Abstract
Several transient receptor potential (TRP) ion channels sense and respond to changes in ambient temperature. Chemical agonists of TRP channels, including menthol and capsaicin, also elicit sensations of temperature change. TRPM8 is a cold- and menthol-sensing ion channel that converts thermal and chemical stimuli into neuronal signals and sensations of cooling/cold. However, the expression and function of TRPM8 receptors in non-neuronal cells and tissues is a relatively unexplored area. Results presented here document the expression and function of a truncated TRPM8 variant in human bronchial epithelial cells. Expression of the TRPM8 variant was demonstrated by RT-PCR, cloning, and immunohistology. Receptor function was characterized using the prototypical TRPM8 agonist, menthol, and exposure of cells to reduced temperature (18°C). The TRPM8 variant was expressed primarily within endoplasmic reticulum membranes of lung epithelial cells and its activation was attenuated by thapsigargin, the cell-permeable TRPM8 antagonist N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide, and shRNA-induced suppression of TRPM8 expression. Activation of the TRPM8 variant in lung cells was coupled with enhanced expression of the inflammatory cytokines IL-6 and IL-8. Collectively, our results suggest that this novel TRPM8 variant receptor may function as a modulator of respiratory physiology caused by cold air, and may partially explain asthmatic respiratory hypersensitivity to cold air.
Keywords: TRP family, TRPM8, menthol, calcium, lung epithelial cells
CLINICAL RELEVANCE
This research characterizes a transient receptor potential melastatin 8 (TRPM8) variant that effects IL-6 and IL-8 expression by lung epithelial cells. These data provide a rationale for translational research evaluating the role of TRPM8 in respiratory distress due to cold air inhalation.
Temperature detection is essential for mammalian homeostasis. Several members of the transient receptor potential (TRP) superfamily of ion channels function as molecular transducers of thermal stimuli (1–3). Thermo-TRP channels, which include TRPV1 (4, 5), TRPV2 (6), TRPV3 (7), TRPV4 (8), TRPM8 (9, 10), and TRPA1 (11), are activated over distinct temperature ranges (1). Combined, these thermo-TRPs account for the detection of the entire spectrum of temperature sensations perceived by mammals.
TRP channels consist of six putative transmembrane (TM)-spanning segments flanked by intracellular N- and C-terminal domains (12, 13) of variable length and an ion-conducting pore-loop region located between TM segments five and six (12–14). Residues within the N- and C-terminal regions are proposed to associate with cellular proteins and cytoplasmic factors (15, 16) that determine channel activation properties and subcellular localization. The sequence similarity between the TRP subfamilies is highest in the sixth TM region and in the highly conserved “TRP” domain, located in the intracellular C-terminal region (2, 12, 17).
TRPM8 (or Trp-p8), a member of the melastatin subfamily, functions as a transducer of innocuous cold stimuli in the somatosensory system. It is a ligand-gated, cation channel, with moderate to high selectivity for calcium (Ca2+) ions (9, 10). TRPM8 is activated by temperatures in the range of 8 to 22°C, with an activation threshold at approximately 22°C, and by chemicals that elicit sensations of cold (18, 19). Chemical agonists of TRPM8 include menthol, eucalyptol, icilin, and several other compounds used commonly in the flavoring and perfumery industry (9, 10, 20–22). Although not identified, endogenous ligands for TRPM8 have been proposed (22). Antagonists of menthol-induced Ca2+ flux through TRPM8 include compounds previously characterized as TRPV1 antagonists, and include N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide (BCTC), thio-BCTC, and capsazepine (CPZ) (20).
TRPM8 transcripts are expressed in a sub-population of cold-responsive primary afferent sensory neurons (17) within the dorsal root and trigeminal ganglia (10), as well as in normal prostate and testicular tissues, and in taste papillae (23–25). TRPM8 expression has been shown to be up-regulated in prostate cancer cells, as well as in several other nonprostatic primary cancers, including melanoma, colorectal carcinomas, and breast carcinomas (25, 26). Studies have demonstrated that TRPM8 is expressed on the plasma and the endoplasmic reticulum (ER) membranes of cells, where it facilitates the influx or release of Ca2+ from extracellular sources and the ER stores, respectively (24, 26, 27). A study by Thebault and coworkers (24) established that TRPM8 was preferentially localized to the ER of androgen-responsive LNCaP prostate cancer cells. The expression of TRPM8 in lung cells has not been evaluated.
The respiratory epithelium constantly interacts with the external environment. When cold air is inhaled, greater than normal heat loss occurs and compensatory thermoregulatory actions ensue. Cold air–provoked respiratory symptoms include bronchial obstruction and inflammation, and these phenomena are common in countries with a cold climate (28). It has not been definitively established whether cold air–induced airway responses are the direct result of temperature changes in the airway, increased airway dehydration due to inhalation of cold air low in humidity, or other causes. We hypothesize that TRPM8 could function as a proximal sensor and mediator of compensatory events in the respiratory tract due to cooling of the airways, and that this TRPM8 action alone, or in combination with other processes, precipitates airway inflammation and obstruction. The most clearly defined physiologic functions of TRPM8 are related to its expression by sensory neurons, and certainly activation of sensory neurons in the airway will play a key role in physiologic responses to cold air inhalation. Currently, it is unclear whether TRPM8 has functions aside from sensing cold by neurons, but its detection in a number of non-neuronal tissues (such as urogenital tissues, taste papillae, etc.) and its up-regulation in cancer cells of the prostate, breast, skin, and so on (23–26, 29, 30) suggest that TRPM8 may have additional novel roles in mammalian physiology.
In this study, we explored the hypothesis that TRPM8 is expressed by human bronchial epithelial cells, and that activation of TRPM8 in these cells is coupled with changes in the expression of representative pro-inflammatory cytokines. We assayed IL-6 and IL-8, two cytokines often cited as being up-regulated in inflamed airways and airways of asthmatics. Based on the results, we conclude that a functional TRPM8 receptor variant is expressed in normal human bronchial epithelial cells, and that this protein may represent a novel mediator of deleterious effects in airways upon exposure to chemical agonists and cold air.
MATERIALS AND METHODS
Chemicals
Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). BCTC was synthesized using published methods (31). Briefly, BCTC was prepared by reacting 4-(tert-butylphenyl)-carbamic acid phenyl ester with 1-(3-chloropyridin-2-yl)piperazine and collecting the precipitate. The product structure was confirmed with 1H nuclear magnetic resonance (NMR) by comparing the NMR values to those in published literature (31).
Cell Culture
Human bronchial epithelial (BEAS-2B) cells (CRL-9609), Chinese hamster ovary (CHO) cells, and DU-145 prostate cancer cells were purchased from American Type Culture Collection (ATCC, Rockville, MD). BEAS-2B cells were cultured in serum-free Lechner and LaVeck (LHC-9) media (BioSource, Camarillo, CA) prepared by fortifying LHC-8 media with retinoic acid (33 nM) and epinephrine (2.75 μM). CHO cells were cultured in F-12 nutrient medium with Kaighn's modification and DU-145 cells were cultured in RPM1 1640 (Gibco/Invitrogen, Carlsbad, CA) media, respectively; both media contained 10% fetal bovine serum (FBS). Normal human bronchial epithelial (NHBE) cells, a primary bronchial epithelial cell line, was purchased from ATCC and cultured in serum-free bronchial epithelial growth media (BEGM; Clonetics/Lonza, Walkersville, MD). Culture flasks (Corning, Inc., Corning, NY) for BEAS-2B and NHBE cells were pre-coated with LHC-basal medium (BioSource) fortified with collagen (30 μg/ml), fibronectin (10 μg/ml), and bovine serum albumin (BSA, 10 μg/ml). Cells were maintained at 37°C, between 30 and 90% confluence, in an air-ventilated and humidified incubator maintained with 5% carbon dioxide. For subculturing, cells were trypsin-dissociated and passaged every 2 to 4 days.
Screening for TRPM8 Expression and Sequencing
Total RNA was extracted from cells using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA). Five micrograms of total RNA was transcribed into cDNA using an Oligo-dT primer and the Superscript-II cDNA synthesis kit (Invitrogen). The following PCR primers were used to assess the expression of TRPM8 mRNA: sense, 5′-CAGACCCCTGGGTACATGGTGGATG-3′; antisense, 5′-GCCTTTCAAGGTTGCATTTTGGGCGAC-3′. The PCR product size was 593 nt, derived from exon 26 through the 3′-UTR of the TRPM8 gene. In addition, multiple combinations of primers (listed in Table 1) were designed to amplify specific TRPM8 exons. A 180-nt portion of β-actin cDNA was simultaneously amplified by PCR using the following primers: sense, 5′-GACAACGGCTCCGGCATGTGCA-3′; antisense, 5′-TGAGGATGCCTCTCTTGCTCTG-3′. β-actin was used as an internal standard to normalize PCR product intensities between samples. PCR products were purified by agarose gel electrophoresis, cloned into the PCR-2.1 TOPO-TA vector (Invitrogen), and sequenced at the University of Utah DNA Sequencing facility. All sequences were verified by comparison to the published TRPM8 sequence (NCBI accession number: NM_024080).
TABLE 1.
PRIMER SEQUENCES USED FOR RT-PCR ANALYSIS OF TRPM8
| TRPM8 Exon-Specific Sense Primers | Primer Sequence |
|---|---|
| 5′-UTR #1 | Sense: 5′-AAAATCCTGCTTGACAAAAACCG-3′ |
| 5′-UTR #2 | Sense: 5′-GACAAAAACCGTCACTTAGG-3′ |
| Exon 2 | Sense: 5′-GCATGAGGAACAGAAGGAATGACAC-3′ |
| Exon 3 | Sense: 5′-ACCAAAGATTCCAAGGCCAC-3′ |
| Exon 4 | Sense: 5′-GCAAGTGTGGCTATGCCCAGAGCCAGC-3′ |
| Exon 5 | Sense: 5′-GCGGAAATCCTTTACGAGCTGC-3′ |
| Exon 6 | Sense: 5′-GAGATAACACCATCAGCAGG-3′ |
| Exon 7 | Sense: 5′-GCTCCGGAATCAGCTAGAGA-3′ |
| Exon 8 | Sense: 5′-GGCAAGATCCCCATTGTGTGT-3′ |
| Exon 9 | Sense: 5′-GCTAGCCTGGTGGAGGTGGAGGA-3′ |
| Exon 10 | Sense: 5′-GCTGGGGATGAAATTGTGAGCAATGC-3′ |
| Exon 11 | Sense: 5′-GACAAGGATAACTGGAATGGGC-3′ |
| Exon 12 | Sense: 5′-CGGAAGTTTCTCACCCATGAT-3′ |
| Exon 13 | Sense: 5′-CGTGTCTCCTATTACTCGGC-3′ |
| Exon 14 | Sense: 5′-CGACATCAATGCTGCTGGGGAGTCCG-3′ |
| Exon 15 | Sense: 5′-GCAGCGATGAAGACTTGGCAGA-3′ |
| Exon 16 | Sense: 5′-GCTGTGGCTTTGTATCAT TTAG-3′ |
| Exon 17 | Sense: 5′-GCTGCTTTGGTACTATGTGGC-3′ |
| Exon 18 | Sense: 5′-GACCTGTGGAATGTGATGGACACGC-3′ |
| Exon 19 | Sense: 5′-GCAGAAACTTAGGACCCAAGATT-3′ |
| Exon 20 | Sense: 5′-GCCAGGCAAGGGATCCTTAGGC-3′ |
| Exon 21 | Sense: 5′-CGGTTCCCCGAGTGGATCACCATC -3′ |
| Exon 22 | Sense: 5′-CGTCCAGAGAACAATGACC-3′ |
| Exon 23 | Sense: 5′-GCCAACGACACCTCAGAGG-3′ |
| Exon 24 | Sense: 5′-GCATCGATTTAGACAACTGGAT-3′ |
| Exon 25 | Sense: 5′-AGAGAGCGGGGTCCTCTTGCT C-3′ |
| TRPM8 Exon-Specific Antisense Primers | |
| Exon 7 | Antisense: 5′-TCTCTAGCTGATTCCGGAGC-3′ |
| Exon 17 | Antisense: 5′-GCCACATAGTACCAAAGCAGC-3′ |
| Exon 18 | Antisense: 5′-GCGTGTCCATCACATTCCACAGGTC-3′ |
| Exon 22 | Antisense: 5′-GGT CAT TGT TCT CCT GGA CG-3′ |
| Exon 23 | Antisense: 5′-CCT CTG AGG TGT CGT TGG C-3′ |
| Exon 26 | Antisense: 5′-AGAGAGCGGGGTCCTCTTGCTGC-3′ |
Definition of abbreviations: TRPM8, transient receptor potential melastatin 8.
Assessment of full-length TRPM8 and TRPM8 variant expression in human tissues was performed, as described above, using commercial total RNA samples from human lung, brain, tongue, kidney, ovary, colon, skin, liver, and testes (Stratgene/Agilent, Santa Clara, CA). Expression of message corresponding to exons 15–18 was used as a marker for full-length TRPM8 expression, while expression of the 593-nt fragment corresponding to exons 26–3′UTR was indicative of both variant and full-length TRPM8 expression.
Cloning and Stable Overexpression of the Truncated TRPM8 Variant
Exon 18–26 of TRPM8 was amplified by PCR from a full-length human TRPM8 open reading frame clone (Clone ID 8992058) (Open Biosystems, Huntsville, AL) using the following primers: sense, 5′-CACCATGGACACGCTGGGGC-3′; antisense, 5′-TTTGATTTTATTAGCAATCTCT-3′ and Phusion GC-rich PCR supermix (New England Biolabs, Ipswich, MA). The 5′CACC before the ATG start codon was used for directed cloning of the PCR product into the pcDNA3.1 D V5/His6 expression vector (Invitrogen) and the V5/His6 epitope tag was included by omitting the natural stop codon of TRPM8. The sequence of the truncated TRPM8 clone was verified and purified plasmid (250 ng) was transfected into CHO cells using Lipofectamine 2000 (Invitrogen) and a lipid:DNA ratio of 4:1 in a single well of a 96-well plate. Over 95% of cells were transfected using pMaxGFP as a reference. Transfected cells were selected for stable expression of the TRPM8 variant by growing cells in the presence of G418/Geneticin (1 mg/ml) for approximately 2 weeks and expansion of the clones in selective media. Gain of function due to expression of the TRPM8 variant in CHO cells was assessed using the fluorometric calcium flux assay and menthol.
TRPM8shRNA Design and Cloning
Short hairpin RNA (shRNA) was used to attenuate the expression of TRPM8 via RNA interference (RNAi) (32). The following oligonucleotides were designed using the siRNA Target Finder algorithm (Ambion, Austin, TX) and from published sequences (33). Exon 4: sense, 5′-GATCCGGCACCCAGATCAA-CCAAATTCAAGAGATTTGGTTGATCTGGGTGCCTTA-3′; antisense, 5′-AGCTTA-AGGCACCCAGATCAACCAAATCTCTTGAATTTGGTTGATCTGGGTGCCG-3′. Exon 8: sense, 5′-GATCCTCTGAGCGCACTATTCATTTTCAAGAGAAATGAATAGTGCGCTCAG-AGAA-3′; antisense, 5′-AGCTTTCTCTGAGCGCACTATTCATTTCTCTTGAAAATGAATAGTGCGCTCAGAG-3′. Exon 18: sense, 5′-GATCCGAAACCTGTCGACAAGC-ACTTCAAGAGAGTGCTTGTCGACAGGTTTCTTA-3′; antisense, 5′-AGCTTAAGAA-ACCTGTCGACAAGCACTCTCTTGAAGTGCTTGTCGACAGGTTTC G-3′. The sense and antisense oligonuclotide pairs were annealed and the resulting duplex DNA was ligated into the pSilencer 4.1 mammalian expression vector (Ambion) at the Bam H1 and Hind III restriction sites. A scrambled, nontargeting shRNA provided with the kit was also prepared as a negative control. Vector constructs were transformed into Escherichia coli cells and selected by ampicillin resistance. Plasmid DNA was isolated from individual colonies using QIAprep spin miniprep kit (Qiagen) and the sequence of the shRNA insert was verified by sequencing the plasmid DNA.
Stable Transfection of BEAS-2B Cells with TRPM8shRNA
Plasmid DNA (1 μg), containing either the TRPM8shRNA or ScrambleshRNA inserts, was transfected into BEAS-2B cells using Effectene transfection reagent (10:1 reagent:DNA) for 24 hours at 37°C. Stably transfected cells were selected by resistance to G418/Geneticin (300 μg/ml). Resistant colonies were visible approximately 2 to 3 weeks after transfection. Individual colonies were harvested, expanded, and screened for reduced expression of TRPM8 mRNA by RT-PCR using the primers described above.
TRPM8 Protein Detection by Immunohistochemistry
NHBE, BEAS-2B, and DU-145 cells were subcultured into 6-well culture plates, grown to approximately 75% confluence, and fixed with ice-cold methanol. Cells were washed three times with tris-buffered saline (TBS) containing 0.1% tween-20 (TBS/T), and nonspecific binding was blocked using a solution of 10% donkey serum and 5% BSA in TBS/T. The cells were rinsed three times with TBS and incubated at 4°C for 18 hours with a rabbit polyclonal IgG antibody fraction specific to human TRPM8 (Abcam, Cambridge, MA), diluted 1:500 in the blocking solution. The cells were washed and treated for 1 hour at room temperature with an Alexa-Fluor 488 conjugated donkey anti-rabbit IgG secondary antibody (Molecular Probes, Eugene, OR) at a dilution of 1:400 in the blocking solution. The nuclei were counter-stained blue using 4′,6-diamidino-2-phenylindole (DAPI) at 1:1,000 dilution in TBS. Controls consisted of untreated cells or cells treated with either primary or secondary antibodies alone. Images were collected using an Olympus (1×51) inverted microscope (Olympus America Inc., Melville, NY), equipped with filters to visualize green fluorescent protein and DAPI. An integrated Hamamatsu digital camera (Bridgewater, NJ) was used to collect image data at ×40 magnification. FITC- and DAPI-stained images were analyzed and superimposed using Olympus MicroSuite-5 and NIH Image-J software packages. Immunoreactivity of TRPM8 was detected as green fluorescence.
Fluorometric Intracellular Calcium Imaging
Cells were subcultured into 96-well culture plates or 35- × 10-mm coated polystyrene culture dishes (Corning Inc.) and grown to approximately 95% confluence. Cells were loaded with the membrane-permeable fluorogenic Ca2+ indicator, Fluo-4 (AM) (Molecular Probes) at a concentration of 2.5 μM for 1 hour at 37°C in media containing 200 μM sulfinpyrazone (Sigma-Aldrich, St. Louis, MO). Cells were washed with media and incubated at 37°C for an additional 30 minutes before analysis. All loading steps were performed in the dark. Images were collected immediately before and 30 seconds after addition of menthol (0–10 mM) or at multiple time points after exposure to cool temperature (18°C). Cool temperature treatments were achieved by placing the culture dishes in a water-bath maintained at the desired temperature (18°C). Inhibition by the antagonist BCTC was evaluated by adding BCTC (5–500 μM) 5 minutes before and during menthol or cold treatments. Changes in cellular fluorescence in response to the treatments were assessed microscopically (×10 objective) on cell populations (∼ 500 cells/field) using a Nikon Diaphot inverted microscope (Nikon Instruments Inc., Melville, NY) equipped with a fluorescence filter set designed to visualize green fluorescent protein. Fluoromicrographs were captured at high resolution using a SPOT Insight QE digital camera interfaced with the SPOT data system software (Diagnostic Instruments, Inc., Sterling Heights, MI). Image quantitation was performed using the NIH Image-J software package. The brightness of the images was normalized, the background fluorescence subtracted, and the mean fluorescence intensity of the images was determined. Data are presented as change in fluorescence intensity normalized to the untreated controls and standard deviation. Experiments were performed in triplicate.
Subcellular Localization of TRPM8 Function
Depletion of the ER Ca2+ stores was accomplished by treating cells with thapsigargin (Sigma-Aldrich) at 1.5 μM for approximately 5 minutes before treatment with menthol. Ca2+ flux caused by activation of cell surface TRPM8 receptors was quantified by treating cells with media containing the plasma membrane impermeable Ca2+ chelator, EGTA (100 μM). Differences in Ca2+ flux observed between the treatment groups were used to determine the relative subcellular distribution of active TRPM8. Data represent changes in fluorescence intensity normalized to the untreated controls and standard deviation. Experiments were performed in triplicate.
Quantitation of IL-6 and 8 Gene Expression
NHBE and BEAS-2B cells were subcultured into 25 cm2 flasks and grown to approximately 95% confluence. Cells were either exposed to cool temperature (18°C) for 0 to 4 hours, followed by a 2-hour recovery at 37°C, or were treated with menthol (2.5 mM) for 0 to 24 hours. The TRPM8 antagonist BCTC (100 μM) was added to cells 5 minutes before treatment. cDNA from the treated cells was obtained as described above. cDNA corresponding to human IL-6 and 8 was amplified by PCR from 1 μl of the cDNA synthesis reaction using GoTaq green PCR mastermix (Promega, Madison, WI) and the following primers. IL-6: sense, 5′-CTTCTCCACAAGCGCCTTC-3′; antisense, 5′-GGCAAGTCTCCTCATTGAATC-3′. IL-8: sense, 5′-GTGGCTCTCTTGGCAGCCTTC-3′; antisense, 5′-CAGGAATCTTGTATTGCATCTG-3′. β-actin was simultaneously amplified as an internal standard. PCR products were resolved using agarose gels and images were captured using a Bio-Rad Gel-Doc imaging system (Bio-Rad Laboratories, Hercules, CA). Product quantification was achieved by determining the relative density of the IL-6 and IL-8 product relative to the β-actin product, using the Gel-Doc density analysis tools. Data are expressed as band density normalized to β-actin relative to the ratios observed for the untreated control cells and standard deviation. Experiments were reproduced a minimum of three times with different passages of cells.
Statistical Analysis
Half maximal effective concentration (EC50) and half maximal lethal dose (LD50) values were obtained by nonlinear regression analysis (Prism 4; GraphPad Software, Inc., San Diego, CA) using the sigmoidal dose–response (variable slope) equation. Statistical analysis was performed using ANOVA and Dunnett's multiple comparisons post-test, with a confidence interval of P ≤ 0.05. The paired t test with a confidence interval of P ≤ 0.05 was also used.
RESULTS
Detection of TRPM8 Transcripts in Lung Epithelial Cells
TRPM8 expression by lung epithelial cells was established using RT-PCR and DNA sequence analysis of PCR products. A 593 nt fragment, unique to human TRPM8, was amplified from DU-145 (positive control) (24), NHBE, and BEAS-2B cDNA (Figure 1A, lanes 1, 2, and 3, respectively). The relative amount of TRPM8 message was roughly equal in all three cell types. Sequence analysis of the cloned 593-nt product confirmed that this product was derived from TRPM8; greater than 99% sequence identity was observed between the PCR products from each cell type and the published human TRPM8 sequence (NCBI accession number: NM_024080).
Figure 1.
Expression of transient receptor potential melastatin 8 (TRPM8) variant mRNA in lung epithelial cells. (A) An agarose gel demonstrating the presence of a 593-nt PCR product amplified from human prostate carcinmoa cells (DU-145) (positive control, lane 1), normal human bronchial epithelial cells (NHBE) (lane 2), and human bronchial epithelial cell line (BEAS-2B) (lane 3) cDNA. Below, the normalized (β-actin) PCR product intensities are shown. Each bar corresponds to the mean normalized product intensity (n = 4) and standard deviation for the gel lanes shown above the bars. (B) Representative agarose gel showing the presence of TRPM8 variant-derived PCR products from NHBE cDNA, using E18F/E26R (lane 1), E20F/E26R (lane 2), E21F/E26R (lane 3), and E22F/E26R (lane 4) primer pairs and gels showing the presence of TRPM8-derived PCR products from LNCaP and normal human prostate cDNA, using E18F/E22R (lane 1), E20F/E23R (lane 2), E14F/E18R (lane 3), E15F/E18R (lane 4), E4F/E7R (lane 5), and E13F/E17R (lane 6) primer pairs. (C) Predicted translation product of the TRPM8 variant cDNA expressed by lung epithelial cells. The putative transmembrane domains (shaded boxes), pore loop region (underlined, italics), TRP domain (open box), residues required for menthol binding and activation (bold, #), and icilin binding and activation (bold, *) are highlighted. The cold-sensing domain resides between TM4 and 5.
Amplification of full-length TRPM8 cDNA from lung cells was unsuccessful despite multiple attempts. Positive amplification of multiple segments of the TRPM8 transcript and the inability to amplify the full-length transcript from lung cell cDNA suggested that a truncated TRPM8 variant form was expressed by human lung cells. Sense primers corresponding to each of the first 25 exons (E1F to E25F) and antisense primers corresponding to exons 7 (E7R), 17 (E17R), 18 (E18R), 22 (E22R), 23 (E23R), and 26 (E26R) were designed (Table 1). Using cDNA prepared from NHBE cells, various combinations of the exon-specific primers were employed to amplify specific TRPM8 exons. Figure 1B shows a gel image with PCR products generated using combinations of exon-specific sense primers and an antisense primer that binds to exon 26 of human TRPM8. The sequence identity of each product was confirmed. This systematic approach revealed a TRPM8 transcript (Figure 1C) encompassing exon 18 to the 3′UTR, which conceptually translated into a 304–amino acid TRPM8 variant (∼ 36 kD) lacking the N-terminal domain, but retaining TM domains 3–6, the pore loop region, the characteristic TRP domain, and the intracellular C-terminal domain. The alternate translational start site (ATG) for this variant occurred 2,400 nt 3′ to the normal translational start site for TRPM8, at amino acid M801 (Figure 1C).
Although exons before exon 18 were absent in NHBE cells, several were detected in LNCaP prostate cancer cell and normal human prostate cell cDNA, which are known to express full-length TRPM8 gene (24, 25) (Figure 1B). Thus, amplification of multiple exons that spanned the full ORF of TRPM8 from prostate cells confirmed that our inability to detect exons 1–17 in lung cells was not a result of faulty procedures.
Analysis of TRPM8 and Truncated TRPM8 Expression in Human Tissues
Expression of full-length and truncated TRPM8 in various human tissues was also assessed (Figure 2A). The 593-nt PCR product corresponding to exon 26 through the 3′-UTR of TRPM8 was detected in brain, liver, kidney, testes, lung, tongue, and colon, with highest abundance in brain and lowest abundance in colon. Expression of full-length TRPM8 was detected by amplifying a segment of the TRPM8 gene corresponding to exons 15 through 18, a portion not expressed in lung cells. The full-length gene was only detected in brain, liver, and testes.
Figure 2.
(A) Expression of full-length and truncated TRPM8 mRNA in human tissues. Amplification of the 593-nt segment (top gel image) of TRPM8 corresponding to exons 26–3′UTR, but not exons 15–18 (bottom gel image) was used as a marker for TRPM8 variant expression. Amplification of message corresponding to exons 15–18 was used as a marker of full-length TRPM8 expression. Tissue sources of RNA are indicated above each lane of the gel image. β-actin (lower band) was used as an internal control. (B) Functional analysis of the cloned TRPM8 variant in Chinese hamster ovary (CHO) cells. Stably overexpressing (squares) and control CHO cells (circles) were treated with increasing concentration of menthol and changes in [Ca2+]i were quantified, as described in Materials and Methods. An asterisk represents statistically significant (paired t test, P ≤ 0.05) increases in [Ca2+]i relative to untreated and control CHO cells (n = 3). No Ca2+ flux was observed in control CHO cells.
Functional Analysis of the Cloned TRPM8 Variant
Before functional characterization of the TRPM8 variant in lung cells, the ability of the truncated receptor to function was assessed. CHO cells stably overexpressing the TRPM8 variant demonstrated dose-dependent menthol-induced calcium flux with an EC50 of approximately 1.5 mM (Figure 2B). Maximum calcium flux was approximately 2.9-fold over control and approximately 57% that of ionomycin, with over 90% of cells responding to menthol at 5 mM. Menthol-induced calcium flux was attenuated by BCTC and was not elicited by icilin (1 mM) (data not shown). Calcium flux was not observed in control CHO cells (Figure 2B).
shRNA-Mediated Attenuation of TRPM8 Expression in Lung Epithelial Cells
Expression of the TRPM8 variant in lung cells was further characterized using shRNA to attenuate receptor expression. BEAS-2B cells were stably transfected with shRNA targeted to exons 4, 8, and 18 of human TRPM8. Changes in TRPM8 mRNA expression in G418/Geneticin-resistant foci, in response to the different shRNAs, were evaluated by RT-PCR using primers that amplified the 593-nt fragment derived from exon 26 and the 3′-UTR. cDNA prepared from normal BEAS-2B cells and BEAS-2B cells stably transfected with the nontargeting ScrambleshRNA were used as controls. A significantly lower quantity of TRPM8 message was observed for BEAS-2B cells stably transfected with TRPM8shRNA directed to exon 18 (Figure 3), but no changes in gene expression were observed in BEAS-2B cells stably transfected with shRNA targeted to either exon 4 or 8 (Figure 3). A total of 10 to 15 colonies derived from each transfection were evaluated. These findings support the conclusion that the full-length TRPM8 transcript is absent in the human lung epithelial cells and that a novel, truncated TRPM8 variant is selectively expressed.
Figure 3.
Attenuated TRPM8 variant mRNA expression in BEAS-2B cells stably overexpressing exon 18–directed TRPM8shRNA. Normalized (β-actin) TRPM8 variant PCR product intensities for untransfected BEAS-2B cells, BEAS-2B cells after stable transfection with either TRPM8shRNA targeted to TRPM8 exons 4, 8, and 18, or nontargeting ScrambleshRNA. Each bar corresponds to the mean product intensity (n = 4). *Values significantly different from untreated cells and ScrambleshRNA-transfected cells (paired t test, P ≤ 0.05).
Expression of TRPM8 Protein in Lung Epithelial Cells
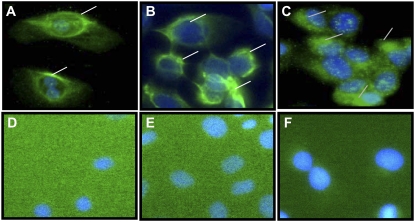
The expression of the TRPM8 variant protein in normal human lung epithelial cells was also evaluated by immunohistochemical analysis using a polyclonal antibody for human TRPM8 (Figure 4). DU-145 cells were used as a positive control and NHBE, BEAS-2B, and DU-145 cells treated with secondary antibody alone served as negative controls. Immunoreactivity for TRPM8 protein was evident in NHBE (Figure 4A), BEAS-2B (Figure 4B), and DU-145 cells (Figure 4C). The pattern of intense green staining within the lung cells suggested localization of the TRPM8 variant within the ER (perinuclear staining), as well as some localization to the plasma membrane (peripheral staining) (Figures 4A and 4B). This distribution was different than that observed for DU-145 cells, which exhibited more diffuse staining throughout the cell and the cell surface (Figure 4C). These results were consistent with published literature (24, 27). No staining for TRPM8 was observed in negative control samples (Figures 4D, 4E, and 4F). Moreover, the expression pattern of the truncated TRPM8 protein did not change in response to 2.5 mM menthol treatment for 8 hours (data not shown).
Figure 4.
Immunohistochemical detection of TRPM8 variant protein in lung epithelial cells. Immunohistochemical staining using a polyclonal antibody to residues 278–292 and 1089–1104 of human TRPM8 demonstrated protein expression within NHBE, BEAS-2B, and throughout DU-145 cells (A, B, and C, respectively). The nuclei were counterstained blue using DAPI. No immunohistochemical staining for TRPM8 was observed in NHBE, BEAS-2B, or DU-145 cells (D, E, and F, respectively) treated with secondary antibody alone.
Characterization and Localization of TRPM8 Variant Activity in Lung Epithelial Cells
Fluorometric Ca2+ flux assays were used to assess TRPM8 variant function in lung cells. Treatment of NHBE, BEAS-2B, and DU-145 cells with menthol produced robust, dose-dependent increases in cytosolic Ca2+ concentrations ranging from 2.5-fold over control for DU-145 cells to approximately 5- to 7-fold over control for NHBE and BEAS-2B cells (Figure 5A). EC50 values for induction of flux by menthol were 1.8 mM, 1.1 mM, and 0.0024 mM for NHBE, BEAS-2B, and DU-145 cells, respectively. The EC50 values for BEAS-2B and NHBE cells were similar to that observed for the stably transfected CHO cells (Figure 2B). Similarly, exposure to 18°C for 0 to 5 minutes produced substantial time-dependent increases in cytosolic Ca2+ concentrations in all three cell types (Figure 5B), ranging from 2.9-fold over control for NHBE cells, to 1.6-fold and 3.7-fold over control for BEAS-2B and DU-145 cells, respectively. In CHO cells, which do not express TRPM8 (2), concentrations of menthol in excess of 10 mM and prolonged (up to 15 min) exposures to cold temperature failed to induce Ca2+ flux (Figures 5A and 5B).
Figure 5.
Characterization of TRPM8 variant function in lung epithelial cells. (A) Concentration-dependent increases in [Ca2+]i by menthol in NHBE (squares), BEAS-2B (triangles), and DU-145 cells (diamonds). No Ca2+ flux was observed in CHO cells (circles). (B) Increased [Ca2+]i in NHBE (squares), BEAS-2B (triangles), and DU-145 cells (diamonds) exposed to cold (18°C) for various time points. No Ca2+ flux was observed in CHO cells (circles). Data represent mean fluorescence values normalized to untreated controls and standard deviation (n = 3). An asterisk represents concentrations at which statistically significant (ANOVA, P ≤ 0.05) increases in [Ca2+]i were observed. (C) TRPM8 variant-mediated Ca2+ flux induced by 3 mM menthol in the presence of EGTA (100 μM) or after depletion of ER Ca2+ with thapsigargin (1.5 μM) in NHBE and BEAS-2B cells. Data represent mean fluorescence values normalized to the untreated controls, and standard deviation (n = 3). Values significantly (paired t test, P ≤ 0.05) different from untreated and menthol-treated cells are represented by * and #, respectively.
Since higher than reported menthol doses were required to induce Ca2+ flux in the lung epithelial cells, cytotoxicity assays were performed. Results from these assays indicated that menthol was essentially nontoxic to cells under conditions that stimulated Ca2+ flux; approximately 20 to 25% loss of cell viability was observed at 15 mM menthol, only after 24 hours of treatment. The LD50 for menthol was 8 mM for DU-145 cells and approximately 10 mM for both NHBE and BEAS-2B cells. The relative doses required to produce Ca2+ flux and cell death, as well as the duration of the assays, clearly suggest that Ca2+ flux was due to activation of TRPM8 or its variant form, and not due to nonspecific disruption of cell membrane integrity. Exposure to cold (18°C) for up to 24 hours was also not lethal to the cells.
Depletion of ER Ca2+ stores with thapsigargin before menthol (3 mM) treatment inhibited menthol-induced Ca2+ flux by approximately 100% in NHBE and BEAS-2B cells (Figures 5C). Conversely, reduction of bio-available extracellular Ca2+ ions with EGTA inhibited menthol-induced Ca2+ flux by only 30 to 50%. These data indicate that the majority of the TRPM8 variant activity was attributable to an intracellular ER-bound subpopulation of receptor. These data are consistent with the immunohistochemical data presented in Figures 4A and 4B, which place the variant receptor primarily within the cells.
Inhibition of TRPM8 Function in Lung Epithelial Cells
NHBE, BEAS-2B, and DU-145 cells were treated with menthol (5 mM, Figure 6A) or cold (18°C, Figure 6B), after pre-treatment with BCTC, an antagonist of TRPM8. Ca2+ flux initiated by menthol was attenuated by 5 μM BCTC in NHBE and BEAS-2B cells, and by 25 μM BCTC in DU-145 cells (Figure 6A). Likewise, 10 μM BCTC blocked cold (18°C)-mediated increases in Ca2+ flux in NHBE and BEAS-2B cells, whereas 5 μM BCTC was sufficient to block the cold responses in DU-145 cells (Figure 6B). In addition, Ca2+ flux caused by menthol (Figure 6C), as well as prolonged (up to 1 h) cold exposure (18°C, Figure 6D), was attenuated in BEAS-2B cells stably transfected with TRPM8shRNA targeting exon 18 (circles), but not in untransfected BEAS-2B cells (squares) and BEAS-2B cells stably transfected with nonspecific ScrambleshRNA (triangles) (Figures 6C and 6D).
Figure 6.
Inhibition of (A) menthol- and (B) cold-induced activation of the TRPM8 variant in lung epithelial cells. (A) NHBE, BEAS-2B, and DU-145 cells were assayed for changes in [Ca2+]i after treatment with menthol (5 mM) with and without BCTC (5 or 25 μM) or (B) after exposure to cold (18°C) for 45 minutes (with a 2-h recovery at 37°C) with or without BCTC (5 or 10 μM). Data in A and B represent fluorescence values normalized to untreated controls for the cell populations and standard deviation (n = 3). * and # represent values significantly different from untreated and menthol- or cold-treated cells, respectively (paired t test, P ≤ 0.05). (C) Dose–response activation of TRPM8 by menthol in BEAS-2B cells (squares), BEAS-2B cells stably transfected with ScrambleshRNA (triangles), or exon 18-specific shRNA (circles). (D) Activation of TRPM8 by cold (18°C, 0–1 h) in BEAS-2B cells (squares), BEAS-2B cells stably transfected with ScrambleshRNA (triangles), or exon 18–specific shRNA (circles). Data in C and D represent fluorescence values normalized to untreated controls for the cell populations and standard deviation (n = 3). *Represents concentrations at which statistically significant (ANOVA, P ≤ 0.05) increases in Ca2+ flux were observed.
Effect of TRPM8 Variant Activation on IL-6 and 8 mRNA Levels
Coupling of TRPM8 variant activation in lung cells with enhanced expression of the inflammatory cytokines IL-6 and 8 was evaluated following treatment with menthol (2.5 mM) or exposure to cold (18°C). Menthol produced approximately 8- and approximately 21-fold increases in IL-6 and IL-8 gene expression after 24 hours of treatment, and cold exposure produced approximately 16- and approximately 28-fold increases after a 4-hour exposure to cold with a 2-hour recovery at 37°C (Figure 7A). Both menthol- and cold-induced increases in IL-6 and 8 mRNA were ablated in BEAS-2B cells stably expressing TRPM8shRNA directed to exon 18 (Figure 7B).
Figure 7.
TRPM8 variant-mediated alterations in IL-6 and 8 transcription in lung epithelial cells. (A) Induction of IL-6 (squares with dashed line) and IL-8 (squares with solid line) mRNA expression by menthol (2.5 mM) and cold (18°C) (IL-6, triangles with dashed line; IL-8 triangles with solid line). Data are presented as PCR product intensity relative to β-actin and untreated controls (n = 3). *Represents statistical significance (ANOVA, P ≤ 0.05). (B) Inhibition of menthol- (2.5 mM; open bars and hatched open bars) and cold- (18°C; shaded and hatched shaded bars) induced IL-6 and IL-8 mRNA expression in BEAS-2B cells stably overexpressing TRPM8shRNA targeted to exon 18. Data represent PCR product intensities normalized to β-actin versus untreated controls (n = 3). Values significantly (paired t test, P ≤ 0.05) different from untreated and menthol-treated cells are represented by * and #, respectively.
DISCUSSION
TRP channels have been firmly established as primary sensors of thermal stimuli in the peripheral nervous system of mammals (34). In sensory neurons, TRPM8 constitutes a key molecular sensor of decreased ambient temperature. However, the function of TRPM8 in nonneuronal tissues, including the lungs, is essentially unknown. Mammals are homeothermic and constantly adjust their physiology to compensate for changes in the ambient temperature.
The surface of human airways is lined with epithelial cells that are directly exposed to inspired air of varying temperatures. We hypothesized that a nonneuronal cold temperature–sensitive pathway existed in the lung. Results from this study demonstrate the expression of a functional truncated TRPM8 receptor variant that detects and responds to cold temperatures and the prototypical TRPM8 agonist menthol in human lung bronchial epithelial cells. It was shown that activation of this TRPM8 variant in lung cells produced efflux of Ca2+ from the ER of cells, as well as cellular uptake of Ca2+, and that these events were coupled with the transcriptional regulation of at least two important immunomodulatory cytokines, IL-6 and IL-8. These results provide strong evidence for the existence of a novel nonneuronal cold temperature–activated signal transduction pathway in human lung epithelial cells and support the hypothesis that this pathway may play a significant role in cold air–induced changes in airway homeostasis.
A previous study profiling the distribution of TRPM8 transcripts in various tissues failed to detect TRPM8 expression in the lung (25). However, this study employed PCR primers that were designed to amplify exons 11 through 14. Results presented in Figures 1 and 2A demonstrated that this region of the TRPM8 gene was absent in lung epithelial cells and in RNA isolated from the whole lung. Expression of this variant was also detected in human tongue, kidney, colon, liver, and testes mRNA samples based on the amplification of the 593-nt PCR product and an absence or significant decrease in amplification of message corresponding to exons 15 to 18 in these tissues (Figure 2A).
A recent study showed that an ER-specific TRPM8 isoform is expressed in the prostate cell ER membrane, and the constitutive activity and subcellular distribution of full-length TRPM8 was regulated, in part, by the expression of a truncated TRPM8 splice variant (27). Although the exact exon composition of the reported TRPM8 variant was not elucidated, it was shown to lack exons 8 and 9. Since a variant of the TRPM8 channel seems to exist in human prostate cells, the existence of another truncated variant in human lung epithelial cells also seems reasonable.
Indeed, an approximately 1,400-nt TRPM8 variant consisting of exons 18 through the 3′UTR of TRPM8 was detected by PCR in lung epithelial cells. The full-length TRPM8 could not be detected, despite exhaustive efforts. In silico analysis of the truncated TRPM8 cDNA predicted a variant protein product composed of 304 amino acids lacking the N-terminal intracellular domain. The expression of the variant protein was verified by immunohistochemistry using a polyclonal antibody raised against both the N- and C-terminal regions of TRPM8. The predicted translational start site of this variant occurred 2,400 inside the normal TRPM8 open reading frame (Figure 1C). We speculate that the absence of the N-terminal domain in this variant protein could significantly affect its localization, function, and coupling in lung cells such that activation of the TRPM8 variant may regulate novel signal transduction pathways that are not observed in cells that express the full-length TRPM8.
The cytoplasmic N-terminus of TRPM8 contains several TRPM homologous sequences (34). Integrity of the N-terminus seems to be a requisite feature for the trafficking of TRPM8 channels (35). However, the novel TRPM8 variant characterized in this study lacks this domain. Despite the apparent substantial protein levels in lung cells, the lack of an N-terminal region in the variant TRPM8 may explain why the majority of this channel resides within the ER of cells (Figure 4) and why significant differences in EC50 values for menthol-induced ion flux were observed between the transfected CHO cells, lung cells, and DU-145 cells (Figure 5A).
The large intracellular C-terminal domain of TRPM8 is required for TRPM8 subunit interactions (15), and is vital for the protein folding and function, including assembly of functional, tetrameric ion channel complexes (36–38). The TRPM8 variant described herein retains the C-terminal and pore-loop domains, and thus should still assemble into a functional tetrameric channel that interacts with regulatory proteins in response to stimuli. Voets and coworkers (39) demonstrated that residues in TM4 and the TM4–5 linker of TRPM8, which appear to function as a voltage sensor region, are also involved in cold sensation. The TRPM8 variant reported here retains both the TM4 and the TM4–5 linker region, and thus should possess the ability to sense cold temperatures. These predictions were validated in Figures 5B, 6B, and 6D, which show cold temperature–induced Ca2+ flux that was inhibited by BCTC and shRNA-induced knockdown of TRPM8 variant expression.
Mutagenesis studies have also identified Y745 within TM2 (40), Y1005, and L1009, which map to the TRP domain in the C-terminus of TRPM8 (39, 40), and H845, R851, and R862 within the TM4 and the TM4–5 linker (39, 41), as residues critical for menthol activation of TRPM8. Mutations in the TM2 domain resulted in strong shifts in the concentration dependence of menthol activation toward higher menthol concentrations (41). The TRPM8 variant identified in human lung cells lacked the TM2 domain, but retained the other substrate-binding elements required for low-affinity menthol binding and receptor activation. These features may also explain why significantly higher doses of menthol were required to maximally activate the TRPM8 variant in lung cells and in stably transfected TRPM8 variant overexpressing CHO cells (Figures 2B and 5A).
Icilin, the synthetic “super-cooling agent” (21), is structurally unrelated to menthol, and activates TRPM8 by binding with approximately 200-fold greater potency (20) to sites distinct from those of menthol. Chuang and colleagues established that sensitivity of TRPM8 to icilin, but not to cold or menthol, depended up on residues, N799, D802, and G805, located in the cytoplasmic loop connecting TM2 and TM3 and within TM3 of rat TRPM8 (21). The TRPM8 variant described herein lacks TM2, and specifically N799, and thus lacks complete icilin binding motif. This change likely explains the lack of icilin-mediated TRPM8 activation in lung cells and transfected CHO cells, even at concentrations up to 5 mM (data not shown).
Acute or chronic exposure to cold air elicits several deleterious effects on the respiratory system (42–44). Physiologic and pathologic respiratory responses are triggered by exposure to cold air, including pro-inflammatory (often IL-6) cytokine secretion. We postulate that these deleterious effects may be mediated, in part, by TRPM8 variant activity in airway epithelial cells exposed to cold air. Additional cold-responsive TRP receptors probably do not augment these cold-induced responses as TRPA1 mRNA was not detected in the lung cells (data not shown); TRPA1 is the only other known cold-sensing TRP channel (11). Cold-mediated activation of the TRPM8 variant in lung epithelial cells substantially increased the expression of IL-6 and -8 mRNA (Figure 7A), two cytokines known to regulate airway responsiveness and inflammation in a number of disease states. In individuals with asthma, IL-6 and -8 are often elevated and cold air is a notorious stimulus of airway inflammation and “asthma attacks.” These facts suggest the possibility that TRPM8 variant-mediated effects on respiratory physiology may contribute to the enigmatic pathogenesis of cold-induced asthma.
In summary, this study shows the existence of a novel, truncated, functional variant of the cold-sensitive TRPM8 receptor channel in lung epithelial cells. Our results lay the groundwork for future investigations into TRPM8 function in nonneuronal temperature transduction pathways in the lungs. It is therefore intriguing to speculate that this TRPM8 variant may control multiple physiologic and pathologic responses of human airways to cold air exposure.
Acknowledgments
The authors thank Dr. Mark Johansen and Dr. N. Shane Cutler for their helpful suggestions and insightful comments. The authors also acknowledge Diane Lanza for technical assistance with these studies.
Support for this work was provided by a grant from the National Heart, Lung, and Blood Institute (HL069813) to C.A.R.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0440OC on May 5, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Caterina MJ, Montell C. Take a TRP to beat the heat. Genes Dev 2005;19:415–418. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. TRP channels as cellular sensors. Nature 2003;426:517–524. [DOI] [PubMed] [Google Scholar]
- 3.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004;430:748–754. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000;288:306–313. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–824. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999;398:436–441. [DOI] [PubMed] [Google Scholar]
- 7.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002;418:186–190. [DOI] [PubMed] [Google Scholar]
- 8.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 2002;22:6408–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002;416:52–58. [DOI] [PubMed] [Google Scholar]
- 10.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002;108:705–715. [DOI] [PubMed] [Google Scholar]
- 11.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003;112:819–829. [DOI] [PubMed] [Google Scholar]
- 12.Huang CL. The transient receptor potential superfamily of ion channels. J Am Soc Nephrol 2004;15:1690–1699. [DOI] [PubMed] [Google Scholar]
- 13.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol 2006;68:685–717. [DOI] [PubMed] [Google Scholar]
- 14.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 2007;87:165–217. [DOI] [PubMed] [Google Scholar]
- 15.Erler I, Al-Ansary DM, Wissenbach U, Wagner TF, Flockerzi V, Niemeyer BA. Trafficking and assembly of the cold-sensitive TRPM8 channel. J Biol Chem 2006;281:38396–38404. [DOI] [PubMed] [Google Scholar]
- 16.Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 2003;22:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci 2001;2:387–396. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Zhang X, McNaughton PA. Modulation of temperature-sensitive TRP channels. Semin Cell Dev Biol 2006;17:638–645. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer 2006;13:27–38. [DOI] [PubMed] [Google Scholar]
- 20.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (flipr) assay. Br J Pharmacol 2004;141:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 2004;43:859–869. [DOI] [PubMed] [Google Scholar]
- 22.Tsuzuki K, Xing H, Ling J, Gu JG. Menthol-induced Ca2+ release from presynaptic Ca2+ stores potentiates sensory synaptic transmission. J Neurosci 2004;24:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res 2005;136:91–98. [DOI] [PubMed] [Google Scholar]
- 24.Thebault S, Lemonnier L, Bidaux G, Flourakis M, Bavencoffe A, Gordienko D, Roudbaraki M, Delcourt P, Panchin Y, Shuba Y, et al. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in lncap human prostate cancer epithelial cells. J Biol Chem 2005;280:39423–39435. [DOI] [PubMed] [Google Scholar]
- 25.Tsavaler L, Shapero MH, Morkowski S, Laus R. TRP-P8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 2001;61:3760–3769. [PubMed] [Google Scholar]
- 26.Mahieu F, Owsianik G, Verbert L, Janssens A, De Smedt H, Nilius B, Voets T. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and golgi. J Biol Chem 2007;282:3325–3336. [DOI] [PubMed] [Google Scholar]
- 27.Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, Gkika D, Roudbaraki M, Bonnal JL, Mauroy B, Shuba Y, et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest 2007;117:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koskela HO. Cold air-provoked respiratory symptoms: the mechanisms and management. Int J Circumpolar Health 2007;66:91–100. [DOI] [PubMed] [Google Scholar]
- 29.Bidaux G, Roudbaraki M, Merle C, Crepin A, Delcourt P, Slomianny C, Thebault S, Bonnal JL, Benahmed M, Cabon F, et al. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: Functional androgen receptor requirement. Endocr Relat Cancer 2005;12:367–382. [DOI] [PubMed] [Google Scholar]
- 30.Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci 2005;25:11322–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tafesse L, Sun Q, Schmid L, Valenzano KJ, Rotshteyn Y, Su X, Kyle DJ. Synthesis and evaluation of pyridazinylpiperazines as vanilloid receptor 1 antagonists. Bioorg Med Chem Lett 2004;14:5513–5519. [DOI] [PubMed] [Google Scholar]
- 32.Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin rnas (shrnas) induce sequence-specific silencing in mammalian cells. Genes Dev 2002;16:948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res 2004;64:8365–8373. [DOI] [PubMed] [Google Scholar]
- 34.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain 2005;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev 2002;82:429–472. [DOI] [PubMed] [Google Scholar]
- 36.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 2006;26:4835–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dragoni I, Guida E, McIntyre P. The cold and menthol receptor TRPM8 contains a functionally important double cysteine motif. J Biol Chem 2006;281:37353–37360. [DOI] [PubMed] [Google Scholar]
- 38.Tsuruda PR, Julius D, Minor DL Jr. Coiled coils direct assembly of a cold-activated TRP channel. Neuron 2006;51:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 2007;3:174–182. [DOI] [PubMed] [Google Scholar]
- 40.Montell C. A mint of mutations in TRPM8 leads to cool results. Nat Neurosci 2006;9:466–468. [DOI] [PubMed] [Google Scholar]
- 41.Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci 2006;9:493–500. [DOI] [PubMed] [Google Scholar]
- 42.Giesbrecht GG. The respiratory system in a cold environment. Aviat Space Environ Med 1995;66:890–902. [PubMed] [Google Scholar]
- 43.Larsson K, Tornling G, Gavhed D, Muller-Suur C, Palmberg L. Inhalation of cold air increases the number of inflammatory cells in the lungs in healthy subjects. Eur Respir J 1998;12:825–830. [DOI] [PubMed] [Google Scholar]
- 44.Davis MS, Malayer JR, Vandeventer L, Royer CM, McKenzie EC, Williamson KK. Cold weather exercise and airway cytokine expression. J Appl Physiol 2005;98:2132–2136. [DOI] [PubMed] [Google Scholar]