Abstract
We studied the diversity of bacteria and host in the H. pylori-human model. The human indigenous bacterium H. pylori diverged along with humans, into African, European, Asian and Amerindian groups. Of these, Amerindians have the least genetic diversity. Since niche diversity widens the sets of resources for colonizing species, we predicted that the Amerindian H. pylori strains would be the least diverse. We analyzed the multilocus sequence (7 housekeeping genes) of 131 strains: 19 cultured from Africans, 36 from Spanish, 11 from Koreans, 43 from Amerindians and 22 from South American Mestizos. We found that all strains that had been cultured from Africans were African strains (hpAfrica1), all from Spanish were European (hpEurope) and all from Koreans were hspEAsia but that Amerindians and Mestizos carried mixed strains: hspAmerind and hpEurope strains had been cultured from Amerindians and hpEurope and hpAfrica1 were cultured from Mestizos. The least genetically diverse H. pylori strains were hspAmerind. Strains hpEurope were the most diverse and showed remarkable multilocus sequence mosaicism (indicating recombination). The lower genetic structure in hpEurope strains is consistent with colonization of a diversity of hosts. If diversity is important for the success of H. pylori, then the low diversity of Amerindian strains might be linked to their apparent tendency to disappear. This suggests that Amerindian strains may lack the needed diversity to survive the diversity brought by non-Amerindian hosts.
Introduction
Humans coevolved with their microbiomes [1]–[3] and persistent human microbes can be markers of human migrations. Viruses such as HPV [4], Hepatitis G [5], RNA retrovirus HTLV-1 [6] and the bacterium Helicobacter pylori [7]–[9] show signs of coevolution with their human host, reflecting the serial founding African-origin model of human evolution due to successive human migrations that consisted of only a subset of the genetic variation available at the source location [10], [11].
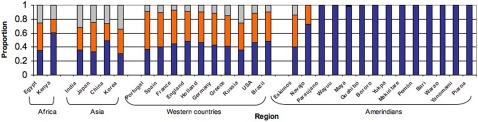
The original human migration out of Africa occurred approx. 60,000 years ago, towards the Middle East and thereafter independently to Europe and Asia [12]. The Americas were populated by humans of East Asian ancestry that crossed the Bering Strait, about 15 thousand years ago. These first Americans suffered a genetic bottleneck [13]–[15], and the reduced genetic diversity in Amerindians is evidenced in the absolute dominance of the O blood group among Amerindians ( Fig. 1 ), their low heterozygosity and the reduced number of mitochondrial DNA haplogroups [16], [17]. However, the diversity of Amerindians has increased through genetic inflow from Europeans and Africans over the last 500 years, leading to an increasing Mestizo population.
Figure 1. Distribution of ABO blood groups in humans from Africa, Asia, Western countries and in Amerindians.
The blue, orange and grey bars represent respectively O, A and B allele frequencies [34]–[43]. The remarkable dominance of O blood groups among Amerindians from South America affects the ABO blood group recognition by H. pylori strains.
H. pylori is a human gastric indigenous bacteria, in spite of being a risk factor for gastric cancer and peptic ulcer disease [18], [19]). A congruent divergence of H. pylori and their human hosts suggests that the quality of host resources influences the diversity of indigenous bacteria and their adaptations. In host-parasite interactions, a restricted gene flow in the host leads to local microbial adaptation [20], [21]. An example of local adaptation is the differing affinity of H. pylori strains to bind blood group antigens expressed in the human gastric mucosa: European H. pylori strains bind all three (A, B and O) blood group antigens, but an important proportion of strains from the are dominantly O blood group Amerindians have higher affinity for O blood antigen [22].
Environmental diversity allows different species to occupy different niches, sustaining coexistence and species diversity. There is a strong body of evidence for the loss of diversity by reduction of niches. In addition to landscape mosaicism, temporal variability also affects diversity. Simulations predict that while spatial variability increases diversity, the strongest increase occur at intermediate levels of temporal variability [23]. When the environment of a species is provided by another species, there is a set of age-changing mosaic niches provided by each individual host for the life of the host. Since microbes circulate in a dynamic population of hosts, the microbe diversity is held at the host population –and not individual- level. The studies that have provided the basis for many ecology theories have come from the field of plant ecology. Plant ecological studies have shown that diversity of plant associated herbivores increases with diversity of plant species [24] but also with population genotypic diversity, within a single species [25]. As such, a direct association between diversities in host and microbiome microbes could be expected. To test this hypothesis, we determined intra-population genetic diversity of strains of the highly diverse gastric bacteria H. pylori and of humans from the Americas and other geographical populations relevant to its peopling.
Results
We determined the intra-population genetic diversity of multilocus sequences of 7 housekeeping genes from 131 strains of the gastric bacteria H. pylori and of 2,232 sequences of the human mtDNA hypervariable segment I. H. pylori strains were selected from human populations relevant to the ancestry of the people of the Americas, with East Asians providing a reference group for Amerindians: 131 H. pylori isolates were from Amerindians, Spanish, West-Africans, South American Mestizos and Koreans. Based on their DNA multilocus sequences, the strains were assigned to one of 6 geographical bacterial populations: hpEastAsia (subpopulations hspAmerind, hspMaori and hspEAsia), hpAsia2, hpEurope, hpNEAfrica, hpAfrica1 (hspWAfrica, hspSAfrica) and hpAfrica2 [7], [26]. Selected human sequences were from similar locations to those where H. pylori strains had been isolated. A total of 1,148 sequences of human mtDNA hypervariable segment I from non-Bantu Africans, Spanish, Koreans, Amerindians (Guahibo, Huitoto, Inuit) and Latin American Mestizos were retrieved from Genbank and from published work. Bacterial and human genetic distance matrixes were generated and compared using non-parametric statistics.
Strain genetic diversity
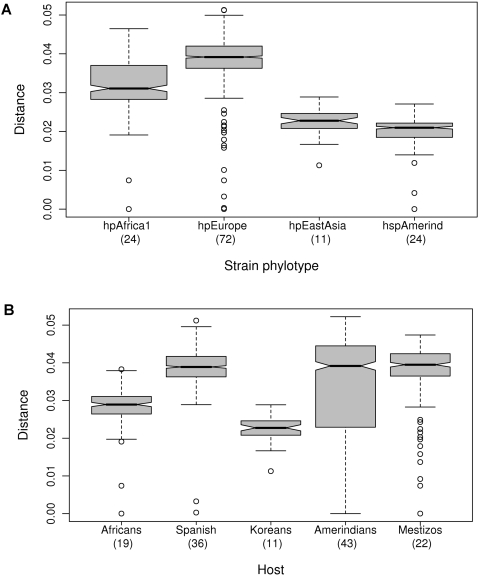
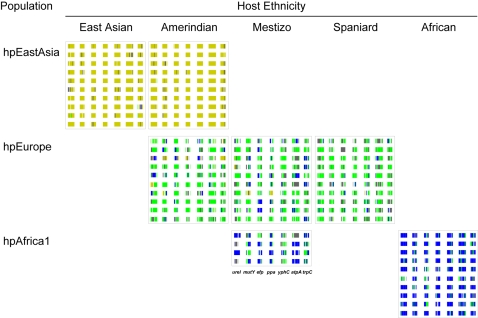
The bacterial strains were assigned to populations according to their multilocus DNA sequences: those from African hosts yielded only hpAfrica1, those from Spanish yielded hpEurope and those from Koreans yielded hspEAsia ( Table 1 ). However, Huitoto and Guahibo Amerindians yielded both hspAmerind and hpEurope strains, and Mestizos yielded hpEurope and hpAfrica1, but not hspAmerind. The least and most diverse strains of H. pylori populations were hspAmerind and hpEurope, respectively ( Figure 2A ). Nonetheless, when grouping the strains by host, strain diversity in Amerindians increased to the levels found in Spanish and Mestizo hosts ( Figure 2B ), consistently with the circulation of mixed strains ( Table 1 ) and with the remarkable mosaicism reflected in the multilocus sequences ( Figure 3 ). As stated previously [7], [26], the ancestry patterns of modern hpEurope strains revealed extensive recombination between the two ancestral populations ancestral Europe1 and ancestral Europe2. Spanish H. pylori ( Figure 3 ) further include components from ancestral hpAfrica1, which possibly reflects the role Africa has played in shaping the Spanish modern human gene pool. Traces of African bacterial ancestry were also detected in strains from Mestizos and Amerindians. The observed mosaicism reflects extensive recombination and results in lower genetic structure.
Table 1. H. pylori assignment to bacterial populations.
| Location/source of human population | Bacterial population | ||||
| No. of strains | hpAfrica1 | hpEurope | hpEAsia | hspAmerind | |
| African | 19 | 19 | |||
| Senegal | 5 | 5 | |||
| Burkina Faso | 14 | 14 | |||
| Europe | 36 | 36 | |||
| Spain | 36 | 36 | |||
| East Asian | 11 | 11 | |||
| Korea | 11 | 11 | |||
| Amerindian | 43 | 19 | 24 | ||
| Inuit (Eskimo) | 13 | 4 | 9 | ||
| Athabaskan (Na-Dene) | 6 | 6 | |||
| Huitoto | 16 | 12 | 4 | ||
| Piaroa | 3 | 3 | |||
| Guahibo | 5 | 3 | 2 | ||
| Mestizo | 22 | 5 | 17 | ||
| Colombia | 12 | 1 | 11 | ||
| Venezuela | 10 | 4 | 6 | ||
| All | 131 | 24 | 72 | 11 | 24 |
Multilocus sequences of the strains were from 7 housekeeping genes (atpA, efp, mutY, ppa, trpC, ureI and yphC). While only the expected populations were identified in African, Spanish and Korean hosts, strains assigned to more than one population were observed among Amerindians and Mestizos.
Figure 2. Pairwise genetic distances between H. pylori strains grouped by bacterial population (2A), or according to their human host (2B).
Differences in pairwise distances among strains in Fig. 2A were significant (Kruskal-Wallis test, p<2.2×10−16, Wilcoxon and Bonferroni; p<10−14) with a decreasing order hpEurope>hpAfrica1>hpEastAsia>hspAmerind. When grouped by the human host from which each strain was isolated (Fig 2B), strain diversity in Amerindians was as high as in Spanish and Mestizos (with no significant differences among them; Wilcoxon Pairwise comparison p>0.7), with a decreasing order Spaniards = Amerindians = Mestizos>Africans>Koreans. The waist of the dress-like box is the median with the waist side openings indicating the 95% interval for the median; the top represents the 3rd quartile and the bottom the 1st quartile. The interval in dashed lines represents a maximum of 1.5× interquartile range and the open circles are outliers. Permutation tests confirmed group differences in strain diversity.
Figure 3. Mosaic structure of the multilocus H. pylori sequences in representative strains.
The ancestral source of each polymorphic nucleotide is shown by a vertical line for each of the seven gene fragments in the multilocus analysis of 10 representative strains from each group (see the legend below Mestizo strains). Individual nucleotides were derived from ancestral Europe1 (grey), ancestral Europe2 (green), ancestral Africa1 (blue) and ancestral EastAsia (yellow). Nucleotides not assigned with >50% probability to any one population are indicated by white lines. African and European components can be observed in hpEurope strains from Spaniards and Mestizos, as well as in hpAfrica1 from Mestizos, while African hpAfrica1 strains and Amerindian hspAmerind strains tested were largely homogeneous.
Human genetic diversity
Host genetic diversity increased in this study in the order: Amerindians<Spaniards<Koreans<Africans<Mestizos (Kruskal-Wallis p<10−14, Figure S4). However, measurement of human diversity through mtDNA underestimates paternal-linked diversity. Mestizos diversity has probably been introduced by both maternal (Amerindian and African) and paternal (European and African) gene flow, but the remarkably low diversity in the Spanish mtDNA may not reflect the true variability of human genetic diversity introduced in Spain by paternal lines.
Discussion
Human evolution has been shaped by the early forms of life in the planet, the microbes. Indeed, evolution of all eukaryotes occurred in a bacterial planet, and microbes found niches on surfaces, invaginations and guts of humans, as well. Helicobacters found a niche in the gastric pouch of mammals [27], including humans. Helicobacter pylori has colonized the human stomach since the beginning of the human history [26], and maintained a tuned evolution with its host, reflecting in its genome some genetic, linguistic and cultural traits of the human population. It can therefore be a useful model to determine how humans modulate the diversity of their microbiomes.
Genetic divergence and convergence
Although most of the data analyzed in this study was already available, they had previously not been analyzed in terms of the associations between genetic diversity of humans and their indigenous microbial population. There are many genetic studies that confirm that human intrapopulation genetic diversity decreases with geographic distance from Africa, and that the Amerindians suffered a genetic bottleneck [12]–[17]. The finding that a low-diversity human population was associated with a low-diversity bacterium could be due to increased genetic drift (on a populations of low effective size), and/or selection by low diversity blood groups on the H. pylori population.
Genetic isolation and drift as well as selection may explain the radiation of humans into different groups, and the concomitant divergence of H. pylori into geographical types, whereby both host and bacteria show patterns of isolation by distance [26]. However, the modern world is increasingly bringing genetic influx into each of the human groups, both at the human and microbiome level. Indigenous microbes that once diverged during most of human history are currently in the process of converging, which erase phylogeographic signals. How this process is occurring is important because some human-evolved bacteria are relevant to human health (for example H. pylori is implicated in gastric diseases), and changes in the dynamics of its coevolution with humans might lead to changes in disease patterns.
Genetic diversity and fitness
There might be greater local genetic variation in parasite populations relative to their hosts. In this case, parasite populations might increase their genetic structure by locally adapting to their hosts [28]. Consistently, mosaic strains would be expected to be genetically more diverse and more generalist. This might explain why hpAfrica1 strains from Mestizos are more diverse than those from African hosts (Kruskal-Wallis p<10−14; Figure S1 ). Recombination obviously requires coexistence of different strains in a single stomach, which we indeed have found to be frequent in patients from Venezuela [29].
Diversity optimizes niche partitioning. In the gastric context, strain mosaicism (intra-genomic diversity) and strain diversity (inter-genomic variants) is likely to be a key factor in the hpEurope strains host range expansion (in both Mestizos and Amerindians). Meanwhile, hspAmerind strains seem to lack the diversity that would be required to survive in the high host variability brought by Mestizos. Displacement of hspAmerind by hpEurope strains could occur by two different means: 1) Strain competition during colonization, in which “generalist” strains will have better chance to colonize diverse niches than “specialist” strains [as in the case of O binder Amerindian strains described above, which would have less success in colonizing non-O blood group Mestizos [22]]; 2) Strain subversion by transformation, in which DNA from hpEurope strains is taken up and recombinant strains become increasingly European. The high component of European ancestry in the mosaic strains from Mestizos and Amerindians, and the relatively low Amerindian component in Mestizos ( Figure 3 ) support this second hypothesis.
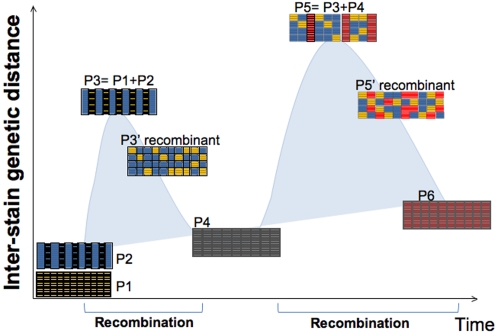
The consumption of antibiotics and other drugs (such as proton pump inhibitors that reduce gastric acidity) might also be currently affecting the bacterial populations by increasing selection pressure in favor of particular variants, thus shaping the genome of indigenous microbes in modern humans. Modern human admixture results not only in increased human diversity but also in increased microbial diversity of the human microbiome. In the long term, however, one can predict that maximum genetic distances will be succeeded by homogenization of the genomic structure in strain variants (all mosaic or recombinant strains) and humans (all Mestizos) and decreased within population genetic distances brought by further recombination and lack of isolation, as illustrated in Figure 4 . Since no influx of genetic variation is expected from outside the global village, low genetic flow in both microbes and hosts will eventually result in decreased human and microbiome diversity.
Figure 4. Development of inter-strain genetic diversity over time as two populations recombine.
In the context of our hypothesis, a low diversity H. pylori population (P1) arose from co-evolution with the isolated Amerindian host population. With the introduction of new H. pylori strains (P2) the new population formed (P3) is more diverse than any of the source populations alone. Selection acts and strains recombine with the consequent homogenization of the population (P3′). The cycle is repeated when new populations (P4) arrive. Given time and isolation (no gene flow), population diversity will be reduced (P6). Based on their mosaic structure and high genetic distances, it seems that current H. pylori from Amerindians and Mestizos are in one of the intermediate states (P3′ or P5′). Arrows indicate introduction of new populations.
To our knowledge, Wirth et al. [30] were the first to analyze both human DNA and H. pylori sequences from 50 patients in Ladakh in northern India. Our results support the need of a study with a true large scale population-based approach involving human and microbe samples from the same hosts in order to better measure the extent in which human populations shape the genomes of H. pylori. As new technology optimizes cost and time of sequencing, there can be new studies performed in individual Homo sapiens and his individual microbiome, using more genes. These studies will be crucial to understand the coevolution of humans and their microbes.
Conclusions
In the analysis of differences in genetic diversity in human groups and the concomitant diversity of a human indigenous microbe, our study provides support to the hypothesis that the distribution of genetic diversity in humans determines the genetic diversity of indigenous microbes. Specifically, we found genetic evidence of i) a decreased diversity of H. pylori strains in the human group with the least genomic diversity; ii) an increased diversity in strains from Mestizos, hybrid strains with mosaic structure assigned to the hpEurope group; iii) a host-range expansion of these hpEurope strains into Mestizos and Amerindian hosts, confirming that niche diversity widens the sets of resources for colonizing species.
Materials and Methods
H. pylori strains
We selected strains from Amerindians and other human populations relevant to the peopling of the Americas. These included, Spanish, West-Africans, Mestizos, and East Asians who provide a reference group for Amerindians.
We analyzed multilocus sequences from 7 housekeeping genes (atpA, efp, mutY, ppa, trpC, ureI and yphC) [7] in 131 H. pylori isolates cultured from 19 Africans (14 from Burkina Fasso, 5 from Senegal), 36 Spanish, 11 Koreans, 43 Amerindians (13 Inuit, 6 Athabaskan, 16 Huitoto, 5 Guahibo and 3 Piaroa), and 22 Mestizos (12 from Colombia and 10 from Venezuela). Sequences were available in http://www.mlst.net/ and the EMBL database except for 14 sequences experimentally obtained in this work, from 8 Amerindians (5 Guahibos, 3 Piaroas) and 6 Venezuelan Mestizos, now available in GenBank (accession numbers EU878038–EU878135) and in the MLST database in Oxford (http://www.mlst.net/).
Strain population assignment was performed as described by Falush et al [7], using the “no admixture” model in STRUCTURE2.0 [31], the proportion of nucleotides being derived from ancestral population was estimated using the “linkage” model in Structure2.0 as described [7], [26].
Human mtDNA sequences
A total of 1,148 sequences of human mtDNA hypervariable segment I were retrieved from Genbank from published work (Table 2), from humans close to the hosts from which H. pylori strains had been cultured: non-Bantu Africans, Spanish, Koreans, Amerindians (Guahibo, Huitoto, Inuit) and South American Mestizos.
Table 2. Sources of human mtDNA sequences analyzed in this study.
| Host group | Number of mtDNA sequences | References |
| Africans | 53 | [44] |
| Hausa (non-Bantu) | 13 | |
| Sierra Leone (non-Bantu) | 40 | |
| Europeans-Spanish | 718 | |
| Andalusia | 130 | Genbank; [45]–[47] |
| North Eastern | 118 | [48] |
| Catalans | 61 | [45]–[47] |
| Baleares | 222 | [49] |
| Galicia | 92 | [50] |
| Canary Island, | 54 | [46] |
| Spain various | 41 | [46], [47] |
| East Asians-Koreans | 64 | [51] |
| East Asians- Amerindians | 143 | |
| Guahibo | 66 | GenBank |
| Huitoto | 7 | [52] |
| Inuit (Eskimo) | 70 | [53] |
| Mestizos a | 170 | [54] |
| African haplotypes | 58 | |
| European haplotypes | 59 | |
| Amerindian haplotypes | 53 | |
| TOTAL | 1,148 |
From Venezuela and Colombia.
Statistical analyses
Genetic distances (pairwise distances) of H. pylori isolates and human mtDNA were analyzed by strain ancestral phylogroups and by human host groups. Distances were calculated using the Kimura 2-parameter model in MEGA3 [32]. Nonparametric tests (Kruskal-Wallis rank sum, Wilcoxon test with Bonferroni adjustment for multiple comparisons) were used to compare strains by bacterial population and by host. In addition, these comparisons were performed using permutation tests based on 5000 permutations. All statistical analyses were performed using R statistical software [33].
Supporting Information
Intrapopulation genetic distance of H. pylori strains hpAfrica1 and hpEurope, by host source. hpAfrica1 strains from Mestizos were much more diverse than those from Africans (Kruskal-Wallis p<10−14). In contrast, neither Mestizos nor Amerindians increased the already high variability hpEurope strains from Spain. Medians are represented as the waist of the dress-like box, and the waist side openings indicate the 95% interval for the median. Above and below the median are the 3rd and 1st quartile respectively. The interval in dashed lines represents a maximum of 1.5× interquartile range and the open circles are outliers. Outliers are mostly low pairwise distances, indicating that similar pair of strains are less common than distant strains.
(0.24 MB TIF)
Genetic distances between mtDNA sequences of diverse human groups. Differences between median distances were significant for each of the human groups (Kruskal-Wallis test p<2.2×10−15; Wilcoxon with Bonferroni adjustment p<10−14). Permutation tests with 5,000 permutations confirmed human group differences (p = 0). Distances decreased in the order: mestizos>Africans>Koreans>Spanish> Amerindians. For explanation of the box plot see Figure S1.
(18.39 MB DOC)
Genetic distances of human mtDNA sequences within Africans, Spanish, Koreans and groups of Amerindians and Mestizos. South American Amerindians studied have a degree of admixture as indicated by their higher genetic diversity than the Inuit. The Mestizos with African or Amerindian haplotypes have increased diversity in relation to Mestizos with European haplotypes. For explanation of the box plot see Figure S1.
(0.26 MB TIF)
Acknowledgments
We are grateful to J. Bertrand Petit for providing Spanish sequences of human mtDNA. We thank Gladys Ramos for her help with Figure 3.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by UPR intramural FIPI grant 880314. B.L. was supported by ERA-Net grant BMBF 0313930B to Mark Achtman.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A. 2007;104:2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho L, Chan SY, Burk RD, Das BC, Fujinaga K, et al. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J Virol. 1993;67:6413–6423. doi: 10.1128/jvi.67.11.6413-6423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loureiro CL, Alonso R, Pacheco BA, Uzcategui MG, Villegas L, et al. High prevalence of GB virus C/hepatitis G virus genotype 3 among autochthonous Venezuelan populations. J Med Virol. 2002;68:357–362. doi: 10.1002/jmv.10211. [DOI] [PubMed] [Google Scholar]
- 6.Miura T, Fukunaga T, Igarashi T, Yamashita M, Ido E, et al. Phylogenetic subtypes of human T-lymphotropic virus type I and their relations to the anthropological background. Proc Natl Acad Sci U S A. 1994;91:1124–1127. doi: 10.1073/pnas.91.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falush D, Wirth T, Linz B, Pritchard JKMS, et al. Traces of Human Migrations in Helicobacter pylori Populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 8.Wirth T, Meyer A, Achtman M. Deciphering host migrations and origins by means of their microbes. Mol Ecol. 2005;14:3289–3306. doi: 10.1111/j.1365-294X.2005.02687.x. [DOI] [PubMed] [Google Scholar]
- 9.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran S, Deshpande O, Roseman CC, Rosenberg NA, Feldman MW, et al. Support from the relationship of genetic and geographic distance in human populations for a serial founder effect originating in Africa. Proc Natl Acad Sci U S A. 2005;102:15942–15947. doi: 10.1073/pnas.0507611102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prugnolle F, Manica A, Balloux F. Geography predicts neutral genetic diversity of human populations. Curr Biol. 2005;15:R159–R160. doi: 10.1016/j.cub.2005.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalli-Sforza LL, Menozzi P, Piazza A. The history and geography of human genes. Princeton, NJ: Princeton University Press; 1994. p. 258. [Google Scholar]
- 13.Bortolini MC, Salzano FM, Thomas MG, Stuart S, Nasanen SP, et al. Y-chromosome evidence for differing ancient demographic histories in the Americas. Am J Hum Genet. 2003;73:524–539. doi: 10.1086/377588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reidla M, Kivisild T, Metspalu E, Kaldma K, Tambets K, et al. Origin and diffusion of mtDNA haplogroup X. Am J Hum Genet. 2003;73:1178–1190. doi: 10.1086/379380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schurr T, Sherry ST. Mitocondrial DNA and Y chromosome diversity and the peopling of the Americas: evolutionary and demographic evidence. Am J Hum Biol. 2004;16:420–439. doi: 10.1002/ajhb.20041. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Lewis CM, Jakobson M, Ramachandran S, Ray N, et al. Genetic Variation and Population Structure in Native American. PLoS Genet. 2007;3:e185. doi: 10.1371/journal.pgen.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Prugnolle F, Manica A, Balloux F. A geographically explicit genetic model of worldwide human-settlement history. Am J Hum Genet. 2006;79:230–237. doi: 10.1086/505436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsonnet J. Gastric adenocarcinoma and Helicobacter pylori infection. West J Med. 1994;161:60. [PMC free article] [PubMed] [Google Scholar]
- 19.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandon S. Local adaptation and host-parasite interactions. Trends Ecol Evol. 1998;13:214–216. doi: 10.1016/s0169-5347(98)01358-5. [DOI] [PubMed] [Google Scholar]
- 21.Kirkpatrick M, Barton NH. Evolution of a species range. Am Nat. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- 22.Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 23.Reineking B, Veste M, Wissel C, Huth A. Environmental variability and allocation trade-offs maintain species diversity in a process-based mofel of succulent plant communities. Ecological Modelling. 2006;199:486–454. [Google Scholar]
- 24.Armbrecht I, Perfecto I, Vandermeer J. Enigmatic biodiversity correlations: Ant diversity responds to diverse resources. Science. 2004;304:284–286. doi: 10.1126/science.1094981. [DOI] [PubMed] [Google Scholar]
- 25.Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, et al. Plant genotypic diversity predicts community structure and governs an ecosystem process. Sience. 2006;313:966–968. doi: 10.1126/science.1128326. [DOI] [PubMed] [Google Scholar]
- 26.Linz B, Balloux F, Moodley Y, Manica A, Liu H, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domínguez-Bello MG, Blaser JM. Evolutionary Biology of Bacterial and Fungal Pathogens. Washington D.C.: ASM Press; 2008. Evolution of Helicobacter and Helicobacter Infections. [Google Scholar]
- 28.Kaltz O, Shykoff JA. Local adaptation in host-parasite systems. Heredity. 1998;81:361–370. [Google Scholar]
- 29.Ghose C, Perez-Perez GI, van Doorn LJ, Dominguez-Bello MG, Blaser MJ. High frequency of gastric colonization with multiple Helicobacter pylori strains in Venezuelan subjects. J Clin Microbiol. 2005;43:2635–2641. doi: 10.1128/JCM.43.6.2635-2641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirth T, Wang X, Linz B, Novick RP, Lum JK, et al. Distinguishing human ethnic groups by means of sequences from Helicobacter pylori: lessons from Ladakh. Proc Natl Acad Sci U S A. 2004;101:4746–4751. doi: 10.1073/pnas.0306629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 33.R. Vienna: R Foundation for Statistical Computing; 2006. Developement Core Team. R: A language and environment for statistical computing. http://wwwr-projectorg. [Google Scholar]
- 34.Martìnez H. Determinación de las frecuencias de grupos sanguíneos y polimorfismos de ADN para dos estratos socioeconómicos de la población de Caracas. 2003. Tesis de Grado, Universidad Central de Venezuela, Caracas - Venezuela.
- 35.Acosta M. Caracterizaciòn genética y composición étnica de la población de Churuguara, Edo. Falcòn, mediante los polimorfismos genéticos: ABO, Rh, VWA, FES/FPS y F13A01. 2002. Tesis de Grado, Universidad Central de Venezuela, Caracas - Venezuela.
- 36.Castro D. Relación entre polimorfismos genéticos e historia en dos poblaciones negras venezolanas. Bol Soc Esp Antrop Biol. 1993;14:21–29. [Google Scholar]
- 37.Zambrano-Guzmàn O. Estudio de la estructura genética de Hoyo de La Cumbre: un pueblo del Avila. 1999. Tesis de Grado, Universidad Central de Venezuela, Caracas - Venezuela.
- 38.Gonzàlez-Coira M, Mora J, Sepúlveda S, Cuevas J, Pérez J, et al. Evolución temporal “aparente” de la frecuencia génica de los sistemas ABO y Rh en una población de Mérida.; 1997. :78–79. [Google Scholar]
- 39.Castro-Guerra D, Zambrano-Guzmàn O. Aporte génico español canario en tres poblaciones semiaisladas venezolanas; estimaciones hechas a partir de los sistemas ABO, Rh y -1-antitripsina. Rev Esp Antrop Biol. 2000;21:111–118. [Google Scholar]
- 40.Rodrìguez-Larralde A, Castro D, Gonzàlez-Coira M, Morales J. Frecuencia génica y porcentaje de mezcla en diferentes àreas geográficas de Venezuela, de acuerdo a los grupos Rh y ABO. Interciencia. 2001;26:8–12. [Google Scholar]
- 41.Layrisse M, Wilbert J. Indian Societies of Venezuela.; Instituto Caribe de Antropología y Sociología FlSdCN, editor. Caracas: Editorial Sucre; 1966. [Google Scholar]
- 42.Arends T. Estructura genética de la población indígena de Venezuela. Caracas, Venezuela: Universidad de las Naciones Unidas; 1992. [Google Scholar]
- 43.http://www.bloodbook.com/world-abo.html. Ratial and ethnic distribution of ABO blood types. 2007.
- 44.Graven L, Passarino G, Semino O, Boursot P, Santachiara-Benerecetti S, et al. Evolutionary correlation between control region sequence and restriction polymorphisms in the mitochondrial genome of a large Senegalese Mandenka sample. Mol Biol Evol. 1995;12:334–345. doi: 10.1093/oxfordjournals.molbev.a040206. [DOI] [PubMed] [Google Scholar]
- 45.Plaza S, Calafell F, Helal A, Bouzerna N, Lefranc G, et al. Joining the pillars of Hercules: mtDNA sequences show multidirectional gene flow in the western Mediterranean. Ann Hum Genet. 2003;67:312–328. doi: 10.1046/j.1469-1809.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- 46.Pinto F, Gonzalez AM, Hernandez M, Larruga JM, Cabrera VM. Genetic relationship between the Canary Islanders and their African and Spanish ancestors inferred from mitochondrial DNA sequences. Ann Hum Genet. 1996;60:321–330. doi: 10.1111/j.1469-1809.1996.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 47.Corte-Real HB, Macaulay VA, Richards MB, Hariti G, Issad MS, et al. Genetic diversity in the Iberian Peninsula determined from mitochondrial sequence analysis. Ann Hum Genet. 1996;60:331–350. doi: 10.1111/j.1469-1809.1996.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 48.Crespillo M, Luque JA, Paredes M, Fernández R, Ramírez E, et al. Mitochondrial DNA sequences for 118 individuals from northeastern Spain. Int J Legal Med. 2000:130–132. doi: 10.1007/s004140000158. [DOI] [PubMed] [Google Scholar]
- 49.Picornell A, Gomez-Barbeito L, Tomas C, Castro JA, Ramon MM. Mitochondrial DNA HVRI variation in Balearic populations. Am J Phys Anthropol. 2005;128:119–130. doi: 10.1002/ajpa.10423. [DOI] [PubMed] [Google Scholar]
- 50.Salas A, Comas D, Lareu MV, Bertranpetit J, Carracedo A. mtDNA analysis of the Galician population: a genetic edge of European variation. Eur J Hum Genet. 1998;6:365–375. doi: 10.1038/sj.ejhg.5200202. [DOI] [PubMed] [Google Scholar]
- 51.Horai S, Murayama K, Hayasaka K, Matsubayashi S, Hattori Y, et al. mtDNA polymorphism in East Asian populations, with special reference to the peopling of Japan. Am J Hum Genet. 1996;59:579–590. [PMC free article] [PubMed] [Google Scholar]
- 52.Torres MM, Bravi CM, Bortolini MC, Duque C, Callegari-Jaques S, et al. A Revertant of the Major Founder Native American Haplogroup C Common in Populations From Northern South America. American Journal of Human Biology. 2006;18:59–65. doi: 10.1002/ajhb.20461. [DOI] [PubMed] [Google Scholar]
- 53.Helgason A, Palsson G, Pedersen HS, Angulalik E, Gunnarsdottir ED, et al. mtDNA variation in Inuit populations of Greenland and Canada: migration history and population structure. Am J Phys Anthropol. 2006;130:123–134. doi: 10.1002/ajpa.20313. [DOI] [PubMed] [Google Scholar]
- 54.Alves-Silva J, da Silva Santos M, Guimarães P, Ferreira A, Bandelt HJ, et al. The Ancestry of Brazilian mtDNA Lineages. Am J Hum Genet. 2000;67:444–461. doi: 10.1086/303004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intrapopulation genetic distance of H. pylori strains hpAfrica1 and hpEurope, by host source. hpAfrica1 strains from Mestizos were much more diverse than those from Africans (Kruskal-Wallis p<10−14). In contrast, neither Mestizos nor Amerindians increased the already high variability hpEurope strains from Spain. Medians are represented as the waist of the dress-like box, and the waist side openings indicate the 95% interval for the median. Above and below the median are the 3rd and 1st quartile respectively. The interval in dashed lines represents a maximum of 1.5× interquartile range and the open circles are outliers. Outliers are mostly low pairwise distances, indicating that similar pair of strains are less common than distant strains.
(0.24 MB TIF)
Genetic distances between mtDNA sequences of diverse human groups. Differences between median distances were significant for each of the human groups (Kruskal-Wallis test p<2.2×10−15; Wilcoxon with Bonferroni adjustment p<10−14). Permutation tests with 5,000 permutations confirmed human group differences (p = 0). Distances decreased in the order: mestizos>Africans>Koreans>Spanish> Amerindians. For explanation of the box plot see Figure S1.
(18.39 MB DOC)
Genetic distances of human mtDNA sequences within Africans, Spanish, Koreans and groups of Amerindians and Mestizos. South American Amerindians studied have a degree of admixture as indicated by their higher genetic diversity than the Inuit. The Mestizos with African or Amerindian haplotypes have increased diversity in relation to Mestizos with European haplotypes. For explanation of the box plot see Figure S1.
(0.26 MB TIF)