Abstract
Fifty-six patients who suffered from chronic persistent tennis elbow of more than six months duration were randomly assigned to two active treatment groups. Group 1 (n = 29) received high-energy extracorporeal shock wave treatment (ESWT; 1,500 shocks) at 18 kV (0.22 mJ/mm2) without local anaesthesia; group 2 (n = 27) underwent percutaneous tenotomy of the common extensor origin. Both groups achieved improvement from the base line at three weeks, six weeks, 12 weeks and 12 months post-intervention. The success rate (Roles and Maudsley score: excellent and good) at three months in the ESWT group was 65.5% and in the tenotomy group was 74.1%. ESWT appeared to be a useful noninvasive treatment method that reduced the necessity for surgical procedures.
Résumé
Cinquante-six patients qui souffraient de douleurs chroniques de type tennis elbow depuis plus de six mois ont été randomisés dans une étude avec deux groupes de traitements. Le groupe 1 (n = 29) a été traité par un traitement physique comprenant 1.500 chocs à 18 kV (0.22 mJ/mm2) après anesthésie locale, le groupe 2 (n = 27) a été traité par ténotomie percutanée de l’insertion de l’extenseur commun. Les deux groupes ont été évalués à 3 semaines, 6 semaines, 12 semaines et 12 mois après l’intervention au traitement. Le taux de bons résultats (selon le score de Roles et Maudsley) pour le groupe ayant bénéficié de traitements physiques a été de 65.5%, alors que le groupe traité par ténotomie présentait 74.1% de bons résultats. Le traitement physique apparaît utile. Il s’agit d’un traitement non invasif qui peut réduire la nécessité d’un traitement chirurgical.
Introduction
Tennis elbow is the term used to describe the pain of uncertain pathogenesis that is centred over the common extensor origin at the lateral aspect of the elbow and that interferes with the activities of daily living, sport and work [4].
The choice of treatment for each individual case remains controversial and is based on the personal experience of the treating physician. Many conservative treatments have been suggested including nonsteroidal anti-inflammatory drugs, ultrasound, low-dose laser therapy, steroid injection, functional brace and manipulative treatment but none has shown consistent results [10].
Most of the patients respond to nonoperative treatment [2]; however, surgical treatment is necessary in 4%–11% of patients when symptoms persist [1, 13].
The outcome of surgical treatment is inconsistent and unpredictable [13]. Of the surgical procedures, percutaneous release of the common extensor origin is a simple procedure with a high success rate; it probably sets up an injury and repair reaction that eliminates the pathological anatomy [4, 6].
Extracorporeal shock wave therapy (ESWT) is a relatively new mode of treatment [3, 7, 11, 16, 19, 22, 24]. It involves focused single-pressure pulses of microsecond duration and was first used for medical purposes in the treatment of renal calculi. In the 1990s, ESWT became popular in Germany for certain soft-tissue disorders, including calcifying tendonitis of the rotator cuff, humeral epicondylitis and plantar fasciitis. It is now employed worldwide for the treatment of musculoskeletal complaints [23].
A prospective randomised study was designed to assess the effectiveness of ESWT for the treatment of recalcitrant lateral humeral epicondylitis (tennis elbow) and to compare its outcome with the outcome of percutaneous release of the common extensor origin. Based on a Medline search and on a review of key journals, no previous study had been conducted to compare these two modalities of treatment. Only one study compared patients undergoing shock-wave therapy and patients undergoing percutaneous partial fasciotomy for chronic heel pain; this showed comparable outcomes [25].
Materials and methods
Sixty-two consecutive patients, with unilateral recalcitrant tennis elbow, were enrolled in a prospective study from November 2004 to September 2005. We followed up 56 patients, who comprised the two study groups, for 12 months post-intervention. Six patients did not complete the one year follow-up (three in each group).
Inclusion criteria These included an established diagnosis of lateral epicondylitis of the elbow with failure of at least six months of conservative treatment including: nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, physical therapy, exercise program and elbow brace.Pain was induced by two or more of these diagnostic tests [19]:
Palpation of the lateral epicondyle.
Resisted wrist extension (Thomsen test).
Chair test. With the shoulder flexed to 60° and the elbow extended, the patient attempts to lift a chair weighing 3.5 kg.
Exclusion criteria Patients were excluded if they were younger than 18 years, had a local infection, malignancy, elbow arthritis, generalised polyarthritis, ipsilateral shoulder dysfunction, neurological abnormalities, radial-nerve entrapment, cardiac arrhythmia or a pacemaker, had received a corticosteroid injection within the previous six weeks or were pregnant.Patients were randomised into two groups by the closed envelope technique. NSAIDs were not allowed concomitantly.The study population consisted of:
(ESWT) comprised 29 patients, 15 were males, the right side was involved in 18 patients and the dominant side was involved in 19 patients, the mean age was 40.14±11.12 years (range: 23–60 years) and the duration of symptoms was 16.72±10.94 months (range: 6–48 months).
(Tenotomy) comprised 27 patients, 18 were males, the right side was involved in 16 patients and the dominant side was involved in 17 patients, the mean age was 39.26±10.10 years (range: 22–59 years) and the duration of symptoms was 18.26±12.67 months (range: 6–60 months).
Group 1 (ESWT) group (n = 29)
The point of maximum tenderness to pressure was demarcated. Ear protection devices were used. Conscious sedation anaesthesia was given to all patients prior to therapy (i.e. no local anaesthesia given). The shock-wave treatments were applied by means of an OssaTron device (High Medical Technology, Kreuzlingen, Switzerland), a device generating repetitive high-energy shock waves by the electrohydraulic method. The device was adjusted to maximise the focused treatment wave (f2) into the common extensor origin.
Each patient received 100 graded shocks (14–18 kV; 0.12–0.22 mJ/mm2) to assess the effectiveness of the anaesthesia, followed by 1,400 shocks at 18 kV (0.22 mJ/mm2) for a total of 1,500 shocks, applied at 4 shocks/s. The total energy delivered was 324.25 J. This power setting was defined as a high-energy treatment protocol [14].
The elbow was manipulated against the treatment head throughout the shock-wave applications. Shock waves were thus applied to the maximum pain site and an area with a radius of 1 cm surrounding it.
Group 2 (operative) group (n = 27)
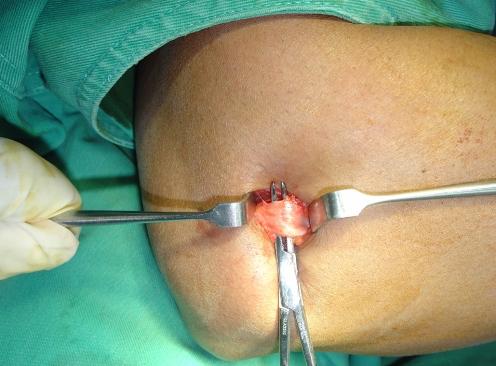
All operations were carried out by using a standard technique [6]. Under general anaesthesia and pneumatic tourniquet, percutaneous release of the common extensor origin was performed through a transverse incision of 1–2 cm made just distal to the lateral epicondyle (Fig. 1). Upon release, the common extensor origin was displaced approximately 1 cm from the lateral condyle and could be palpated by finger tip. Haemostasis was achieved by pressure on the wound after tourniquet deflation. The incision was left open to drain and to avoid haematoma formation. A bulky dressing and a posterior plaster splint were applied to the elbow for one week. Range of motion was advised within the limits of pain.
Fig. 1.
Common extensor origin prior to division
Outcomes
Pain was determined by using a visual analogue scale ranging from 0 (no pain) to 100 (maximal pain). Examination included night pain, resting pain, pressure pain, Thomsen test and the chair test at three, six, 12 weeks and 12 months.
Grip strength was compared with the normal side and classified [12] as: 1= equal strength on both sides, 2= up to 25% reduction, 3= up to 50% reduction and 4= up to 75% reduction of grip strength compared with the unaffected side.
At the end of follow-up, patients assessed their level of residual pain compared with that before treatment according to the criteria of Roles and Maudsley [17] as follows:
Excellent—no pain, full movement, full activity.
Good—occasional discomfort, full movement, full activity.
Acceptable—some discomfort after prolonged activities.
Poor—pain limiting activity.
Success is defined as an excellent or good score based on Roles and Maudsley [17].
Statistical analysis
STATA version 9 was used for sample size calculation and statistical analysis. The required sample size after setting the power to 80% to detect a Thomsen score difference of 5 as statistically significant at the 5% level was 52. Each group required at least 26 participants. Descriptive analysis was conducted to explore the characteristics of the participants at baseline. The median, the 25th and the 75th interquartile percentiles of the different pain scores, the mean and the standard deviation of age and the percentages of the gender distribution by intervention type were calculated.
To compare the different pain scores across the different time periods, Friedman’s analyses were carried out. Post hoc tests were used to compare the pain scores between one time period and the one that which preceded it. Since post hoc tests were used several times, the significance level was divided by the number of planned comparisons and each two sample test was accordingly performed at the reduced level.
A Kruskal-Wallis test was used to compare the pain scores between the two intervention groups at the different time periods, with Cochran’s Q-test for success (categorical data).
Fisher’s exact test was used to compare the number of patients who had an improvement of at least 50% in Thomsen test at 12 weeks and those who achieved an improvement of at least 80% at 12 months.
Results
Baseline demographics and the different scores of the study participants are presented in Table 1.
Table 1.
Comparison of baseline characteristics and various scores across compared groups. Values are medians and interquartile ranges, numbers of participants and their percentages, and means±SD of age.
| Test | ESWT, n = 29 | Operative, n = 27 |
|---|---|---|
| Night pain | 33.0 (23.5–45.5) | 30.0 (19–50) |
| Rest pain | 30.0 (17.5–40) | 25.0 (15–40) |
| Pressure | 58.0 (43.5–72.5) | 60.0 (50–72) |
| Thomsen test | 51.0 (40–70) | 52.0 (42–65) |
| Chair test | 48.0 (39–59) | 48.0 (40–61) |
| Grip | 2 (2–3) | 2 (2–3) |
| Male | 15 (52.0%) | 18 (66.70%) |
| Age | 40.14 (11.12) | 39.26 (10.05) |
Each group achieved improvement at each follow-up, in all parameters measured. This improvement between one follow-up and the previous one is shown in Table 2.
Table 2.
Comparison between operative and ESWT groups across time. Values are median (25th and 75th percentiles).
| Test and time in weeks | ESWT, n = 29 | Operative, n = 27 |
|---|---|---|
| Night pain | ||
| 0 | 33 (23.50–45.50) | 30 (19–52) |
| 3 | 13 (10–21.50)* | 15 (9–22)* |
| 6 | 9 (3–20)* | 10 (5–13)* |
| 12 | 5 (0–12)* | 5 (0–12)* |
| 52 | 0 (0–10)* | 5 (0–10) |
| <0.01 | <0.01 | |
| Rest pain | ||
| 0 | 30 (17.5–40) | 15 (25–40) |
| 3 | 15 (7.5–23.5)* | 15 (8–20)* |
| 6 | 10 (0–20)* | 10 (3–15)* |
| 12 | 5 (0–12.5) | 3 (0–10) |
| 52 | 5 (0–10) | 3 (0–10) |
| <0.01 | <0.01 | |
| Pressure pain | ||
| 0 | 58 (43.5–72.5) | 60 (50–72) |
| 3 | 30 (19.50–41.00)* | 31 (19–44)* |
| 6 | 19 (13.50–33.00)* | 20 (10–29)* |
| 12 | 15 (8.00–32.00)* | 15 (8–25)* |
| 52 | 10 (5 .00–27.00)* | 10 (7–20)* |
| <0.01 | <0.01 | |
| Thomsen test | ||
| 0 | 51 (40–70) | 52 (42–65) |
| 3 | 30 (20–43.50)* | 28 (21–39)* |
| 6 | 18 (8–30.5)* | 20 (10–27)* |
| 12 | 15 (5–32)* | 12 (5–17)* |
| 52 | 12 (5–25)* | 10 (3–19)* |
| <0.01 | <0.01 | |
| Grip strength | ||
| 0 | 2 (2–3) | 2 (2–3) |
| 3 | 2 (1–2) | 2(1–2) |
| 6 | 2 (1–2) | 2(1–2) |
| 12 | 1 (1–2)* | 1(1–2)* |
| 52 | 1 (1–2) | 1(1–2) |
| <0.01 | <0.01 | |
| Chair test | ||
| 0 | 48 (39.00–59.50) | 48 (40–61) |
| 3 | 23 (15–37.00)* | 21 (17–35)* |
| 6 | 18 (5.50–33.50)* | 17 (9–31)* |
| 12 | 15 (2.50–25)* | 11 (5–25)* |
| 52 | 10 (1–18.50)* | 9 (2–14)* |
| <0.01 | <0.01 | |
| Roles and Maudsley score | ||
| 0 | 1 (1–1) | 1 (1–1) |
| 3 | 2 (2–3.5)* | 3 (2–4)* |
| 6 | 3 (2–4)* | 3 (2–4) |
| 12 | 3 (2–4) | 3 (2–4) |
| 52 | 3 (2–4) | 3 (2–4) |
| <0.01 | <0.01 | |
*Significantly different from the preceding time period
The majority of improvements were achieved and maintained in both groups between the third and 12th week post-intervention and continued to a lesser extent for up to one year.
A minimum of 50% improvement of Thomsen score at 12-weeks was achieved in 21/29 patients in ESWT group and 23/27 patients in the tenotomy group [Fisher’s exact test, P = 0.334, RR = 0.850 (95% CI=0.646–1.118)].
At 12-month follow-up, an 80% improvement in the Thomsen score was achieved in 14/29 patients of the ESWT group and 17/27 patients of the tenotomy group [Fisher’s Exact test, P = 0.296, RR = 0.767 (95% CI=0.477–1.1233)].
No significant differences between the ESWT and operative groups were detected across the different time periods for any measured parameter (Table 3).
Table 3.
Comparison of different scores across ESWT and operative groups at different time periods
| Test and treatment | Baseline | 3 weeks | 6 weeks | 12 weeks | 1 year |
|---|---|---|---|---|---|
| Night pain | |||||
| ESWT (n=29) | 33 (23.5–45.5) | 13 (10–21.5) | 9 (3–20) | 5 (0–12) | 0 (0–10) |
| Tenotomy (n=27) | 30 (19 –50) | 15 (9–22) | 10 (5–13) | 5 (0–12) | 5 (0–10) |
| P value | 0.67 | 0.97 | 0.79 | 0.69 | 0.87 |
| Rest pain | |||||
| ESWT | 30(17.50–40) | 15 (7.5–23.5) | 10 (0–20) | 5 (0–12.5) | 5 (0–10) |
| Tenotomy | 25 (15–40) | 15 (8–20) | 10 (3–15) | 3 (0–10) | 3 (0–10) |
| P value | 0.35 | 0.64 | 0.68 | 0.91 | 0.81 |
| Pressure pain | |||||
| ESWT | 58 (43.5–72.5) | 30 (19.5–41) | 19 (13.5–33) | 15 (8–32) | 10 (5–27) |
| Tenotomy | 60 (50–72) | 31 (19–44) | 20 (10–29) | 15 (8–25) | 10 (7–20) |
| P value | 0.49 | 0.88 | 0.98 | 0.93 | 0.77 |
| Thomsen test | |||||
| ESWT | 51 (40–70) | 30 (20–43.5) | 18 (8–30.5) | 15 (5–32) | 12 (5–25) |
| Tenotomy | 52 (42–65) | 28 (21–39) | 20 (10–27) | 12 (5–17) | 10 (3–19) |
| P value | 0.92 | 0.95 | 0.99 | 0.59 | 0.56 |
| Chair test | |||||
| ESWT | 48 (39–59.5) | 23 (15–37) | 18 (5.5–33.5) | 15 (2.5–25) | 10 (1–18.5) |
| Tenotomy | 48 (40–61) | 21 (17–35) | 17 (9–31) | 11 (5–25) | 9 (2–14) |
| P value | 0.87 | 0.96 | 0.89 | 0.99 | 0.82 |
| Grip strength | |||||
| ESWT | 2 (2–3) | 2 (1–2) | 2 (1–2) | 1(1–2) | 1 (1–2) |
| Tenotomy | 2 (2–3) | 2(1–2) | 2(1–2) | 1(1–2) | 1 (1–2) |
| P value | 0.59 | 0.94 | 0.88 | 0.61 | 0.56 |
| Roles and Maudsley score | |||||
| ESWT | 1(1–1) | 2 (2–3) | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| Tenotomy | 1(1–1) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| P value | 0.99 | 0.32 | 0.35 | 0.24 | 0.16 |
| Success (Roles and Maudsley excellent and good results) | |||||
| ESWT | – | 14 (48.3%) | 17 (58.6%) | 19 (65.5%) | 18 (62.10%) |
| Tenotomy | – | 16 (59.3%) | 17 (63.0%) | 20 (74.1%) | 21 (77.80%) |
| P value | – | 0.44 | 0.79 | 0.57 | 0.25 |
The baseline characteristics and the baseline pain scores of the six participants who were lost to follow-up were not statistically different from those who remained until the end of the study (data not shown; P > 0.05). Therefore, the missing information was random and the beta coefficients that represented the average score differences were not biased estimates.
Three patients showed no effect after ESWT at three weeks and their results were considered as poor. These patients received a second treatment 30 days after the first and were regarded as failures at all follow-up periods.
The success rate (number of patients who achieved good and excellent scores in the Roles and Maudsley test) at three weeks was 14 (48.3%) and 16 (59.3%), P = 0.44, in the ESWT and tenotomy groups, respectively. This number increased to 17 (58.6%) and 17 (63%), P = 0.79, at six weeks and to 19 (65.5%) and 20 (74.1%), P = 0.57 at 12 weeks. At the one year follow-up, the numbers were 18 (62.1%) and 21 (77.8%), P = 0.25 for the ESWT and tenotomy groups, respectively (Table 3).
Discussion
Many articles have been published regarding the treatment of tennis elbow [1, 2, 10]. Over 40 different modalities of treatment, used either alone or in combination, have been reported [10].
The use of shock wave therapy for lateral epicondylitis in the literature is highly controversial [9, 18, 19, 24]. A meta-analysis of ESWT in the musculoskeletal system, conducted by Ogden et al. [14] and involving 1672 patients with lateral epicondylitis in eleven prospective studies, showed success rates of 48%–72%. In contrast, several studies have concluded that ESWT has no benefit over placebo [3, 7].
Our study shows that ESWT is indeed an effective treatment of chronic lateral epicondylitis. The overall results in ESWT group at one year are 62% excellent to good and 38% fair to poor. A substantial improvement of symptoms is achieved between three to 12 weeks after treatment and this improvement is maintained at the one year follow-up. Our results are comparable with those reported in the literature [9, 16, 18, 19, 22].
We report three cases of failure of treatment attributable to failure of response to the first treatment of ESWT at three weeks follow-up. We managed these patients by giving a second treatment session, which yielded two good and one fair result. This effect can be explained by one of the following: the cumulative effects of the second dose of ESWT [9, 15] or the dose-dependent changes that have been shown in the tendon and paratenon after shock waves in an experimental Achilles tendon model in rabbit [21].
This study shows clearly that the effect of ESWT starts after application with a gradual increase of effects afterwards: at three months, 21/29 patients achieved an improvement of at least 50%. Three months has been reported to be the time by which most, but not, all of effects of ESWT appear [9, 18].
The overall results of the tenotomy group at one year were 77.4% excellent to good and 22.6% fair to poor. This is comparable with the results reported by Grundberg and Dobson [6].
Positive outcomes have been confirmed in many clinical studies. Petrone et al. [16] have evaluated the effects of three doses of low ESWT (0.06 mJ/mm2) without local anaesthesia; they have found a significant benefit to shock wave treatment over placebo in pain reduction, functional activity scores, activity-specific evaluation and overall impression of disease state. Rompe et al. [22] have reported low-energy ESWT results in patients with epicondylitis secondary to playing tennis; their results are similar to our findings with a success rate of 65% (25/38 patients in the treatment group). Levitt and Alvarez [11] have reported that 11/20 patients showed an improvement of at least 50% by six weeks, with 14 improving by three months and 16 improving by six months. Haake et al. [7] have evaluated the use of low-dose ESWT with local anaesthesia and have found no difference between shock wave therapy and sham treatment. In our study, we have used high-energy shock wave without local anaesthesia.
The mechanism of action of shock waves is not fully understood and has been explained by many theories including direct stimulation of healing, neovascularisation, direct suppressive effects on nociceptors and a hyperstimulation mechanism that blocks the gate-control mechanism [23]. The considerable differences between the results of various studies may be explained by a number of factors including machine design, intensity, focal energy, geometry of the shock-wave focus, treatment frequency, localisation methods, duration and severity of symptoms, sample sizes, heterogeneous study populations, surrogate outcome measure and type of anaesthesia [23].
The apparatus used for the current study employed electrohydraulic shock-wave generation. Electrohydraulic application is based on one treatment in most patients [9, 14], whereas electromagnetic and piezoelectric devices routinely use three to six treatments [7, 15, 16, 18, 19, 22].
Electrohydraulic shock-wave generation was the first shock-wave method approved by the Food and Drug Administration for musculoskeletal use [8]. Although electrohydraulic lithotriptors are regarded as being more clinically effective in renal stone fragmentation compared with electromagnetic or piezoelectric devices [5], no direct comparative study has been made of the different machines in musculoskeletal applications, and no information exists with regard to the relative efficacy of one method of shock-wave generation over others [15].
Therapy with high-energy waves is considered unpleasant by patients [20]. We have not employed local anaesthesia. Instead, we use a conscious sedation type of anaesthesia, as a local anaesthetic possibly influences the effect of ESWT in the form of altering the tissue effect of the shock wave therapy, thereby interfering with hyper-stimulation analgesia and preventing clinical refocusing (a local anaesthetic may inhibit the aiming of the treatment head at the point of maximal tenderness)[16].
No major side effects have been observed in our study in the ESWT group. One patient developed parasthesia and two patients developed myalgia but all made a full recovery within one month.
Both ESWT and tenotomy groups showed comparable results with respect to the reduction of pain, restoration of grip strength and overall rating of the disease state. The lack of statistical significance is not attributable to the absence of a suitable sample size or type-II errors. We believe that ESWT is a comparable method of treatment to that of operative intervention.
Conclusion
In patients who had experienced the failure of conventional treatment of lateral epicondylitis, shock wave therapy can be a potentially helpful additional management. Our study has revealed comparable results of high-energy ESWT in patients with chronic tennis elbow when compared with percutaneous tenotomy of the common extensor origin. Analyses of three month and one year responses to ESWT support the continuing effect of therapy with one application of 1,500 impulses and without the use of local anaesthesia. ESWT appears to be a useful noninvasive treatment method that reduces the necessity for surgical procedures.
References
- 1.Boyd HB, McLeod AC., Jr Tennis elbow. J Bone Joint Surg Am. 1973;55:1183–1187. [PubMed] [Google Scholar]
- 2.Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55:1177–1182. [PubMed] [Google Scholar]
- 3.Crowther MA, Bannister GC, Huma H, Rooker GD. A prospective, randomized study to compare extracorporeal shock-wave therapy and injection of steroid for the treatment of tennis elbow. J Bone Joint Surg Br. 2002;84:678–679. doi: 10.1302/0301-620X.84B5.12741. [DOI] [PubMed] [Google Scholar]
- 4.Dunkow PD, Jatti M, Muddu BN. A comparison of open and percutaneous techniques in the surgical treatment of tennis elbow. J Bone Joint Surg. 2004;86 B:701–704. doi: 10.1302/0301-620X.86B5.14469. [DOI] [PubMed] [Google Scholar]
- 5.Fuselier HA, Prats L, Fontenot C, Gauthier A., Jr Comparison of mobile lithotripters at one institution: Healthtronics Lithotron, Dornier MFL-5000, and Dornier Doli. J Endourol. 1999;13:539–542. doi: 10.1089/end.1999.13.539. [DOI] [PubMed] [Google Scholar]
- 6.Grundberg AB, Dobson JF. Percutaneous release of the common extensor origin for tennis elbow. Clin Orthop. 2000;376:137–140. doi: 10.1097/00003086-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Haake M, Konig IR, Decker T, Riedel C, Buch M, Muller HH. Extracorporeal shock wave therapy clinical trial group. Extracorporeal shock wave therapy in the treatment of lateral epicondylitis: a randomized multicenter trial. J Bone Joint Surg Am. 2002;84:1982–1991. doi: 10.2106/00004623-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Henney JE. From the food and drug administration: shock wave for heel pain. JAMA. 2000;284:2711. doi: 10.1001/jama.284.21.2711. [DOI] [PubMed] [Google Scholar]
- 9.Ko JY, Chen HS, Chen LM. Treatment of lateral epicondylitis of the elbow with shock waves. Clin Orthop. 2001;387:60–67. doi: 10.1097/00003086-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Labelle H, Guibert R, Newman N, Fallaha M, Rivard CH. Lack of scientific evidence for the treatment of lateral epicondylitis of the elbow. J Bone Joint Surg Br. 1992;74:646–651. doi: 10.1302/0301-620X.74B5.1388172. [DOI] [PubMed] [Google Scholar]
- 11.Levitt RL, Alvarez R (1998) FDA study in the United States of musculo-skeletal shock wave therapy for lateral epicondylitis and heel pain syndrome. First Congress of the European Society for Musculoskeletal Shockwave Therapy. Izmir, Turkey, 9
- 12.Mucha C, Wannske M. Ergebnisse einer kontrollierten Studie zur physikalischen Therapie der Epicondylopathia humeri. Physik Therapie. 1989;10:564–573. [Google Scholar]
- 13.Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832–839. [PubMed] [Google Scholar]
- 14.Ogden JA, Alvarez R, Levitt R, Cross GL, Marlow M. Shock wave therapy for chronic proximal plantar fasciitis. Clin Orthop. 2001;387:47–59. doi: 10.1097/00003086-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Ogden JA, Toth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop. 2001;387:8–17. doi: 10.1097/00003086-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Pettrone FA, McCall BR. Extracorporeal shock wave therapy without local anesthesia for chronic lateral epicondylitis. J Bone Joint Surg Am. 2002;87:1297–1304. doi: 10.2106/JBJS.C.01356. [DOI] [PubMed] [Google Scholar]
- 17.Roles NC, Maudsley RH. Radial tunnel syndrome. J Bone Joint Surg [Br] 1972;54:499–508. [PubMed] [Google Scholar]
- 18.Rompe JD, Hopf C, Kullmer K, Heine J, Burger R. Analgesic effect of extracorporeal shock-wave therapy on chronic tennis elbow. J Bone Joint Surg Br. 1996;78:233–237. [PubMed] [Google Scholar]
- 19.Rompe JD, Hopf C, Kullmer K, Heine J, Burger R, Nafe B. Low-energy extracorporeal shock wave therapy for persistent tennis elbow. Int Orthop. 1996;20:23–27. doi: 10.1007/s002640050021. [DOI] [PubMed] [Google Scholar]
- 20.Rompe JD, Küllmer K, Riehle HM, Herbsthofer B, Eckardt A, Bürger R, Nafe B, Eysel P. Effectiveness of low-energy extracorporeal shock waves for chronic plantar fasciitis. Foot Ankle Surg. 1996;2:215–221. doi: 10.1016/S1268-7731(96)80004-X. [DOI] [Google Scholar]
- 21.Rompe JD, Kirpatrick CJ, Kullmr K, Schwitalle M, Krischek O. Dose related effects of shock waves on rabbit tendon achillis: a sonographic and histological study. J Bone Joint Surg Br. 1998;80:546–552. doi: 10.1302/0301-620X.80B3.8434. [DOI] [PubMed] [Google Scholar]
- 22.Rompe JD, Decking J, Schoellner C, Theis C. Repetitive low-energy shock wave treatment for chronic lateral epicondylitis in tennis players. Am J Sports Med. 2004;32:734–743. doi: 10.1177/0363546503261697. [DOI] [PubMed] [Google Scholar]
- 23.Speed CA. Extracorporeal shock-wave therapy in the management of chronic soft tissue conditions. J Bone Joint Surg Br. 2004;86:165–171. doi: 10.1302/0301-620X.86B2.14253. [DOI] [PubMed] [Google Scholar]
- 24.Wang CJ, Chen HS. Shock wave therapy for patients with lateral epicondylitis of the elbow: a one- to two-year follow-up study. Am J Sports Med. 2002;30:422–425. doi: 10.1177/03635465020300031901. [DOI] [PubMed] [Google Scholar]
- 25.Weil LS, Jr, Roukis TS, Weil LS, Borrelli AH. Extracorporeal shock wave therapy for the treatment of chronic plantar fasciitis: indications, protocol, intermediate results, and a comparison of results to fasciotomy. J Foot Ankle Surg. 2002;41:166–172. doi: 10.1016/S1067-2516(02)80066-7. [DOI] [PubMed] [Google Scholar]