LUNG MORPHOMETRY IN NEWBORN MICE
| Age | WT | VEGF TG | P Value | |
|---|---|---|---|---|
| Lm (μm)* | PN7† | 112.1 ± 13.6 | 143.1 ± 8.7 | <0.05 |
| PN14† | 71.86 ± 6.84 | 71.52 ± 5.2 | 0.97 | |
| Lm (μm)* | PN7† | 63.7 ± 2.95 | 104.0 ± 6.04 | <0.001 |
| PN14† | 50.6 ± 1.9 | 56.2 ± 3.2 | 0.14 |
Definition of abbreviations: PN, Postnatal Day; VEGF TG, vascular endothelial growth factor transgene positive; WT, wild-type littermate.
Independent evaluations using two different techniques, as described in Materials and Methods.
VEGF was turned on at birth (PN1). The noted values represent assessments in a minimum of four animals.
VEGF induction before Embryonic Day (E)18 (day of vaginal plug noted as E0) led to nonviability. However, after that period, VEGF induced lung maturation. When VEGF was turned on at E20, the cord lengths (Lm) were significantly increased in the VEGF TG lungs at PN7 (WT versus VEGF TG, mean ± SEM, 87.1 ± 5.4 versus 137.5 ± 5.7 μm, P < 0.001). Interestingly, if VEGF was turned on at birth (PN1) for 3 days and then off for 4 days, the increase in cord lengths was still obvious (67.03 ± 4.97 versus 101.0 ± 4.97 μm, P = 0.03). Importantly, VEGF induction from birth for up to 2 weeks did not result in increased mortality, compared with WT littermate controls (data not shown). Furthermore, there were no differences in lung histopathologic or morphometric evidences of maturation at that stage (latter data shown in Table 1). Our data strongly suggests that VEGF appears to have maturational effect within a specific developmental window (after E18 to PN7).
Effect of NOS Inhibition on VEGF Production
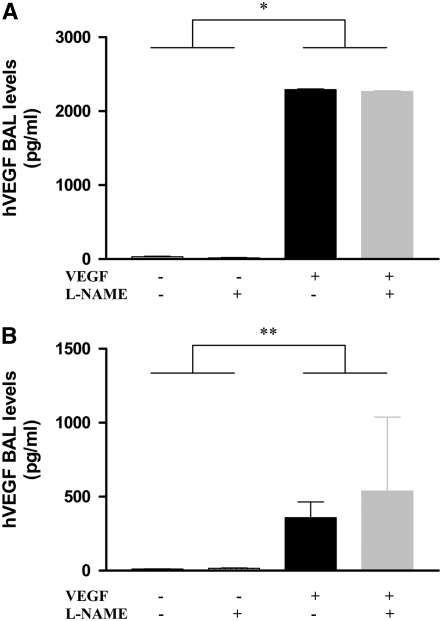
NOS inhibition could alter VEGF effects in the lung by altering the production of VEGF or altering its effector capacity. To differentiate among these options, we compared the levels of VEGF in BAL fluids from WT and TG mice treated with l-NAME or its vehicle control. As can be seen in Figure 7, similar levels of BAL VEGF were seen in TG mice treated with l-NAME or vehicle control in the adult (Figure 7A) as well as the NB (Figure 7B) animals. These studies demonstrate that interventions that inhibit NOS function alter the effects of VEGF in the lung by altering the VEGF effector pathway activation.
Figure 7.
Effect of NOS inhibition on VEGF production. l-NAME treatment of VEGFTG+ mice in the adult (A) or newborn (B) lung did not alter BAL hVEGF levels. Mice were on dox water and/or l-NAME as described in Materials and Methods. The data are representative of experiments conducted in a minimum of four mice. *P ≤ 0.0001; **P ≤ 0.01.
Role of VEGF in the Developing Human Lung and Response to HALI
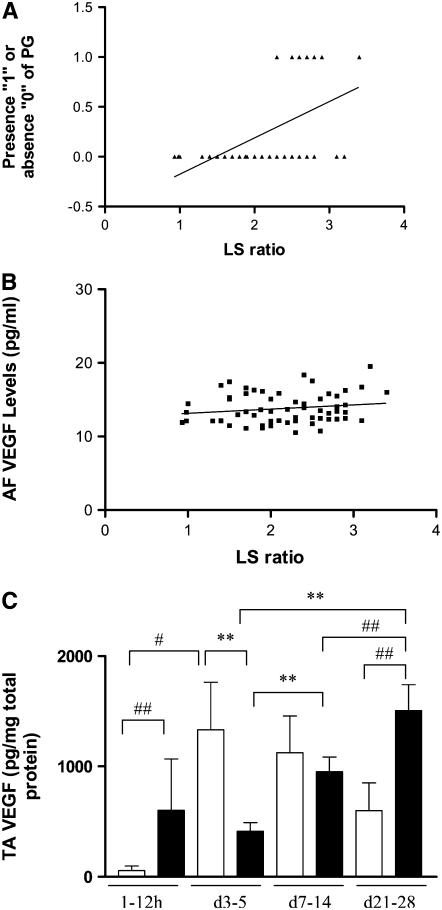
To evaluate if our data of the effect of VEGF in enhancing the maturation of the surfactant system in the developing murine lung had some relevance in the human context, we analyzed human AF that had been sent to our clinical laboratory for evaluation of pulmonary maturity. This was a convenience sample, as it was mostly sent from mothers who were in late gestation (≥ 34 wk), where the AF was sampled to assess lung maturity of the infant before the decision of delivering the infant. Presence of PG correlated strongly with L/S ratios (P < 0.0001). There was also a significant positive correlation of L/S ratios with AF VEGF levels (P < 0.03) (Figures 8A and 8B).
Figure 8.
VEGF levels in human amniotic fluid (AF) and tracheal aspirate (TA). AF samples from mothers with infants in late gestation (≥ 34 wk) with a lecithin/sphingomyelin (L/S) ratio of less than 3.5 (n = 58). Presence of PG (“0” absent, “1” present) correlated strongly with L/S ratios (R = 0.55, P < 0.0001) (A). There was a significant positive correlation of L/S ratios with AF VEGF levels (R = 0.26, P < 0.03) (B). TA VEGF levels (C) from premature babies with RDS with (n = 9; solid bars) and without (n = 7; open bars) an adverse outcome (BPD /Death). The noted values represent assessments in a minimum of four infants at each time point. **P ≤ 0.01; #P < 0.02; ##P ≤ 0.05.
Our studies also noted that VEGF is an important mediator of HALI that regulates oxidant-induced pulmonary cell death in the developing murine lung. To evaluate the human disease relevance of these findings, studies were undertaken to determine if lung VEGF levels had a role in neonates exposed to hyperoxia. VEGF was readily apparent in TA from premature babies being ventilated for RDS. A subset of neonatal patients with RDS develop lung injury and pulmonary edema, subsequently leading to BPD and even death. Interestingly, the levels of TA VEGF measured in the first 12 hours of life were significantly higher in babies that subsequently developed BPD or death (Figure 8C). Subsequent measurements of VEGF revealed a significant decrease at Days 3 to 5 (compared with patients with no BPD) followed by significantly increased levels by Days 21 to 28 (Figure 8C). These studies demonstrate that VEGF levels appear to follow a specific pattern in conditions characterized by exposure to high concentrations of oxygen and ALI in neonatal patients.
DISCUSSION
Our data show that there are significant developmental differences in the NO-mediated effects of VEGF, some of which are NO dependent, others NO independent.
We, and others, have reported that VEGF causes enhanced pulmonary angiogenesis, edema, and vascular permeability (9, 15, 28). Not surprisingly, there was evidence of pulmonary hemorrhage/hemosiderosis in both the NB and adult lungs. The evidence of pulmonary hemosiderosis confirms data reported in a transgenic model overexpressing VEGF164 in a different strain of mice (29). In addition, we noted that VEGF-induced pulmonary endothelial permeability (as assessed by lung wet/dry weight ratios) in the NB lung was also NOS dependent, similar to what we have reported earlier in the adult lung (15).
Supplemental oxygen, while increasing tissue oxygen delivery, can cause pulmonary injury when administered at high concentrations. Previous studies from our laboratory demonstrated that VEGF overexpression in the murine lung diminished the toxic consequences of exposure to 100% oxygen (11). They also demonstrated that this effect was due, at least in part, to the ability of the Bcl-2 family gene A1 to inhibit hyperoxia-induced cell death (11). Our present studies demonstrate that NO plays an important role in this cytoprotective response because, in the absence of NOS induction, the VEGF-induced survival advantage was markedly diminished. In addition, the ability of VEGF to stimulate the expression of A1 was also diminished in the absence of NO elaboration. When viewed in combination, these studies demonstrate that VEGF induces A1 in the adult murine lung via an NO-dependent pathway and that this pathway plays an important role in mediating the cytoprotective effects of this important cytokine.
In striking contrast to the results reported in the adult transgenic mice (11), we found that induction of VEGF in the NB did not effect their survival. Surprisingly, the NB lungs had increased HALI, as evidenced by histopathology, TUNEL, and 8-OhDG staining (Figure 4). This highlights the importance of evaluating the effects of cytokines in HALI models in a developmental-specific manner (18, 30). These results are in contradiction to some earlier reports suggesting a beneficial effect of VEGF in neonates with hyperoxia exposure (31, 32). In the report by Kunig and coworkers, the study was conducted in neonatal rats that were exposed to hyperoxia after PN2 for 12 days and then administered recombinant VEGF (31). In the study by Thebaud and colleagues, neonatal rats were exposed to hyperoxia for 14 days, with VEGF administration done at PN4 (32). Besides the obvious difference of using rat models, the timing and dose of VEGF, and the developmental stage of the lung can explain the variable effects of VEGF. Interestingly, Kunig and coworkers have recently reported that VEGF administration leads to lung injury in the form of pulmonary edema in their rodent model (33). Recently, we have reported increased IL-6 levels to have a detrimental effect in HALI in the neonatal lung, in contrast to what has been seen in the adult (34). Since VEGF is known to induce IL-6 (35, 36), this could be one potential mechanism to explain the differences in the NB and adult lungs to HALI.
Surfactant is a mixture of phospholipids and SP that lower the surface tension at the air–water interface and prevent alveolar collapse. In preterm neonates, RDS is caused by a deficiency of surfactant due to immaturity of alveolar type II epithelial cells (25). VEGF can stimulate lung development and maturation (25, 26, 37), at least in part, via its ability to stimulate phospholipid production and SP elaboration in vivo and in vitro (25, 27, 38). As a result, VEGF has been proposed as a therapeutic option that can be administered to enhance lung maturation in the pre- or postnatal time periods (25). However, studies using inducible overexpression TG systems have raised issues that question the safety of this approach. Specifically, in one study, the overexpression of VEGF in the lung in the later stages of gestation proved to be fatal, causing prominent vascular abnormalities, hemorrhage, and vascular obstruction of airway lumens (39). Similarly, neonatal VEGF also increased mortality with tissue inflammation and pulmonary hemorrhage (29). The same group of investigators found no effect on SP-B protein and marked alveolar remodeling at PN14 and in adult mice (29). In striking contrast, our studies demonstrate that VEGF is a potent stimulator of SP production in the adult and NB (at PN7) lungs, with no evidence of alveolar remodeling at PN14 or in adult mice (9, 15). This discrepancy could be explained by the inherent genetic differences in different strains of mice used, as has been shown in multiple other model systems (40–43), as well as the timing. Importantly, our studies also demonstrate that the effects on surfactant were not mediated by NO, as they were not altered by the NOS-based intervention that was employed.
Our data on human AF VEGF levels that correlate well with the surfactant phospholipids support our contention that VEGF does have a role to play in lung maturation in the human context. Our data are in accord with another report that VEGF levels may reflect pulmonary maturity as evidenced by the significant positive correlation (P = 0.03) of TA VEGF levels from premature babies with their AF L/S ratios (44). In a recent study, preterm infants with more severe RDS had lower VEGF detected by immunhistochemistry (45), while another study reported that cord blood VEGF elevation was significantly correlated with absence of RDS (46).
We found increased TA VEGF levels in the first 12 hours of life in babies with RDS who had an adverse outcome. Others (47) have not found such an association. This could be, however, due to the variable timing or measurement technique that was used or the different characteristics of the patient population studied.
Interestingly, we found that the pattern seen in the babies with no BPD shows an initial low VEGF level, followed by a substantial increase, with a subsequent decrease or plateau. In the babies with an adverse outcome (BPD/death), there was an initial spike, followed by a decline, and then a subsequent rise. As can be noted in Figure 8, Day 7 to 14 and Day 21 to 28 VEGF levels were significantly higher than Day 3 to 5 VEGF levels, and Day 21 to 28 VEGF levels were significantly higher than Day 7 to 14 VEGF levels, for the babies who had an adverse outcome (BPD/death). These patterns/relationships held true even when analyzed by one-way ANOVA analyses, with P < 0.04 for the “no BPD” group and P = 0.02 for the “BPD/died” group. This is in accord increased VEGF staining in the baboon model of BPD (48) as well as human infants with BPD (45, 49). The pattern of TA VEGF levels in babies with an adverse outcome that we have found has been shown to occur during acute myocardial infarction in human adults (50), ALI in adult animals (51, 52), and HALI in NB animals (53), but to our knowledge, our data provide evidence, for the first time, in the human NB lung. We speculate that the initial increase in VEGF levels could potentially contribute to lung injury/pulmonary edema leading to cellular damage/death, which in turn, results in decreased VEGF levels. Subsequently, there is a surge in VEGF levels associated with lung repair.
In summary, these studies demonstrate that, in contrast to the adult lung, VEGF is a potent stimulator of nNOS (in addition to iNOS and eNOS) in the murine NB lung. We report that VEGF-induced pulmonary hemosiderosis is NO dependent. In contrast, the VEGF-induced surfactant production in the adult and NB lung is NO independent. Similarly, the VEGF-induced lung maturational effect noted in the NB lung is NO independent. While the enhanced survival in hyperoxia in the adult was partly NO dependent, there was enhanced HALI in the NB. Our data show that there are significant developmental differences in the NO-mediated effects of VEGF, some of which are NO dependent, others NO independent.
Exaggerated VEGF production has been proposed to play an important role in the pathogenesis of a wide variety of diseases, including tumor neovascularization, asthma, pulmonary edema, atherosclerosis, and the retinopathies of the NB and the diabetic (5, 9, 51, 54–57). The present studies suggest that the pathologic effects of VEGF in these disorders may be controlled by interventions that control NO production. In contrast, a relative deficiency of VEGF has been proposed to contribute to the pathogenesis of diseases like RDS in the newborn (25, 46). Our human data is in accord with the same. The present studies suggest that the treatment of this disorder can be optimally accomplished when VEGF is combined with NOS inhibitors. Our study also suggests that timing of delivery of VEGF could be critical for its effects. This establishes the VEGF-NO pathway as a worthwhile focus for future investigations in VEGF-mediated disorders that can be exploited to maximize the utility and safety of VEGF as a therapeutic agent.
This work was supported in part by grants 0755843T (V.B.) from the American Heart Association; HL-74195 (V.B.), HL-64642, HL-61904, and HL-56389 (J.A.E.) from the NHLBI; and HD-07049 (J.H.N.) from the NICHD of the National Institutes of Health.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0024OC on April 25, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–676. [DOI] [PubMed] [Google Scholar]
- 2.Zelzer E, Olsen BR. Multiple roles of vascular endothelial growth factor (VEGF) in skeletal development, growth, and repair. Curr Top Dev Biol 2005;65:169–187. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod 2001;64:1033–1040. [DOI] [PubMed] [Google Scholar]
- 4.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes Disord 2005;29:1308–1314. [DOI] [PubMed] [Google Scholar]
- 5.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 2005;112:1813–1824. [DOI] [PubMed] [Google Scholar]
- 6.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 2004;26:943–954. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson-Berka JL. Vasoactive factors and diabetic retinopathy: vascular endothelial growth factor, cycoloxygenase-2 and nitric oxide. Curr Pharm Des 2004;10:3331–3348. [DOI] [PubMed] [Google Scholar]
- 8.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006;290:L209–L221. [DOI] [PubMed] [Google Scholar]
- 9.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000;106:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, Chen Q, Zhu Z, Zhong M, Nakayama K, et al. Bcl-2-related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest 2005;115:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol 1996;270:H411–H415. [DOI] [PubMed] [Google Scholar]
- 13.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997;99:2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuwerke SM, Kaza AK, Tribble CG, Kron IL, Laubach VE. Inhibition of compensatory lung growth in endothelial nitric oxide synthase-deficient mice. Am J Physiol Lung Cell Mol Physiol 2002;282:L1272–L1278. [DOI] [PubMed] [Google Scholar]
- 15.Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, Ma B, Baluk P, Lin MI, McDonald DM, et al. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci USA 2006;103:11021–11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornecki A, Tsuchida S, Ondiveeran HK, Engelberts D, Frndova H, Tanswell AK, Post M, McKerlie C, Belik J, Fox-Robichaud A, et al. Lung development and susceptibility to ventilator-induced lung injury. Am J Respir Crit Care Med 2005;171:743–752. [DOI] [PubMed] [Google Scholar]
- 17.Bhandari V. Developmental differences in the role of interleukins in hyperoxic lung injury in animal models. Front Biosci 2002;7:d1624–d1633. [DOI] [PubMed] [Google Scholar]
- 18.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med 2006;41:4–18. [DOI] [PubMed] [Google Scholar]
- 19.Bhandari V, Choo-Wing R, Homer RJ, Elias JA. Increased hyperoxia-induced mortality and acute lung injury in IL-13 null mice. J Immunol 2007;178:4993–5000. [DOI] [PubMed] [Google Scholar]
- 20.Homer RJ, Zheng T, Chupp G, He S, Zhu Z, Chen Q, Ma B, Hite RD, Gobran LI, Rooney SA, et al. Pulmonary type II cell hypertrophy and pulmonary lipoproteinosis are features of chronic IL-13 exposure. Am J Physiol Lung Cell Mol Physiol 2002;283:L52–L59. [DOI] [PubMed] [Google Scholar]
- 21.Kirwin SM, Bhandari V, Dimatteo D, Barone C, Johnson L, Paul S, Spitzer AR, Chander A, Hassink SG, Funanage VL. Leptin enhances lung maturity in the fetal rat. Pediatr Res 2006;60:200–204. [DOI] [PubMed] [Google Scholar]
- 22.Ray P, Tang W, Wang P, Homer R, Kuhn C III, Flavell RA, Elias JA. Regulated overexpression of interleukin 11 in the lung: use to dissociate development-dependent and -independent phenotypes. J Clin Invest 1997;100:2501–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 2000;23:162–167. [DOI] [PubMed] [Google Scholar]
- 24.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82:527–532. [PubMed] [Google Scholar]
- 25.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 2002;8:702–710. [DOI] [PubMed] [Google Scholar]
- 26.McGrath-Morrow SA, Cho C, Cho C, Zhen L, Hicklin DJ, Tuder RM. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol 2005;32:420–427. [DOI] [PubMed] [Google Scholar]
- 27.Raoul W, Chailley-Heu B, Barlier-Mur AM, Delacourt C, Maitre B, Bourbon JR. Effects of vascular endothelial growth factor on isolated fetal alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L1293–L1301. [DOI] [PubMed] [Google Scholar]
- 28.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 2000;22:657–664. [DOI] [PubMed] [Google Scholar]
- 29.Le Cras TD, Spitzmiller RE, Albertine KH, Greenberg JM, Whitsett JA, Akeson AL. VEGF causes pulmonary hemorrhage, hemosiderosis, and air space enlargement in neonatal mice. Am J Physiol Lung Cell Mol Physiol 2004;287:L134–L142. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest 2004;114:669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 2005;289:L529–L535. [DOI] [PubMed] [Google Scholar]
- 32.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005;112:2477–2486. [DOI] [PubMed] [Google Scholar]
- 33.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L1068–L1078. [DOI] [PubMed] [Google Scholar]
- 34.Choo-Wing R, Nedrelow JH, Homer RJ, Elias JA, Bhandari V. Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2007;293:L142–L150. [DOI] [PubMed] [Google Scholar]
- 35.Le Gouill S, Podar K, Amiot M, Hideshima T, Chauhan D, Ishitsuka K, Kumar S, Raje N, Richardson PG, Harousseau JL, et al. VEGF induces Mcl-1 up-regulation and protects multiple myeloma cells against apoptosis. Blood 2004;104:2886–2892. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Chen SL, Chen WM, Liu JW. Zhonghua Nei Ke Za Zhi 2005;44:85–88. (The interaction of vascular endothelial growth factor and interleukin-6 in multiple myeloma.). [PubMed] [Google Scholar]
- 37.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 2002;283:L555–L562. [DOI] [PubMed] [Google Scholar]
- 38.Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol 2001;281:L1001–L1010. [DOI] [PubMed] [Google Scholar]
- 39.Akeson AL, Cameron JE, Le Cras TD, Whitsett JA, Greenberg JM. Vascular endothelial growth factor-A induces prenatal neovascularization and alters bronchial development in mice. Pediatr Res 2005;57:82–88. [DOI] [PubMed] [Google Scholar]
- 40.Leikauf GD, McDowell SA, Bachurski CJ, Aronow BJ, Gammon K, Wesselkamper SC, Hardie W, Wiest JS, Leikauf JE, Korfhagen TR, et al. Functional genomics of oxidant-induced lung injury. Adv Exp Med Biol 2001;500:479–487. [DOI] [PubMed] [Google Scholar]
- 41.Rosenblum Lichtenstein JH, Molina RM, Donaqhey TC, Brain JD. Strain differences influence murine pulmonary responses to Stachybotrys chartarum. Am J Respir Cell Mol Biol 2006;35:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dodd-o JM, Hristopoulos ML, Welsh-Servinsky LE, Tankersley CG, Pearse DB. Strain-specific differences in sensitivity to ischemia-reperfusion lung injury in mice. J Appl Physiol 2006;100:1590–1595. [DOI] [PubMed] [Google Scholar]
- 43.Iwakawa M, Noda S, Ohta T, Oohira C, Tanaka H, Tsuji A, Ishikawa A, Imai T. Strain dependent differences in a histological study of CD44 and collagen fibers with an expression analysis of inflammatory response-related genes in irradiated murine lung. J Radiat Res (Tokyo) 2004;45:423–433. [DOI] [PubMed] [Google Scholar]
- 44.Lassus P, Ristimaki A, Ylikorkala O, Viinikka L, Andersson S. Vascular endothelial growth factor in human preterm lung. Am J Respir Crit Care Med 1999;159:1429–1433. [DOI] [PubMed] [Google Scholar]
- 45.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 2001;164:1981–1987. [DOI] [PubMed] [Google Scholar]
- 46.Tsao PN, Wei SC, Chou HC, Su YN, Chen CY, Hsieh FJ, Hsieh WS. Vascular endothelial growth factor in preterm infants with respiratory distress syndrome. Pediatr Pulmonol 2005;39:461–465. [DOI] [PubMed] [Google Scholar]
- 47.Ambalavanan N, Novak ZE. Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates. Pediatr Res 2003;53:240–244. [DOI] [PubMed] [Google Scholar]
- 48.Asikainen TM, Ahmad A, Schneider BK, White CW. Effect of preterm birth on hypoxia-inducible factors and vascular endothelial growth factor in primate lungs. Pediatr Pulmonol 2005;40:538–546. [DOI] [PubMed] [Google Scholar]
- 49.De Paepe ME, Mao Q, Powell J, Rubin SE, Dekoninck P, Appel N, Dixon M, Gundogan F. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med 2006;173:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pannitteri G, Petrucci E, Testa U. Coordinate release of angiogenic growth factors after acute myocardial infarction: evidence of a two-wave production. J Cardiovasc Med (Hagerstown) 2006;7:872–879. [DOI] [PubMed] [Google Scholar]
- 51.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol 2004;97:1605–1617. [DOI] [PubMed] [Google Scholar]
- 52.Mura M, Han B, Andrade CF, Seth R, Hwang D, Waddell TK, Keshavjee S, Liu M. The early responses of VEGF and its receptors during acute lung injury: implication of VEGF in alveolar epithelial cell survival. Crit Care 2006;10:R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol 2003;285:L161–L168. [DOI] [PubMed] [Google Scholar]
- 54.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res 2005;65:550–563. [DOI] [PubMed] [Google Scholar]
- 55.Vannay A, Dunai G, Banyasz I, Szabo M, Vamos R, Treszl A, Hajdu J, Tulassay T, Vasarhelyi B. Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr Res 2005;57:396–398. [DOI] [PubMed] [Google Scholar]
- 56.Malik RA, Li C, Aziz W, Olson JA, Vohra A, McHardy KC, Forrester JV, Boulton AJ, Wilson PB, Liu D, et al. Elevated plasma CD105 and vitreous VEGF levels in diabetic retinopathy. J Cell Mol Med 2005;9:692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax 2006;61:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]