Abstract
Host complement is widely distributed throughout mammalian body fluids and can be activated immediately as part of the first line of defense against invading pathogens. The agent of Lyme disease, Borrelia burgdorferi sensu lato (s.l.), is naturally resistant to that innate immune defense system of its hosts. One resistance mechanism appears to involve binding fluid-phase regulators of complement to distinct borrelial outer surface molecules known as CRASPs (complement regulator acquiring surface proteins). Using sensitive molecular biology techniques, expression patterns of all three classes of genes encoding the CRASPs of B. burgdorferi sensu stricto (BbCRASPs) have been analyzed throughout the natural tick-mammal infection cycle. Each class shows a different expression profile in vivo and the results are summarized herein. Studies on the expression of B. burgdorferi genes using animal models of infection have advanced our knowledge on the ability of the causative agent to circumvent innate immune defenses, the contributions of CRASPs to spirochete infectivity, and the pathogenesis of Lyme disease.
Keywords: Borrelia burgdorferi, Gene regulation, CRASP, Factor H, Tick, Infection cycle
Introduction
Borrelia burgdorferi sensu lato (s.l.) has a complicated enzootic life cycle. To perpetuate, spirochetes depend on a vertebrate host, often a small mammal or a bird, and a vector tick of the genus Ixodes. Those ticks have three postembryonic stages: larva, nymph, and adult, each of which takes only one blood meal. Bacteria from an infected reservoir host may be acquired by feeding larvae, which then colonize the tick midgut and are retained through the molt into the nymph stage. As an infected nymph feeds, spirochetes are transmitted into the blood and skin of the host and subsequently spread into distant host tissues to establish disseminated and persistent infection (Stanek and Strle, 2003; Wormser, 2006). Occasionally, infected ticks feed on humans, which may lead to the development of Lyme disease (Lyme borreliosis). Dissecting molecular mechanisms underlying the ability of B. burgdorferi s.l. to persistently infect immunocompetent mammalian hosts is crucial to understanding Lyme disease pathogenesis and the development of improved therapies to prevent and treat these infections. Components of the host complement system are widely distributed throughout body fluids and can be activated spontaneously to mediate potent responses to infections (Janeway et al., 1999). Complement was originally discovered as a powerful enhancer of antibody-mediated killing, but it also constitutes an important part of innate immunity. Complement can be activated on surfaces of invading organisms through the alternative pathway, before the specific adaptive response develops. By this pathway, spontaneous activation of C3 on cell surfaces triggers a cascade of enzymatic events leading to formation of the membrane-attack complex as well as opsonization and inflammatory response. Yet, like many other blood-borne pathogens, most infectious isolates of B. burgdorferi s.l. are naturally resistant to this arm of the host innate immune defenses (Brade et al., 1992; Breitner-Ruddock et al., 1997; van Dam et al., 1997). B. burgdorferi s.l. produces several different outer surface proteins collectively termed CRASPs (complement regulator-acquiring surface proteins). These lipoproteins share affinities for the host fluid phase negative regulators of complement factor H and/or FHL-1 (factor H-like protein 1) (Hellwage et al., 2001; Kraiczy et al., 2001, 2003, 2004a, 2004b; Alitalo et al., 2002, 2005; Stevenson et al., 2002; McDowell et al., 2003; Hartmann et al., 2006; Kraiczy and Würzner, 2006; Herzberger et al., 2007). Those two host proteins promote breakdown of C3b and inactivation of the alternative pathway C3 convertase (Janeway et al., 1999; Kraiczy and Würzner, 2006). Mice and many other mammals do not produce FHL-1, while humans do, so the ability of CRASPs to bind FHL-1 may play a role in human disease, although not in infection of natural reservoir hosts. Some CRASPs also bind other, similar serum proteins such as FHR-1 (factor H-related protein 1) (Park and Wright, 1996; Hellwage et al., 1999, 2006; Zipfel et al., 2002, 2007; McRae et al., 2005; Haupt et al., 2007).
Genetic analyses of Lyme disease spirochetes led to division of B. burgdorferi s.l. into several genospecies, with names including B. burgdorferi sensu stricto (s.s.), B. garinii, B. spielmanii, B. afzelii, and others (Baranton et al., 1992; LeFleche et al., 1997). Since this review primarily discusses the CRASPs of B. burgdorferi s.s., for clarity in reading we will refer to that organism as B. burgdorferi and to Lyme disease spirochetes in general as B. burgdorferi s.l. The CRASPs produced by each borrelial genospecies are indicated by the genus and species initials, e.g. B. burgdorferi CRASPs are designated BbCRASP, those of B. afzelii are BaCRASP, etc. B. burgdorferi produces up to five different BbCRASPs, which are reviewed herein. At least two of the BbCRASPs contribute to complement resistance in vitro (Brooks et al., 2005; Hartmann et al., 2006) but the question of why B. burgdorferi produces multiple distinct factor H-binding proteins remains unsolved. Confounding matters further, both wild-type and factor H-deficient mice can be infected by Lyme disease spirochetes to equal degrees (Woodman et al., 2007), suggesting that factor H-binding is not essential for efficient mammalian infection.
Those data suggest five, non-exclusive possibilities:
Each class of CRASP is expressed at different times during the spirochete infectious cycle.
Co-expressed CRASPs cooperate with each other in their functions.
Binding of factor H by B. burgdorferi is not the only mechanism used by the Lyme disease spirochete to avoid being killed by complement.
Binding of factor H is not be the only function of BbCRASPs, and therefore not the only mechanism by which BbCRASPs contribute to complement resistance and/or mammalian infection.
Binding of factor H and other, similar host proteins by BbCRASPs serves other, unrelated functions, such as adherence to host tissues.
Studies of B. burgdorferi transmission from infected ticks into mammalian hosts using animal models have greatly advanced our knowledge on the ability of spirochetes to circumvent innate immune defenses. Shedding more light on the regulation of BbCRASP expression during the tick-mammal infection cycle can assist in determination of their additional functions and may help in developing efficient vaccines that will directly target these proteins at the time they are produced. Alternative therapeutic strategies could involve specific disruption of signaling pathways triggering synthesis or coordinating action of BbCRASPs.
BbCRASP-encoding genes
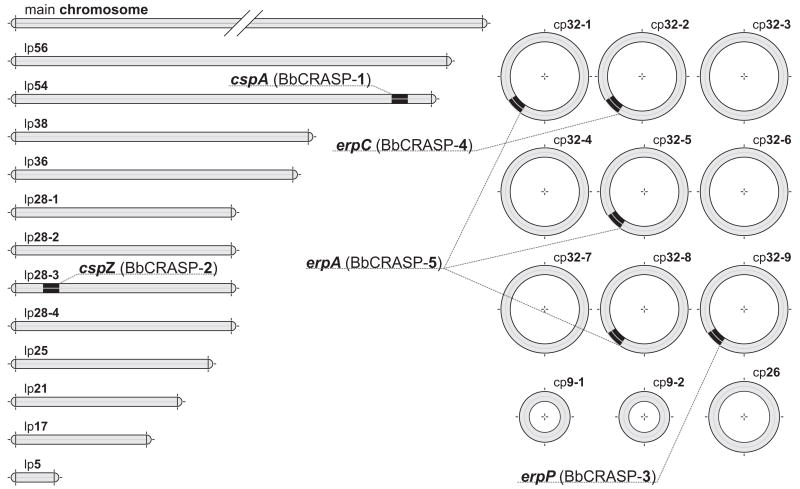
BbCRASP-1 is encoded by cspA, a gene located on the linear DNA replicon lp54 (Fig. 1). Although the genome of every Lyme disease spirochete carries multiple genes that share homology to cspA, only cspA is capable of binding factor H (Fraser et al., 1997; Casjens et al., 2000; Kraiczy et al., 2006). The B. afzelii BaCRASP-1 is also encoded by an orthologous gene, likewise located on its lp54 homolog (Wallich et al., 2005). The cspZ gene, encoding BbCRASP-2, is located on another linear DNA element, lp28-3, but is not closely related to any other gene within the B. burgdorferi genome (Fraser et al., 1997; Casjens et al., 2000; Hartmann et al., 2006). Orthologous genes have been identified in all other Lyme disease spirochete genospecies, although it is not yet clear whether or not the encoded proteins bind factor H and FHL-1 (Rogers and Marconi, 2007, and our unpublished results). BbCRASP-3, -4, and -5 lipoproteins all belong to the Erp paralog family, and their respective genes are named erpP, erpC, and erpA, or, collectively, ospE (Stevenson et al., 1996, 2002; Casjens et al., 2000; Hellwage et al., 2001; Alitalo et al., 2002, 2004; Kraiczy et al., 2003, 2004a; Metts et al., 2003). erp loci are all located on borrelial prophages which replicate as circular episomes known as cp32s (Stevenson et al., 2001, 2006).
Fig. 1.
BbCRASP-encoding genes in the genome of B. burgdorferi strain B31. The segmented genome of B. burgdorferi B31 is composed of a linear main chromosome and at least 24 other linear and circular replicons (Fraser et al., 1997; Casjens et al., 2000; Miller et al., 2000a). Several publications have referred to BbCRASP-encoding genes of the strain B31 by the open reading frame (ORF) numbers assigned following sequencing and annotation of the genome (Casjens et al., 2000). The sequenced B31 subculture had lost cp32-2, cp32-5, and other DNAs, so not all genes known to exist in strain B31 were given ORF numbers. BbCRASP-1 is encoded by cspA (ORF BBA68) gene located on the linear DNA element lp54. BbCRASP-2 is encoded by cspZ (ORF BBH06) carried by another linear replicon, lp28-3. Genes erpP (ORF BBN38, encoding BbCRASP-3) and erpC (encoding BbCRASP-4) are carried by plasmids cp32-9 and cp32-2, respectively. Strain B31 can contain three identical copies of erpA, encoding BbCRASP-5, on prophages cp32-1 (ORF BBP38), cp32-5, and cp32-8 (ORF BBL39).
Tick nymphs and the process of transmission
Bacteria colonizing the midguts of unfed nymphs generally do not produce detectable levels of BbCRASPs (Miller et al., 2003; von Lackum et al., 2005; Bykowski et al., 2007) (Fig. 2). When infected ticks begin to feed, B. burgdorferi begins production of Erp proteins (Miller et al., 2003), but BbCRASP-1 or -2 remain largely undetectable during this time (von Lackum et al., 2005; Bykowski et al., 2007, and our unpublished results). Ingested host complement is ineffective inside the tick midgut, presumably due to components of tick saliva that block complement activation (Ribeiro, 1987; Lawrie et al., 1999; Wikel, 1999; Valenzuela et al., 2000; Rathinavelu et al., 2003; Schroeder et al., 2007).
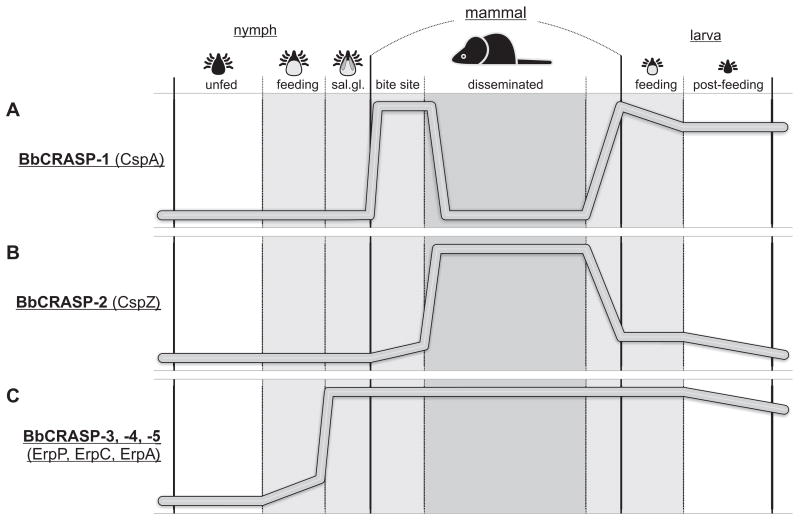
Fig. 2.
Comparison of BbCRASP in vivo expression profiles (relative levels of each BbCRASP or gene transcript) during the mammal-tick infection cycle. (A) BbCRASP-1 likely functions only during stages when bacteria are moving from the infected nymphs to the mammalian host and again to naïve larval ticks (von Lackum et al., 2005; Bykowski et al., 2007). Limited antibody responses to this lipoprotein have been observed (Wallich et al., 2005; McDowell et al., 2006; Rossmann et al., 2006). (B) BbCRASP-2 is produced predominantly during established mammalian infection and induces strong antibody production by the host (Hartmann et al., 2006; Bykowski et al., 2007, and our unpublished results). (C) Erp proteins are produced during all stages between transmission from infected nymphs to acquisition by subsequently feeding larvae (Das et al., 1997; Gilmore et al., 2001; McDowell et al., 2001; Hefty et al., 2002; Liang et al., 2002; Miller et al., 2003, 2005, 2006; Miller and Stevenson, 2006). Infected mammals mount rapid antibody responses to Erp proteins which persist at high levels over several months of infection (Lam et al., 1994; Akins et al., 1995; Suk et al., 1995; Wallich et al., 1995; Stevenson et al., 1998; Miller et al., 2000b; Hefty et al., 2001, 2002; McDowell et al., 2001). Please see text for additional details.
As ticks continue feeding, bacteria cross the gut epithelium, migrate through the hemolymph, target and penetrate the salivary glands, and are deposited into the bite wound with tick saliva (Benach et al., 1987; Ribeiro et al., 1987; Zung et al., 1989). In host dermis at the bite site, almost all bacteria produce detectable levels of BbCRASP-1 and all examined Erp proteins (Miller et al., 2003; von Lackum et al., 2005). However, only a small percentage of bacteria produce detectable BbCRASP-2 levels in skin at the tick bite site (Bykowski et al., 2007).
Disseminated mammalian infection
At the time of disseminated mammalian infection, B. burgdorferi resides extracellularly at low densities in a variety of tissues (Schwan et al., 1999), making it very difficult to directly examine bacterial protein production. However, at this stage transcript levels can be assessed by the highly sensitive method of quantitative RT-PCR (Miller, 2005). Analyses of antibody production can also serve to assess borrelial protein expression during mammalian infection. Transcripts from erp genes have been detected in various tissues of infected laboratory animals, including non-human primates, throughout the course of disseminated and persistent infection (Miller et al., 2003, 2005; Miller and Stevenson, 2006). B. burgdorferi-infected mice mount rapid antibody responses to Erp proteins (Lam et al., 1994; Akins et al., 1995; Suk et al., 1995; Wallich et al., 1995; Stevenson et al., 1998; Miller et al., 2000b; Hefty et al., 2001, 2002; McDowell et al., 2001). Erp-directed antibodies persist at high levels and periodically increase during prolonged infection, suggesting sustained exposure (and re-exposure) to Erp proteins throughout chronic infection (Miller et al., 2003).
In contrast, cspA transcripts become undetectable within two weeks of establishing mammalian infection (Wallich et al., 2003; Lederer et al., 2005; McDowell et al., 2006; Bykowski et al., 2007). Humans and laboratory mice produce limited antibody responses to BbCRASP-1, consistent with brief exposure of that protein to host immune systems (McDowell et al., 2006; Rossmann et al., 2006).
By two weeks of mammalian infection, transcription of cspZ increases dramatically and is significantly higher than in bacteria residing in ticks or during laboratory cultivation (Bykowski et al., 2007). Humans and laboratory animals infected with B. burgdorferi produce robust antibody responses to BbCRASP-2, also indicating substantial production of that protein during vertebrate infection (Hartmann et al., 2006, and our unpublished results).
Acquisition of bacteria by larval ticks
Almost all of the bacteria acquired by feeding larvae produce BbCRASP-1, indicating that cspA expression is re-stimulated during the mammal-tick transmission stage (von Lackum et al., 2005). BbCRASP-2 protein is expressed in low abundance at this time, as cspZ undergoes transcriptional repression (Bykowski et al., 2007). Erp proteins are produced by essentially all recently-acquired borreliae in the feeding larval midgut. Following completion of larval feeding and detachment from the host, B. burgdorferi reduces levels of all BbCRASPs (Miller et al., 2003; von Lackum et al., 2005; Bykowski et al., 2007)
Molecular mechanisms underlying expression of BbCRASPs
Transcription start sites for cspA and cspZ have not yet been mapped, and the sequences upstream from open reading frames show no obvious similarities to each other or to erp loci. All the erp coding sequences, including erpA, erpC, and erpP, are preceded by almost identical 5′-non-coding sequences, including binding sites for at least 3 distinct DNA-binding proteins (Babb et al., 2004, 2006). Co-regulation of erp genes on different cp32 plasmids suggests the existence of similar molecular mechanisms that could coordinate expression of genes encoding BbCRASP-3, -4, and -5 at the transcriptional level (Stevenson et al., 1998; Babb et al., 2001; El-Hage and Stevenson, 2002; Babb et al., 2004, 2006).
Potential for direct or indirect effects on the synthesis of BbCRASPs by the two B. burgdorferi alternative RNA polymerase sigma factors RpoS (σS) and RpoN (NtrA, σ54), has recently been ruled out (Bykowski et al., 2007). All data indicate that each of the BbCRASP-encoding genes are transcribed using the housekeeping sigma, RpoD (σ70) and that the alternative sigma factors do not directly influence expression of these genes.
Conclusions
Three types of genetically distinct but functionally related B. burgdorferi factor H-binding lipoproteins have been described to date. Each class has a distinct expression pattern during the mammal-tick infection cycle. BbCRASP-1 is produced exclusively during stages of bacterial transmission from mammal to tick and vice versa, while BbCRASP-2 is produced during established mammalian infection. Erp proteins are up-regulated when nymphal ticks begin to feed on mammals, are produced at all stages of mammalian infection, and are then repressed following acquisition by feeding ticks. All other genospecies of Lyme disease spirochetes contain cp32 prophage elements and erp genes, with at least some encoded proteins sharing the ability to bind factor H (our unpublished results). These erp operons contain the conserved 5′-non-coding regions as do the B. burgdorferi erp loci, suggesting that they also share the same expression patterns. Likewise, it is likely that the BaCRASP-1-encoding gene of B. afzelii has an expression profile similar to that of its B. burgdorferi ortholog, due to similarities between those genes’ promoter elements (Wallich et al., 2005). As noted above, other genospecies possess orthologs of cspZ, but the 5′-non-coding regions of cspZ genes identified in strains of B. garinii are significantly different from those of B. burgdorferi, so it is very possible that these genes are controlled through different mechanisms (our unpublished results). Those differences indicate that continued studies of CRASPs produced by the many different types of Lyme disease spirochetes are warranted. Since factor H-deficient and wild type mice can be infected with B. burgdorferi to essentially identical levels, the possible binding of factor H to the surface of bacteria may not be the only mechanism employed to avoid complement-mediated killing (Woodman et al., 2007). Progress in dissecting other mechanisms of B. burgdorferi complement resistance, defining novel ligands or roles for each BbCRASP, and studies on interactions between factor H-binding proteins may bring novel explanations for the diverse BbCRASP regulation patterns. Completing these analyses will have a significant impact on our understanding of the infectious properties of Lyme disease spirochetes.
Acknowledgments
Research in our laboratories is funded by US National Institutes of Health grant R01-AI44254 to B. Stevenson, Deutsche Forschungsgemeinschaft grant Kr3383/1-1 to P. Kraiczy, and Deutsche Forschungsgemeinschaft grant Wa533/7-1 to R. Wallich.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akins DR, Porcella SF, Popova TG, Shevchenko D, Baker SI, Li M, Norgard MV, Radolf JD. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- Alitalo A, Meri T, Lankinen H, Seppälä I, Lahdenne P, Hefty PS, Akins D, Meri S. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J Immunol. 2002;169:3847–3853. doi: 10.4049/jimmunol.169.7.3847. [DOI] [PubMed] [Google Scholar]
- Alitalo A, Meri T, Chen T, Lankinen H, Cheng Z-Z, Jokiranta TS, Seppälä IJT, Lahdenne P, Hefty PS, Akins DR, Meri S. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J Immunol. 2004;172:6195–6201. doi: 10.4049/jimmunol.172.10.6195. [DOI] [PubMed] [Google Scholar]
- Alitalo A, Meri T, Comstedt P, Jeffery L, Tornberg J, Strandin T, Lankinen H, Bergström S, Cinco M, Vuppala SR, Akins DR, Meri S. Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur J Immunol. 2005;35:3043–3053. doi: 10.1002/eji.200526354. [DOI] [PubMed] [Google Scholar]
- Babb K, El-Hage N, Miller JC, Carroll JA, Stevenson B. Distinct regulatory pathways control the synthesis of Borrelia burgdorferi infection-associated OspC and Erp surface proteins. Infect Immun. 2001;69:4146–4153. doi: 10.1128/IAI.69.6.4146-4153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb K, McAlister JD, Miller JC, Stevenson B. Molecular characterization of Borrelia burgdorferi erp promoter/operator elements. J Bacteriol. 2004;186:2745–2756. doi: 10.1128/JB.186.9.2745-2756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb K, Bykowski T, Riley SP, Miller JC, DeMoll E, Stevenson B. Borrelia burgdorferi EbfC, a novel, chromosomally-encoded protein, binds specific DNA sequences adjacent to erp loci on the spirochete’s resident cp32 prophages. J Bacteriol. 2006;188:4331–4339. doi: 10.1128/JB.00005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti JC, Assous M, Grimont PAD. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Benach JL, Coleman JL, Skinner RA, Bosler EM. Adult Ixodes dammini on rabbits: a hypothesis for the development and transmission of Borrelia burgdorferi. J Infect Dis. 1987;155:1300–1306. doi: 10.1093/infdis/155.6.1300. [DOI] [PubMed] [Google Scholar]
- Brade V, Kleber I, Acker G. Differences of two Borrelia burgdorferi strains in complement activation and serum resistance. Immunobiology. 1992;185:453–465. doi: 10.1016/S0171-2985(11)80087-2. [DOI] [PubMed] [Google Scholar]
- Breitner-Ruddock S, Würzner R, Schulze J, Brade V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med Microbiol Immunol. 1997;185:253–260. doi: 10.1007/s004300050038. [DOI] [PubMed] [Google Scholar]
- Brooks CS, Vuppala AM, Jett AM, Alitalo A, Meri S, Akins DR. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol. 2005;175:3299–3308. doi: 10.4049/jimmunol.175.5.3299. [DOI] [PubMed] [Google Scholar]
- Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, Kraiczy P, Stevenson B. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete’s mammal-tick infection cycle. Infect Immun. 2007;75:4227–4236. doi: 10.1128/IAI.00604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser C. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- Das S, Barthold SW, Stocker Giles S, Montgomery RR, Telford SR, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Invest. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Stevenson B. Simultaneous coexpression of Borrelia burgdorferi Erp proteins occurs through a specific, erp locus-directed regulatory mechanism. J Bacteriol. 2002;184:4536–4543. doi: 10.1128/JB.184.16.4536-4543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidmann J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Gilmore RD, Jr, Mbow ML, Stevenson B. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 2001;3:799–808. doi: 10.1016/s1286-4579(01)01435-6. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, Miller JC, Stevenson B, Wallich R, Zipfel PF, Kraiczy P. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol. 2006;61:1220–1236. doi: 10.1111/j.1365-2958.2006.05318.x. [DOI] [PubMed] [Google Scholar]
- Haupt K, Wallich R, Kraiczy P, Brade V, Skerka C, Zipfel PF. Binding of human FHR-1 to serum resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J Infect Dis. 2007;196:124–133. doi: 10.1086/518509. [DOI] [PubMed] [Google Scholar]
- Hefty PS, Jolliff SE, Caimano MJ, Wikel SK, Radolf JD, Akins DR. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect Immun. 2001;69:3618–3627. doi: 10.1128/IAI.69.6.3618-3627.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefty PS, Brooks CS, Jett AM, White GL, Wikel SK, Kennedy RC, Akins DR. OspE-related, OspF-related, and Elp lipoproteins are immunogenic in baboons experimentally infected with Borrelia burgdorferi and in human Lyme disease patients. J Clin Microbiol. 2002;40:4256–4265. doi: 10.1128/JCM.40.11.4256-4265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwage J, Jokiranta TS, Koistinen V, Vaarala O, Meri S, Zipfel PF. Functional properties of complement factor H-related proteins FHR-3 and FHR-4: binding to the C3d region of C3b and differential regulation by heparin. FEBS Lett. 1999;462:345–352. doi: 10.1016/s0014-5793(99)01554-9. [DOI] [PubMed] [Google Scholar]
- Hellwage J, Meri T, Heikkilä T, Alitalo A, Panelius J, Lahdenne P, Seppälä IJT, Meri S. The complement regulatory factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem. 2001;276:8427–8435. doi: 10.1074/jbc.M007994200. [DOI] [PubMed] [Google Scholar]
- Hellwage J, Eberle F, Babuke T, Seeberger H, Richter H, Kunert A, Hartl A, Zipfel PF, Jokiranta TS, Józsi M. Two factor H-related proteins from the mouse: expression, analysis and functional characterization. Immunogenetics. 2006;58:883–893. doi: 10.1007/s00251-006-0153-y. [DOI] [PubMed] [Google Scholar]
- Herzberger P, Siegel C, Skerka C, Fingerle V, Schulte-Spechtel U, van Dam A, Wilske B, Brade V, Zipfel PF, Wallich R, Kraiczy P. Human pathogenic Borrelia spielmanii sp nov resist complement-mediated killing by direct binding of immune regulators factor H and FHL-1. Infect Immun. 2007;75:4817–4825. doi: 10.1128/IAI.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Capra JD. Immunobiology. 4. Elsevier Science Ltd; New York: 1999. [Google Scholar]
- Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel PF. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur J Immunol. 2001;31:1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::aid-immu1674>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Hellwage J, Skerka C, Kirschfink M, Brade V, Zipfel PF, Wallich R. Immune evasion of Borrelia burgdorferi: mapping of a complement inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur J Immunol. 2003;33:697–707. doi: 10.1002/eji.200323571. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Hartmann K, Hellwage J, Skerka C, Brade V, Zipfel PF, Wallich R, Stevenson B. Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int J Med Microbiol. 2004a;293(Suppl 37):152–157. doi: 10.1016/s1433-1128(04)80029-9. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Hellwage J, Skerka C, Becker H, Kirschfink M, Simon MM, Brade V, Zipfel PF, Wallich R. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J Biol Chem. 2004b;279:2421–2429. doi: 10.1074/jbc.M308343200. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Rossmann E, Brade V, Simon MM, Skerka C, Zipfel PF, Wallich R. Binding of human complement regulators FHL-1 and factor H to CRASP-1 orthologs of Borrelia burgdorferi. Wien Klin Wochenschr. 2006;11:669–676. doi: 10.1007/s00508-006-0691-1. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Würzner R. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol Immunol. 2006;43:31–44. doi: 10.1016/j.molimm.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Lam TT, Nguyen TPK, Montgomery RR, Kantor FS, Fikrig E, Flavell RA. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie CH, Randolph SE, Nuttall PA. Ixodes ticks: serum species sensitivity of anticomplement activity. Exp Parasitol. 1999;93:207–214. doi: 10.1006/expr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Lederer S, Brenner C, Stehle T, Gern L, Wallich R, Simon MM. Quantitative analysis of Borrelia burgdorferi gene expression in naturally (tick) infected mouse strains. Med Microbiol Immunol. 2005;194:81–90. doi: 10.1007/s00430-004-0218-1. [DOI] [PubMed] [Google Scholar]
- LeFleche A, Postic D, Girardet K, Péter O, Baranton G. Characterization of Borrelia lusitaniae sp nov by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002;196:275–280. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Sung SY, Price G, Marconi RT. Demonstration of the genetic stability and temporal expression of select members of the Lyme disease spirochete OspF protein family during infection in mice. Infect Immun. 2001;69:4831–4838. doi: 10.1128/IAI.69.8.4831-4838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Wolfgang J, Tran E, Metts MS, Hamilton D, Marconi RT. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect Immun. 2003;71:3597–3602. doi: 10.1128/IAI.71.6.3597-3602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Hovis KM, Zhang H, Tran E, Lankford J, Marconi RT. Evidence that BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect Immun. 2006;74:3030–3034. doi: 10.1128/IAI.74.5.3030-3034.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae JL, Duthy TG, Griggs KM, Ormsby RJ, Cowan PJ, Cromer BA, McKinstry WJ, Parker MW, Murphy BF, Gordon DL. Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J Immunol. 2005;174:6250–6256. doi: 10.4049/jimmunol.174.10.6250. [DOI] [PubMed] [Google Scholar]
- Metts MS, McDowell JV, Theisen M, Hansen PR, Marconi RT. Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection. Infect Immun. 2003;71:3587–3596. doi: 10.1128/IAI.71.6.3587-3596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, Bono JL, Babb K, El-Hage N, Casjens S, Stevenson B. A second allele of erpA in Borrelia burgdorferi strain B31 is located on the previously undetected circular plasmid cp9–2. J Bacteriol. 2000a;182:6254–6258. doi: 10.1128/jb.182.21.6254-6258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, El-Hage N, Babb K, Stevenson B. Borrelia burgdorferi B31 Erp proteins that are dominant immunoblot antigens of animals infected with isolate B31 are recognized by only a subset of human Lyme disease patient sera. J Clin Microbiol. 2000b;38:1569–1574. doi: 10.1128/jcm.38.4.1569-1574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, von Lackum K, Babb K, McAlister JD, Stevenson B. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect Immun. 2003;71:6943–6952. doi: 10.1128/IAI.71.12.6943-6952.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC. Example of real-time quanititative reverse transcription-PCR (Q-RT-PCR) analysis of bacterial gene expression during mammalian infection: Borrelia burgdorferi in mouse tissues. In: Coico R, Kowalik T, Quarles J, Stevenson B, Taylor R, editors. Current Protocols in Microbiology. Wiley; Hoboken: 2005. p. 1D.3. [DOI] [PubMed] [Google Scholar]
- Miller JC, Naryan K, Stevenson B, Pachner AR. Expression of Borrelia burgdorferi erp genes during infection of non-human primates. Microb Pathog. 2005;39:27–33. doi: 10.1016/j.micpath.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Miller JC, Stevenson B. Borrelia burgdorferi erp genes are expressed at different levels within tissues of chronically infected mammalian hosts. Int J Med Microbiol. 2006;296(Suppl 40):185–194. doi: 10.1016/j.ijmm.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Miller JC, von Lackum K, Woodman ME, Stevenson B. Detection of Borrelia burgdorferi gene expression during mammalian infection using transcriptional fusions that produce green fluorescent protein. Microb Pathog. 2006;41:43–47. doi: 10.1016/j.micpath.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Park CT, Wright SD. Plasma lipopolysaccharide-binding protein is found associated with a particle containing apolipoprotein A-I, phospholipid, and factor H-related proteins. J Biol Chem. 1996;271:18054–18060. doi: 10.1074/jbc.271.30.18054. [DOI] [PubMed] [Google Scholar]
- Rathinavelu S, Broadwater A, de Silva AM. Does host complement kill Borrelia burgdorferi within ticks? Infect Immun. 2003;71:822–829. doi: 10.1128/IAI.71.2.822-829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JMC. Ixodes dammini: salivary anti-complement activity. Exp Parasitol. 1987;64:347–353. doi: 10.1016/0014-4894(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC, Mather TN, Piesman J, Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae) J Med Entomol. 1987;24:201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- Rogers EA, Marconi RT. Delineation of species-specific binding properties of the CspZ protein (BBH06) of the Lyme disease spirochetes: Evidence for new contributions to Borrelia pathogenesis. Infect Immun. 2007 doi: 10.1128/IAI.00850-07. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann E, Kitiratschky V, Hofmann H, Kraiczy P, Simon MM, Wallich R. Borrelia burgdorferi complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes is expressed in humans and induces antibody responses restricted to nondenatured structural determinants. Infect Immun. 2006;74:7024–7028. doi: 10.1128/IAI.01028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H, Daix V, Gillet L, Renauld JC, Vanderplasschen A. The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes and Infection. 2007;9:247–250. doi: 10.1016/j.micinf.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Burgdorfer W, Rosa PA. Borrelia. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. American Society for Microbiology; Washington, DC: 1999. pp. 746–758. [Google Scholar]
- Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;362:1639–1647. doi: 10.1016/S0140-6736(03)14798-8. [DOI] [PubMed] [Google Scholar]
- Stevenson B, Tilly K, Rosa PA. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Bono JL, Schwan TG, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Zückert WR, Akins DR. Repetition, conservation, and variation: the multiple cp32 plasmids of Borrelia species. In: Saier MH, García-Lara J, editors. The Spirochetes: Molecular and Cellular Biology. Horizon Press; Oxford: 2001. pp. 87–100. [Google Scholar]
- Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun. 2002;70:491–497. doi: 10.1128/IAI.70.2.491-497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Bykowski T, Cooley AE, Babb K, Miller JC, Woodman ME, von Lackum K, Riley SP. The Lyme disease spirochete Erp lipoprotein family: structure, function and regulation of expression. In: Cabello FC, Godfrey HP, Hulinska D, editors. Molecular Biology of Spirochetes. IOS Press; Amsterdam: 2006. pp. 354–372. [Google Scholar]
- Suk K, Das S, Sun W, Jwang B, Barthold SW, Flavell RA, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JG, Charlab R, Mather TN, Ribeiro JMC. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- van Dam AP, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, Dankert J. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun. 1997;65:1228–1236. doi: 10.1128/iai.65.4.1228-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lackum K, Miller JC, Bykowski T, Riley SP, Woodman ME, Brade V, Kraiczy P, Stevenson B, Wallich R. Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect Immun. 2005;73:7398–7405. doi: 10.1128/IAI.73.11.7398-7405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R, Brenner C, Kramer MD, Simon MM. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R, Jahraus O, Stehle T, Tran TT, Brenner C, Hofmann H, Gern L, Simon MM. Artificial-infection protocols allow immunodetection of novel Borrelia burgdorferi antigens suitable as vaccine candidates against Lyme disease. Eur J Immunol. 2003;33:708–719. doi: 10.1002/eji.200323620. [DOI] [PubMed] [Google Scholar]
- Wallich R, Pattathu J, Kitiratschky V, Brenner C, Zipfel PF, Brade V, Simon MM, Kraiczy P. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect Immun. 2005;73:2351–2359. doi: 10.1128/IAI.73.4.2351-2359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK. Tick modulation of host immunity: an important factor in pathogen transmission. Int J Parasitol. 1999;29:851–859. doi: 10.1016/s0020-7519(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Woodman ME, Cooley AE, Miller JC, Lazarus JJ, Tucker K, Bykowski T, Botto M, Hellwage J, Wooten RM, Stevenson B. Borrelia burgdorferi binding of host complement regulator factor H is not required for efficient mammalian infection. Infect Immun. 2007;75 doi: 10.1128/IAI.01923–01906. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP. Hematogenous dissemination in early Lyme disease. Wien Klin Wochenschr. 2006;118:634–637. doi: 10.1007/s00508-006-0688-9. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, Kraiczy P, Noris M, Remuzzi G. Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans. 2002;30:971–978. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Edey M, Heinen S, Józsi M, Richter H, Misselwitz J, Hoppe B, Routledge D, Strain L, Hughes AE, Goodship JA, Licht C, Goodship THJ, Skerka C. Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genetics. 2007;3:e41. doi: 10.1371/journal.pgen.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung JL, Lewengrub S, Rudzinska MA, Spielman A, Telford SR, Piesman J. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini. Can J Zool. 1989;67:1737–1748. [Google Scholar]