Abstract
The Compress® implant (Biomet, Warsaw, IN) is an innovative device developed to enable massive endoprosthetic fixation through the application of compressive forces at the bone-implant interface. This design provides immediate, stable anchorage and helps to avoid the long-term complication of aseptic loosening secondary to stress shielding and particle-induced osteolysis seen in conventional, stemmed megaprostheses. The purpose of our study was to evaluate the in vivo biological effects of the high compressive forces attained. Twelve consecutive Compress® patients undergoing revision surgery for infection, periprosthetic fracture, or local tumour recurrence were reviewed in order to exclude the possibility of osteonecrosis at the prosthetic interface. Compressive forces ranged from 400–800 lb. Duration of implantation averaged 3.3 years (range 0.4–12.2 years). Two patients with infection demonstrated loosening at the bone-prosthetic interface; otherwise, there was no radiographic evidence of prosthetic failure in any of the patients. No patient demonstrated histological evidence of osteonecrosis. In fact, new woven bone and other findings consistent with viable bone were noted in all of the retrieved specimens.
Résumé
La prothèse Compress® (Biomet, Warsaw, In) est une endo-prothèse massive, innovante, développée pour permettre une fixation avec des forces de compression au niveau des interfaces os-implant. Le dessin de l’implant permet une stabilité immédiate au niveau de l’ancrage et semble éviter des complications, à long terme, comme le descellement aseptique, secondaire à un stress shielding rencontré de façon habituelle dans les méga prothèses. Le but de cette étude est d’évaluer les effets biologiques in vivo de ces forces de compression. 11 prothèses consécutives de type Compress® ont été réalisées chez 11 patients, nécessitant une réintervention pour infection, pour fracture périprothétique ou pour récidive d’une tumeur locale. Les forces de compression ont été évaluées de 400 à 800 lb. Le temps d’implantation moyen a été de 2.5 ans (0.4 à 6.5 ans). Deux patients ont présenté un descellement infectieux à l’interface os-prothèse, il n’a pas été mis en évidence, sur le plan radiographique d’échecs de cet implant chez aucun des patients. Aucun patient n’a également montré de façon évidente des phénomènes d’ostéonécrose histologique et, l’analyse des prothèses explantées a montré qu’il existait au contact de celles-ci un os vivant.
Introduction
Compress® technology has been developed in order to facilitate long-term osseointegration of massive endoprostheses used in oncological limb salvage reconstructions. A series of stacked belleville washers provides spring-like compressive force at the bone-prosthetic interface, resulting in immediate, stable fixation of the implant. The use of a medullary device of relatively low stiffness allows for direct stress transfer to the bone during normal cyclical loading, and this fact, together with ongoing compliant force delivered to the bone interface, results in bone hypertrophy according to Wolff’s law [1, 4]. With the canal thus sealed from inflammatory particulate debris, osteolysis is avoided (Figs. 1 and 2).
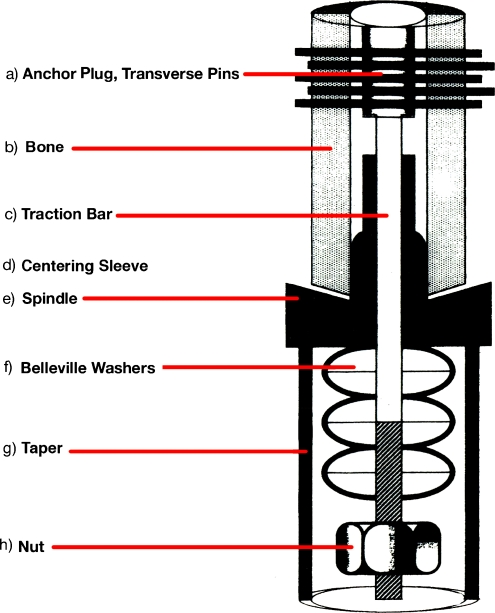
Fig. 1.
Schematic diagram of the Compress® implant, demonstrating belleville washers stacked over a traction bar housed within the endoprosthetic taper at the bone-prosthetic interface
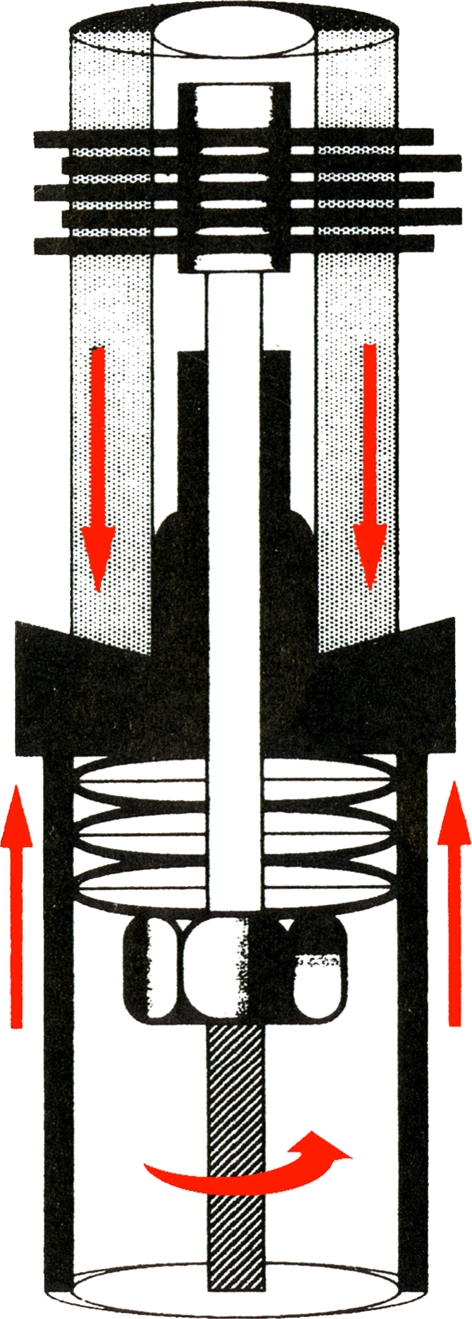
Fig. 2.
Graphic illustration of the means by which immediate, stable fixation is achieved at the bone-prosthetic interface, as a nut is tightened against the belleville washers
Initial clinical results of the Compress® implant have been very encouraging, with early follow-up showing no difference in prosthetic survivorship when comparison is made to patients with conventional cemented prostheses [2]. A multi-institutional prospective cohort study of the Compress® distal femoral device resulted in federal Food and Drug Administration approval in December 2003. In the course of that study, reviewers questioned whether the high compressive forces (400–800 lb) employed would result in osteonecrosis at the bone-prosthetic interface, and ultimately, in prosthetic failure. An early report of a single retrieval specimen showed stable fixation of the Compress® implant [3]. This paper was undertaken in order to address concerns regarding osteonecrosis and failure by reviewing a larger cohort with Compress® implants removed for reasons of infection, periprosthetic fracture, or local recurrence.
Methods
The research protocol was approved by the institution’s Committee on Human Research. All operations were undertaken at a single academic medical centre from September 2002 through March 2007. The orthopaedic oncology database was examined in order to identify implant retrievals performed for reasons of infection, local tumour recurrence, or fracture. The medical records of these patients were assessed in order to collect demographic data, including patient age, sex, initial surgical indication, location of the Compress® implant, compression force, and reason for retrieval. From this data, implantation duration was calculated.
Plain radiographs (AP and lateral views) of the affected extremity obtained prior to retrieval were analysed for evidence of osseointegration versus prosthetic loosening. Signs of osseointegration included bone hypertrophy at the prosthetic interface, extracortical bridging, and absence of radiolucent lines. Radiographs were interpreted by staff radiologists as well as by the attending orthopaedic surgeon.
In each case, representative full cross-sections of the bone at the level of the bone-prosthetic interface were fixed, decalcified and submitted for routine histological analysis. Formalin-fixed, paraffin-embedded 4-μm sections were stained with haematoxylin and eosin. The bone-prosthetic interface was examined for the presence of intact osteocytes, osteoblast nuclei, bone marrow, elements of new woven bone, recurrent neoplasm, and signs of infection. These components were interpreted by an attending pathologist with expertise in musculoskeletal pathology.
Results
The database review identified 11 patients and 12 consecutive Compress® retrieval specimens. One patient (implants 5 and 6) underwent removal of ipsilateral distal femoral and proximal tibial Compress® implants with a Burstein-Lane II constrained knee replacement [7]. There were six male and five female patients. Initial indications for Compress® placement included osteosarcoma (5), failed tumour implant (3), and others (one each of failed arthroplasty, arthrodesis takedown, septic arthritis, and recurrent sarcoma). Compress® locations included distal femur (8), proximal tibia (2), proximal femur (1), and proximal humerus (1). Reasons for retrieval included prosthetic infection (9), local tumour recurrence (2), and periprosthetic fracture (1). Patient age at the time of retrieval averaged 37 years (range 10–65). Compression forces at the bone-prosthetic interface were 400 lbs (5), 600 lbs (2), and 800 lbs (5). Duration of implantation averaged 3.3 years (range 0.4–12.2) (Table 1).
Table 1.
Patient data
| Implant | Indication for Compress® implant | Location of Compress® implant | Reason for retrieval | Age at time of retrieval (years) | Compression force (lb) | Duration of implantation (years) |
|---|---|---|---|---|---|---|
| 1 | Failed oncology implant | Distal femur | Infection | 50 | 400 | 5.3 |
| 2 | Osteosarcoma | Distal femur | Fracture | 17 | 800 | 1.7 |
| 3 | Osteosarcoma | Distal femur | Infection | 10 | 400 | 1.1 |
| 4 | Failed total hip replacement | Distal femur | Infection | 64 | 400 | 1.3 |
| 5 | Knee arthrodesis takedown | Distal femur | Infection | 43 | 800 | 3.1 |
| 6 | Failed oncology implant | Proximal tibia | Infection | 43 | 600 | 0.4 |
| 7 | Septic prosthetic arthritis | Distal femur | Infection | 21 | 800 | 0.8 |
| 8 | Recurrent chondrosarcoma | Proximal femur | Tumor recurrence | 59 | 800 | 2.6 |
| 9 | Failed oncology implant | Distal femur | Infection | 65 | 800 | 6.5 |
| 10 | Osteosarcoma | Proximal humerus | Tumor recurrence | 15 | 600 | 1.2 |
| 11 | Osteosarcoma | Proximal tibia | Infection | 15 | 400 | 3.2 |
| 12 | Osteosarcoma | Distal femur | Infection | 44 | 400 | 12.2 |
| Average (range) | 37 (10–65) | 600 (400–800) | 3.3 (0.4–12.2) |
Radiographic review revealed two patients (implants 7 and 11) with loosening at the bone-prosthetic interface secondary to infection. Otherwise, all implants demonstrated evidence of bone hypertrophy at the prosthetic interface, signifying stable fixation; there were no signs of osteolysis such as radiolucent lines or endosteal scalloping (Fig. 3).
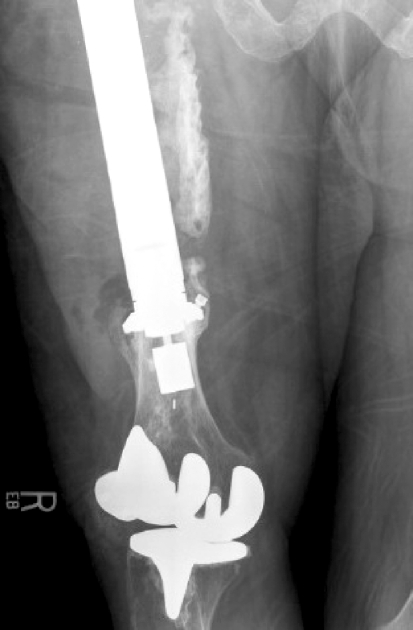
Fig. 3.
AP femoral radiograph demonstrating stable compressive osseointegration with hypertrophic bone formation at the interface of implant 4, retrieved for reasons of prosthetic infection 1.3 years after a 64-year-old woman underwent revision of a failed long-stem total hip replacement with the Compress® device. The radiograph demonstrates a stable pattern of osseointegration despite infection. The versatility of the Compress® implant is revealed by the ability to successfully fix megaprostheses to an extremely short distal femoral metaphyseal segment above a pre-existing total knee replacement
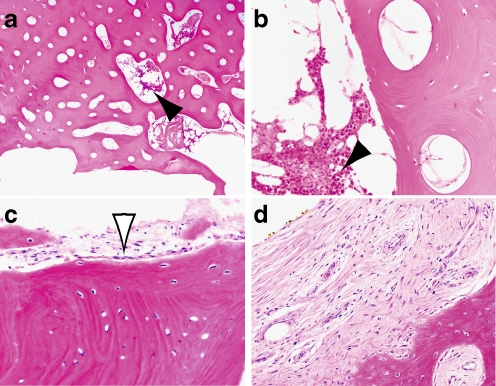
In the remaining 10 cases, gross pathological inspection at the time of retrieval revealed exuberant new bone formation at the bone-prosthetic interface, and evidence of solid osseointegration (Fig. 4). Histological review confirmed the ongoing process of osseointegration, including signs of newly formed woven bone, Haversian canals, intact trabeculae, osteocytes in lacunae, and osteoblasts rimming intraosseous vessels (Fig. 5). No specimen manifested any signs of osteonecrosis. All specimens retrieved for the reason of deep prosthetic infection showed evidence of acute inflammation. However, even implants 7 and 11, which demonstrated preoperative radiographic and intraoperative gross evidence of loosening, showed no osteonecrosis.
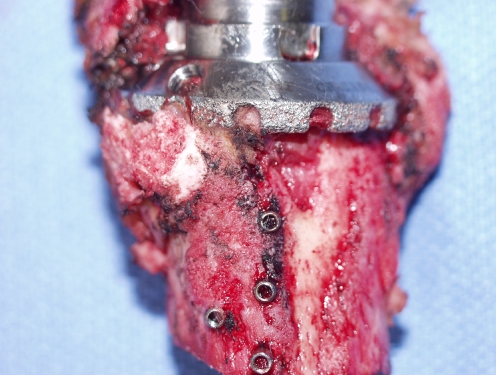
Fig. 4.
Photograph of anterior aspect of distal femoral retrieval specimen (implant 4) demonstrating gross evidence of new bone formation and stable, solid osseointegration at bone-prosthetic interface
Fig. 5.
Histology of bone at prosthetic interface from three patients (a, b patient 8, c patient 2, d patient 3). Viable osteocytes are diffusely present in all three patients immediately adjacent to the medullary cavity. Viable bone marrow (solid arrowheads) and osteoblastic rimming (open arrowhead) are demonstrated around intraosseous vessels. Woven bone and loose fibrosis was also identified at the interface (d). Haematoxylin and eosin; original magnification 40× (a) and 200× (b–d)
Discussion
Bone is a living organ and tissue that responds to mechanical stress in a biological fashion according to Wolff’s Law [6]. It has long been recognised in the field of orthopaedic traumatology that healing of fractures can be hastened by the application of compressive force via plate osteosynthesis [11]. In the field of orthopaedic oncology, as in arthroplasty, the challenge for the past 30 years has been to achieve stable long-term endoprosthetic fixation in the face of aseptic loosening secondary to stress shielding and particle-induced inflammation [8, 10, 12]. Compress® technology was developed in an attempt to capitalise on the healing response of bone to compressive force. Insertion of a medullary anchoring component of comparatively low stiffness allows stress transfer directly to the bone-prosthetic interface during normal cyclical loading, thus obviating stress-shielding encountered with conventional (cemented and uncemented) stemmed devices. In addition, the application of axial compressive force by the stacked belleville washer spring provides not only immediate stability, but also induces bone hypertrophy at the prosthetic interface [3, 4].
During the initial phases of Compress® clinical trials, some reviewers expressed concern that the high compressive forces (400–800 lb) generated by the device would prohibitively increase the risk of osteonecrosis at the bone-prosthetic interface. However, the Compress® implant was designed according to what is known regarding the predictable manner in which bone responds to applied force [5, 9]. Furthermore, both anecdotal and empirical clinical evidence suggests that the Compress® device fails for reasons of aseptic loosening infrequently, and that the results are comparable to those with cemented stems, at least in the short term [2]. For this reason, the retrieval specimens obtained to date have been obtained largely for reasons of infection, local recurrence, and periprosthetic fracture, rather than for aseptic loosening. In reporting on a single retrieval specimen, Bini et al. demonstrated stable Compress® osseointegration using backscatter electron microscopy [3]. Our study was undertaken to report on the outcomes with a much larger number of Compress® retrieval specimens, so as to determine histological evidence of osseointegration versus osteonecrosis at the bone-prosthetic interface.
None of the 12 Compress® specimens reported here demonstrated osteonecrosis. In fact, all bone-prosthetic interfaces were characterised by viable bone, even in two patients with prosthetic loosening secondary to infection. Thus, axial compressive forces, up to 800 lb, have proven to be not only safe, but also conducive to bone formation. Further studies are certainly necessary to examine bone specimens retrieved in cases of aseptic loosening, as these patients accrue. Studies from single and multiple centres are underway to assess this particular mode of failure.
References
- 1.Avedian R, Goldsby RE, Kramer MJ, O’Donnell RJ (2007) Effect of chemotherapy on initial compressive osseointegration of oncologic endoprostheses. Clin Orthop Relat Res, Epub March 15 [DOI] [PubMed]
- 2.Bhangu AA, Kramer MJ, Grimer RJ, O’Donnell RJ. Early distal femoral endoprosthetic survival: cemented stems versus the Compress® implant. Int Orthop. 2006;30:465–472. doi: 10.1007/s00264-006-0186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bini SA, Johnston JO, Martin DL. Compliant prestress fixation in tumor prostheses: interface retrieval data. Orthopedics. 2000;23(7):707–712. doi: 10.3928/0147-7447-20000701-18. [DOI] [PubMed] [Google Scholar]
- 4.Cristofolini L, Bini SA, Toni A. In vitro testing of a novel limb salvage prosthesis for the distal femur. Clin Biomech. 1998;13:608–615. doi: 10.1016/S0268-0033(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 5.Lanyon LE, Hampson WGJ, Goodship AE, Shah JS. Bone deformation recorded in vivo from strain gauges attached to the human tibial shaft. Acta Orthop Scand. 1975;46:256–268. doi: 10.3109/17453677508989216. [DOI] [PubMed] [Google Scholar]
- 6.Mullender MG, Huiskes R. Proposal for the regulatory mechanism of Wolff’s law. J Orthop Res. 1995;13(4):503–512. doi: 10.1002/jor.1100130405. [DOI] [PubMed] [Google Scholar]
- 7.Muschler GF, Levine MJ, Ihara K, Otis JC, Lane JM, Burstein AH, Healey JH. A custom distal femoral prosthesis for reconstruction of large defects following wide excision for sarcoma: results and prognostic factors. Orthopedics. 1995;18(6):527–538. doi: 10.3928/0147-7447-19950601-04. [DOI] [PubMed] [Google Scholar]
- 8.Naudie DDR, Ammeen DJ, Engh GA, Rorabeck CH. Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg. 2007;15(1):53–64. doi: 10.5435/00124635-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Reilly DT, Burstein AH. The mechanical properties of cortical bone. J Bone Jt Surg. 1974;56-A(5):1001–1022. [PubMed] [Google Scholar]
- 10.Ries MD. Complications in primary total hip arthroplasty: avoidance and management: wear. In: Ferlic DC, editor. Instructional course lectures, vol 52. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2003. pp. 257–265. [PubMed] [Google Scholar]
- 11.Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11:118–126. doi: 10.1007/s00776-005-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward WG, Sr, Dorey F, Kelly C, Kabo JM, Wirganowicz PZ, Eckardt JJ. Lessons from massive tumor endoprostheses: implications for future tumor and total joint endoprostheses. Semin Arthroplasty. 1999;10(3):124–132. [Google Scholar]