Abstract
Lactoferrin possesses a wide range of immunomodulatory activities, including promotion of the delayed type hypersensitivity response (DTH) towards BCG (Bacillus Calmette Guerin) antigens. Addition of Lactoferrin as an adjuvant to the BCG vaccine was previously demonstrated to augment protection against subsequent mycobacterial challenge, with concomitant development of a strong T cell helper type 1 (TH1) immunity. Because generation of TH1 immunity is in large part dependent on the balance of monocytic pro- and anti-inflammatory cytokines, the effect of Lactoferrin on leukocytes was investigated. Lactoferrin enhanced proinflammatory responses in a dose-dependant manner from splenocyte and adherent (F4/80+) splenocyte populations, bone marrow derived monocytes (BMM), and J774A.1 cultured cells. In all scenarios tested, Lactoferrin induced a strong increase in the ratio of IL-12:IL-10 production from LPS stimulated cells. Examination of Lactoferrin effects on BCG infected J774A.1 cells and on BMM revealed similar immunomodulatory effects, with particularly strong increase in IL-12 production. Furthermore, immunization of mice with BCG admixed with Lactoferrin led to increased generation of CD4+ cells expressing IFN-γ upon restimulation with BCG antigens. These results provide molecular evidence to support the role of Lactoferrin as an adjuvant candidate to augment development of DTH response to vaccine antigens.
Keywords: Lactoferrin, Macrophage, IL-12, Vaccine adjuvant
Introduction
Lactoferrin is an 80 kDa iron-binding protein belonging to the transferrin family of non-heme iron binding proteins, found primarily in mucosal secretions (e.g. milk) and secondary granules of neutrophils. Lactoferrin is an integral part of the innate immune system [30], with immunomodulatory activities on leukocytes to increase phagocytic activity of neutrophils [32], enhance activation of peritoneal macrophages [14], increase natural killer cell activity [11, 41], and promote maturation of T cells [12] and B cells [51]. Furthermore, Lactoferrin is capable of enhancing delayed type hypersensitivity (DTH) responses, as measured by foot pad swelling, to a variety of antigens, including sheep red blood cells (SRBC), ovalbumin (OVA), and Mycobacterium bovis Calmette Guerin (BCG) [8, 49, 50]. The generation of DTH, a T cell mediated response, against BCG with Lactoferrin suggests the ability of Lactoferrin to augment innate function to promote specific T cell responses against complex antigens.
BCG is the current vaccine for tuberculosis, a disease caused by the intracellular bacterium Mycobacterium tuberculosis (MTB). While BCG has been in use for nearly 85 years, the current vaccine has limited efficacy in controlling MTB infection in adults [5, 16]; there are only a small number of experimental vaccine alternatives that surpass the efficacy of the existing BCG vaccine [6]. Successful control of MTB infection relies on mycobacterial antigen-specific cellular immunity; namely, a T cell dominated DTH response that is often characterized by high induction of IFN-γ, an indication of T cell helper type-1 (TH1) activation [19]. Recently, Lactoferrin as an adjuvant was demonstrated to augment the BCG vaccine to protect against subsequent challenge with virulent MTB [25], with increased IFN-γ recall response to BCG antigens. However, the molecular mechanisms underlying this observation were not defined.
The generation of TH1 immunity is dependent on professional antigen presenting cells (APC) such as macrophages, to produce IL-12, a mediator that promotes naïve T cell development towards the TH1 phenotype [39, 40]. In addition, IL-12 is also a co-stimulator that can maximize secretion of IFN-γ from TH1 cells [35] and activate IFN-γ producing cells from memory T cells [22]. Production of IL-12 is, in part, regulated by IL-10, which is produced by both macrophages and T cells. A high IL-10 level compared to IL-12 will lead to an environment that decreases promotion of TH1 generation [17, 34]. Thus, a high IL-12:IL-10 ratio from activated macrophages is indicative of a cytokine environment that is favored towards promotion of TH1 immunity. A limited number of studies have shown increasing IL-12 with concurrent decrease of IL-10 levels following administration of Lactoferrin [44, 46, 47]. Lactoferrin also demonstrated direct in vivo stimulatory activity in naïve mice with recovered peritoneal cells from mice injected with Lactoferrin exhibiting increased in TNF-α, IL-6, and IL-12 production [1]. None of those studies specifically addressed how Lactoferrin functions as an adjuvant to augment subsequent adaptive responses.
The goal of these studies is to examine in vitro the effect of bovine Lactoferrin on cytokine production from activated and non-activated leukocyte populations [42], with focus on adjuvant activation to elicit cytokines that can drive T cell differentiation. The effects of Lactoferrin will also be examined on BCG infected macrophages, monitoring the production of cytokine mediators that would promote the generation and stimulation of T cell immunity for use in future vaccination studies. In addition, immunization experiments were performed to examine Lactoferrin to promote generation of antigen-specific TH1 responses from CD4+ T cells, as a functional in vivo endpoint for Lactoferrin mediation of response.
Materials and methods
Animals
Female C57BL/6 mice (6 weeks, Jackson Laboratories, Bar Harbor, ME) 20–25 g initial body weight, were used for splenocyte isolation, bone marrow derived monocyte generation, and immunization procedures. All in vivo experiments were conducted under approved guidelines of the animal ethics committee at the University of Texas, Health Science Center at Houston, protocol HSC-AWC-05-060.
Lactoferrin, LPS, BCG, and other reagents
Low endotoxin bovine milk lactoferrin (<1 EU/mg, <20% iron saturated, >95% purity) was provided by PharmaReview Corporation (Houston, TX). Bacterial lipopolysaccha-ride (LPS) (Escherichia coli, Serotype 0111:B4, 3 × 106 EU/mg) was purchased from Sigma Chemical Company (St Louis, MO). Mycobacterium bovis Calmette Guerin (BCG), Pasteur strain (TMC 1011) was purchased from American Type Cell Culture (ATCC, Manassas, VA). All chemicals were of analytical grade.
In vitro cell culture (splenocytes, Adherent F4/80+ cells, J774A.1 cells, and bone marrow derived monocytes)
Whole splenocytes were isolated from C57BL/6 mice as previously reported. Briefly, spleens were minced, and red blood cells were lysed with ACK buffer (Cambrex Bio Sciences, East Rutherford, NJ). To enrich for macrophages, whole splenocytes were depleted of the non-adherent population by incubating cells in T-75 tissue flasks for 2 h prior to use. The adherent population was enriched 140% for F4/80 (a pan macrophage marker) from whole splenocyte populations. Overall contamination of CD3+ cells (T cells) was approximately 10.7%, as demonstrated by fluorescent activated cell sorting (FACS) by staining cells with CD3-PE (R&D Systems, Minneapolis, MN) and F4/80-FITC (Cell Sciences, Canton, MA) [7]. Single cell suspensions were cultured in DMEM complete base [Dulbecco's Modified Eagles Medium (DMEM, Sigma) with 2.2 g/L sodium bicarbonate (Sigma), 0.05 g/L HEPES (Sigma), and 0.05 g/L l-arginine (Sigma)] supplemented with 10% fetal bovine serum (FBS, Sigma), antibiotics [100 μg/mL penicillin G (Sigma) and 50 μg/mL gentamycin sulfate (Sigma)], and 0.005% 2-mercaptoethanol (2Me, Gibco, Carlsbad, CA). Total splenocytes (2 × 106 cells/mL) and adherent splenocytes (1 × 106 cells/mL) were cultured with or without LPS (400 ng/mL). Increasing concentrations of Lactoferrin (1, 10, 100 μg/mL) were added to splenocytes after 5 min with LPS (when indicated) and incubated at 37°C with 5% CO2 for 48 h. Supernatants were collected and stored at −20°C for analysis by ELISA in triplicate. LPS dose response was performed to realize optimal versus suboptimal stimulation for all cell subsets and cell lines.
Bone marrow cells were isolated from C57BL/6 mice (6 weeks, Jackson Laboratories, Bar Harbor, ME) by flushing the femur with McCoy's medium supplemented with antibiotics—100 μg/mL penicillin G (Sigma) and 50 μg/mL gentamycin sulfate (Sigma). Collected cells were treated with ACK buffer (Cambrex Bio Sciences, East Rutherford, NJ) to lyse the red blood cells. Resulting cells were differentiated for 7 days at 1 × 106 cells/mL in McCoy's medium, supplemented with 2.2 g/L sodium bicarbonate, 10% FBS, and GM-CSF (10 ng/mL) (Cell Sciences, Canton, MA). At day 7, non-adherent cells were washed off and adherent cells (estimated at 5 × 105 cells/well) rested overnight in DMEM complete media—supplemented with 50 mg/L HEPES (Sigma), 50 mg/L l-arginine (Sigma), 2.2 g/L sodium bicarbonate (Sigma), and 10% FBS (Sigma)—at 37°C with 5% CO2. Macrophages remained non-infected or infected with BCG with and without Lactoferrin (100 μg/mL). Supernatants were collected post-infection, and stored at 20°C for analysis by ELISA.
Murine-derived macrophage cell line J774A.1 was purchased from ATCC (#TIB-67). Macrophage cells were grown in DMEM complete base with 10% FBS. Macrophages were plated at 5 × 105 cells/mL, rested for 4 h at 37°C with 5% CO2, then washed with 1× PBS (Dulbecco's phosphate buffered saline 10×, Cellgro, Herndon, VA). Macrophages were cultured with or without LPS (1, 10 ng/mL) in the absence or presence of increasing concentrations of Lactoferrin (1, 10, 100 μg/mL), which was added 5 min after the addition of LPS (when indicated). Supernatants were collected at 24 and 72 h and stored at −20°C for analysis by ELISA in triplicate.
BCG infection
Infection with Mycobacterium bovis Calmette Guerin (BCG), Pasteur strain TMC 1011 was accomplished as reported previously [26]. Briefly, organisms were grown in Dubos base (without addition of glycerol) with 10% supplement (5% BSA and 7.5% dextrose in saline) on an orbital shaker at 37°C for 2 weeks before use. Bacteria taken during log phase growth were resuspended in 1× PBS, sonicated for 10 s to dislodge any clumping that might have occurred, and diluted to 3 × 108 organisms/mL, estimated using McFarland Equivalence Turbidity Standards (Remel, Lenesa, KS). Cells were maintained at 37°C with 5% CO2 in DMEM complete base supplemented with 10% FBS. Before use in infections, cells were adjusted to 5 × 105 cells/mL in 24-well tissue culture plates. J774A.1 macrophages were infected with MOI (multiplicity of infection) of 10:1 (#organisms:#cells). Supernatants were collected after infection to assess cytokine production, measured by ELISA.
Enzyme linked immunosorbent assay (ELISA)
Supernatants were assayed for cytokine production using the DuoSet ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. Briefly, the captured antibody was coated onto Costar EIA/RIA 1 × 8 Stripwell™ plates (Corning Inc., Corning, NY) in 1× PBS at 100 μL/well and incubated at room temperature overnight. Plates were washed with wash buffer (0.05% v/v Tween 20 in 1× PBS) three times (3×). Blocking buffer (1% w/v bovine serum albumin, BSA, 5% w/v sucrose, 0.05% w/v sodium azide in 1× PBS) was added at 400 μL/well and incubated at room temperature for at least 1 h. After washing, isolated supernatants were loaded in triplicate at 100 μL/well along with dilutions of cytokine standards and incubated at room temperature for 2 h. After washing, detection antibody diluted in manufacturer reagent diluent (R&D Systems, 1% BSA in 1× PBS) was added at 100 μL/well and incubated at room temperature for 2 h. Plates were washed and Streptavidin-HRP diluted at 1:200 was added at 100 μL/well; the plate was further incubated at room temperature for 20 min away from direct light. SureBlue™ TMB microwell peroxidase substrate 1-component (KPL, Gaithersburg, MD) was added at 100 μL/well; 50 μL/well of stop solution (2 N H2SO4) was added after 30 min of dark incubation. Plates were read at 450 nm subtracting background at 570 nm. Concentration was calculated by generating a standard curve fit. The following cytokines were measured by ELISA using triplicate wells; TNF-α, IL-6, IL-12p40, IL-10 and IFN-γ. The lower assay limits of detection were approximately 15–25 pg/mL.
Intracellular staining
C57BL/6 mice remained non-immunized or were immunized and boosted at 2 weeks with BCG (1 × 106 CFU/mouse) or with BCG/Lactoferrin (100 μg/mouse) in 1× PBS subcutaneously (s.c.) at the base of the tail. At 8 weeks post-boost, splenocytes were examined for specific recall responses, as described with slight modifications [25, 45]. Briefly, isolated splenocytes were cultured in DMEM complete medium supplemented with β-Me, antibiotics, and IL-2 (100 ng/mL), and stimulated with heat-killed BCG (1:1 or 10:1). After 24 or 72 h, splenocytes were restimulated with ConA (2 μg/mL) or with Ionomycin (250 ng/mL, Sigma) and phorbol 12-myristate 13-acetate (PMA, 10 ng/mL, Sigma) in the presence of golgi plug (BD Biosciences Pharmingen, San Diego, CA) for 4–6 h. Intracellular staining was conducted as per the manufacturer's instructions (BD). Non-specific surface antigens were blocked with CD16/CD32 FcγIII/II receptor (1 μg/1 × 106 cells), and were then stained with CD4-FITC (1 μg/1 × 106 cells/50 μL). Splenocytes were washed with staining buffer (1% BSA in 1× PBS), then fixed and permeablized with 250 μL/sample BD Cytofix/Cytoperm. Cells were further stained with IFN-γ-PE (0.5 μg/1 × 106 cells/50 μL) and subsequent analysis was performed using Coulter Flow-Centre™ (EPICS XL-MCL). Results represent cells gated to exclued non-specific isotype matched control staining. Graphs were generated with WinMDI 2.8.
Statistical analysis
All experiments were completed at least three times with supernatants analyzed each time in triplicate. The flow cytometric analysis was completed twice, using 4–6 mice per experiment. Average values and standard deviations of groups are shown. Statistical analysis was carried out using one way ANOVA; differences were considered significant at P ≤ 0.05.
Results
Lactoferrin affects inflammatory and TH1 mediators from LPS activated splenocytes
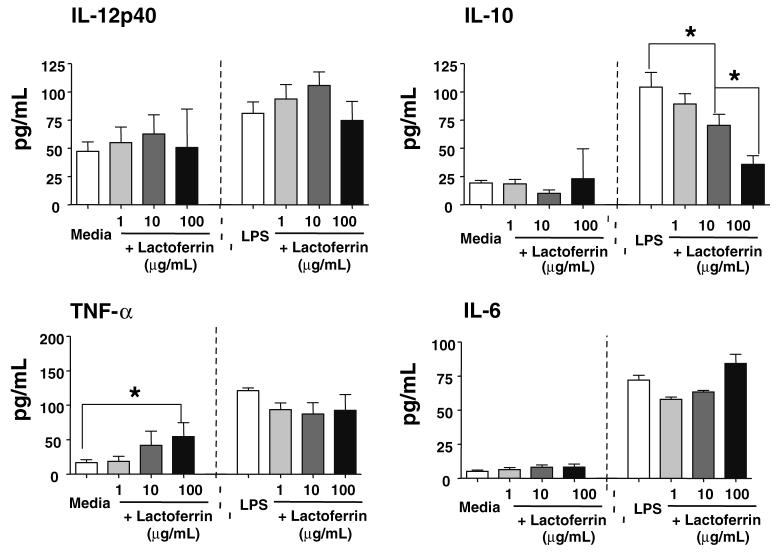
Previous studies on the in vivo effect of Lactoferrin to modulate monocytic cytokine production examined cytokine production from isolated peritoneal cells. Mice treated with Lactoferrin yielded activated peritoneal cells with increased production of TNF-α, IL-6 and IL-12 [1]. The immunomodulatory activities of Lactoferrin were further examined in vitro on murine splenocytes under activation by LPS. Splenocytes were stimulated with LPS (400 ng/mL), a potent stimulant of macrophages involving TLR-4 and CD14 receptors. Increasing concentrations of Lactoferrin (1, 10, 100 μg/mL) were added to LPS activated splenocytes or naïve splenocytes and monitored for production of inflammatory mediators, IL-12p40, IL-10, TNF-α and IL-6 (Fig. 1).
Fig. 1.
Examination of the effect of Lactoferrin on production of proinflammatory mediators from LPS stimulated splenocytes. Splenocytes were stimulated with LPS (400 ng/mL) with or without increasing concentrations of Lactoferrin (1, 10, 100 μg/mL). Supernatants were collected at 48 h and analyzed by ELISA for production of IL-12p40 and IL-10 (top) or TNF-α and IL-6 (bottom); average value with standard deviation shown. *P < 0.05
IL-12p40 production was slightly increased from splenocytes stimulated with LPS (81 ± 10 pg/mL) compared to unstimulated splenocytes. Production of IL-12p40 from LPS stimulated splenocytes was further increased with the addition of Lactoferrin at 1 μg/mL (94 ± 13 pg/mL) and 10 μg/mL (106 ± 12 pg/mL). In contrast, there was a major reduction in IL-10 production from LPS stimulated splenocytes when Lactoferrin was added to the cultures. LPS stimulation alone enhance IL-10 production (104 ± 13 pg/mL) compared to the media control (19 ± 2 pg/mL). The addition of Lactoferrin decreased splenic IL-10 production in a concentration-dependent manner: 10 μg/mL (70 ± 10 pg/mL) and 100 μg/mL (36 ± 8 pg/mL).
Addition of Lactoferrin (100 μg/mL) to naïve splenocytes led to a slight, but significant (P < 0.05) increase in TNF-α production (54 ± 28 pg/mL), and no significant changes in IL-6, IL-12p40, or IL-10 production. LPS stimulated splenocytes increased production of TNF-α (121 ± 4 pg/mL), but no significant changes were observed with the addition of Lactoferrin. Similarly, IL-6 production was increased with LPS stimulation (72 ± 4 pg/mL), and no changes were observed with the addition of Lactoferrin.
The effect of Lactoferrin on splenic populations was further investigated on adherent splenocytes enriched for cells expressing F4/80, a pan macrophage marker. The increased IL-12 was produced from this population. Comparison of the ratio of IL-12:IL-10 from LPS stimulated adherent splenocytes demonstrated that addition of Lactoferrin caused a modest but significant (P < 0.05) increase in IL-12:10 ratios in a concentration-dependent manner (Table 1).
Table 1.
Lactoferrin increases IL-12:10 ratio from LPS stimulated splenocytes
| Lactoferrin (μg/mL) | – | 1 | 10 | 100 |
|---|---|---|---|---|
| LPS | + | + | + | + |
| Splenocytes | 0.78 ± 0.10 | 1.05 ± 0.14 | 1.50 ± 0.17* | 2.09 ± 0.47* |
| F4/80+Enriched | 1.28 ± 0.11 | 1.14 ± 0.07 | 0.93 ± 0.01 | 2.03 ± 0.15 |
Splenocytes and F4/80+ enriched adherent cells from C57BL/6 mice were stimulated with LPS (400 ng/mL) with or without increasing concentrations of Lactoferrin (1, 10, 100 μg/mL). Supernatants were collected and analyzed by ELISA. Concentrations of IL-12p40 were divided by IL-10. Average ratio with standard deviation shown for three experiments, each done in triplicate
P < 0.05 relative to non-Lactoferrin treated controls
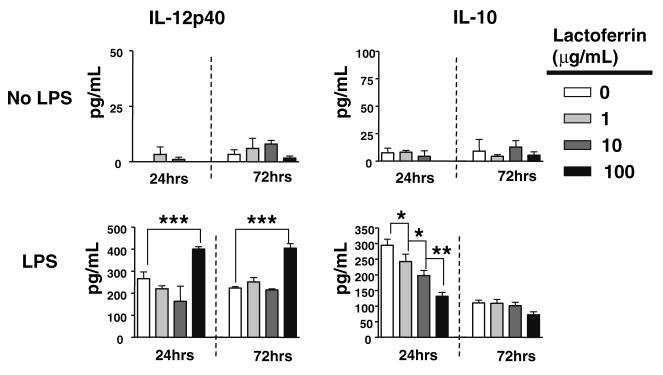
Lactoferrin modulation of cytokine production from J774A.1 macrophage cells lines activated by LPS
J774A.1 macrophages were stimulated with LPS at a suboptimal (1 ng/mL) or stimulatory (10 ng/mL) dose with or without increasing concentrations of Lactoferrin (1, 10, 100 μg/mL). Lactoferrin alone only slightly affected J774A.1 macrophages grown under normal conditions, with little change in IL-12p40 and IL-10 (Fig. 2, top), or TNF-α and IL-6 (not shown), without further mitogenic stimulation. However, upon stimulation with LPS, Lactoferrin was able to mediate proinflammatory responses. Production of IL-12p40 and IL-10 were greatly affected by Lactoferrin (Fig. 2, bottom). Suboptimal dose of LPS (1 ng/ mL) stimulation generated production of IL-12p40 from J774A.1 macrophages at 24 h (266 ± 31 pg/mL) and 72 h (224 ± 6 pg/mL); addition of 100 μg/mL of Lactoferrin further increased IL-12p40 production at 24 h (400 ± 11 pg/mL) and 72 h (404 ± 21 pg/mL). J774A.1 macrophages did not produce significant levels of IL-10 without stimulation with LPS at the higher concentration (10 ng/mL). J774A.1 macrophages stimulated with 10 ng/mL of LPS increased production IL-10 at 24 h (294 ± 19 pg/mL). Addition of Lactoferrin to LPS (10 ng/mL) stimulated J774A.1 macrophages decreased the production of IL-10 in a concentration-dependent manner: 1 μg/mL (242 ± 24 pg/mL), 10 μg/mL (198 ± 16 pg/mL), 100 μg/mL (131 ± 12 pg/mL). Overall, the ratio of production of IL-12:IL-10 was increased when cells were stimulated with LPS (1 and 10 ng/mL) in the presence of Lactoferrin at 72 h. A complete table of response is given in Table 2 (top, middle).
Fig. 2.
Effects of Lactoferrin on cytokine production from macrophages stimulated with suboptimal concentration of LPS. J774A.1 murine macrophages were left untreated as media control (top), or stimulated with LPS at 1 ng/mL (bottom), with or without increasing concentrations of Lactoferrin (1, 10, 100 μg/mL). Supernatants were collected at 24 and 72 h and analyzed by ELISA for production of IL-12 (left), or IL-10 (right); average value with standard deviation shown. *P < 0.05, **P < 0.01
Table 2.
Lactoferrin induces an increase in IL-12:IL-10 ratio from LPS-stimulated or BCG infected macrophages
| IL-12:IL-10 ratio | ||||
|---|---|---|---|---|
| Lactoferrin (μg/mL) | – | 1 | 10 | 100 |
| LPS (ng/mL) | 1 | 1 | 1 | 1 |
| Average ratio | 18.64 | 15.04 | 10.25* | 28.88* |
| Standard deviation | 0.47 | 1.20 | 0.21 | 1.46 |
| Lactoferrin (μg/mL) | – | 1 | 10 | 100 |
| LPS (ng/mL) | 10 | 10 | 10 | 10 |
| Average ratio | 19.80 | 19.99 | 21.57 | 30.27+ |
| Standard deviation | 0.01 | 0.01 | 0.01 | 0.01 |
| Lactoferrin (μg/mL) | – | 1 | 10 | 100 |
| BCG (MOI) | 10:1 | 10:1 | 10:1 | 10:1 |
| Average ratio | 6.57 | 8.81* | 16.56* | 11.65* |
| Standard deviation | 0.51 | 0.42 | 1.02 | 0.72 |
J774A.1 macrophages were stimulated with 1 ng/mL LPS (top) or 10 ng/mL LPS (middle) with or without increasing concentrations of Lactoferrin (1, 10, 100 μg/mL). Alternatively, cells were infected with BCG (MOI 10:1) (bottom) with or without Lactoferrin. Supernatants were collected at 72 h and analyzed by ELISA. Ratio was calculated by dividing concentration of IL-12p40 by IL-10.
P < 0.05 relative to non-Lactoferrin treated controls
At 72 h post stimulation, 100 μg/mL of Lactoferrin significantly elevated production of TNF-α (167 ± 18 pg/mL) compared to LPS alone (101 ± 17 pg/mL). Similarly, IL-6 was further elevated with the addition of 100 μg/mL of Lactoferrin (757 ± 45 pg/mL), compared to LPS alone (634 ± 15 pg/mL) (not shown).
Lactoferrin mediation of IL-12 and IL-10 production from BCG infected macrophages
The activity of Lactoferrin was further examined on cytokine production from BCG infected macrophages. J774A.1 macrophages were infected with BCG at MOI of 10:1 with or without increasing concentrations of Lactoferrin (1, 10, 100 μg/mL) (Fig. 3, top). Bone marrow derived macrophages were examined using 100 μg/mL Lactoferrin (Fig. 3, bottom). For both cell populations, IL-12p40 production from BCG infected cells was significantly enhanced by the addition of Lactoferrin. By 72 h, BCG infected J774A.1 macrophages in the presence of Lactoferrin (100 μg/mL) increased production of IL-12p40 in a concentration-dependent manner, with the highest IL-12p40 concentration observed at 100 μg/mL of Lactoferrin (179 ± 11 pg/mL), compared to non-Lactoferrin treated controls (116 ± 9 pg/mL). There was little to no production of IL-10 at any time point, regardless of infection status or addition of Lactoferrin. Examination of the ratio of production of IL-12:IL-10 from BCG infected macrophages (Table 2, bottom) demonstrated similar properties as shown for cells stimulated with LPS; increasing concentrations of Lactoferrin led to increased IL-12:IL-10 ratios from infected cells. Similarly, the bone marrow derived macrophages were able to increase IL-12 production with concurrent reduction in IL-10 when Lactoferrin was added during BCG infection.
Fig. 3.
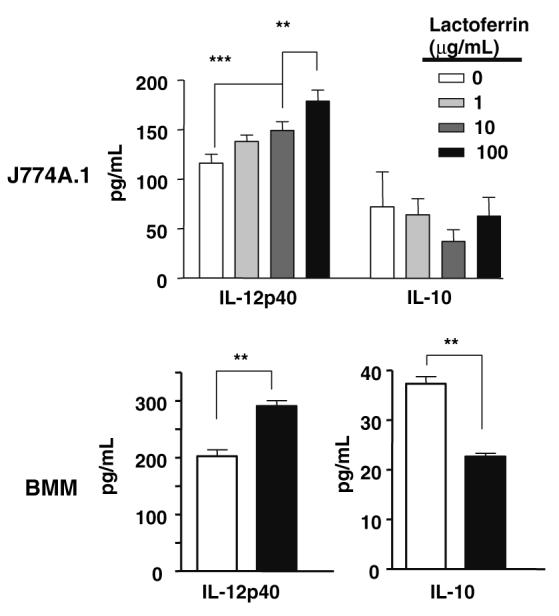
Lactoferrin activity on inflammatory cytokine production from BCG stimulated macrophages. J774A.1 murine macrophages (top) or bone marrow derived macrophages (bottom) were infected with live BCG at MOI 10:1 with and without indicated concentrations of Lactoferrin. Supernatants collected were analyzed by ELISA for IL-12p40, and IL-10; average values with standard deviation shown. **P < 0.01, ***P < 0.001
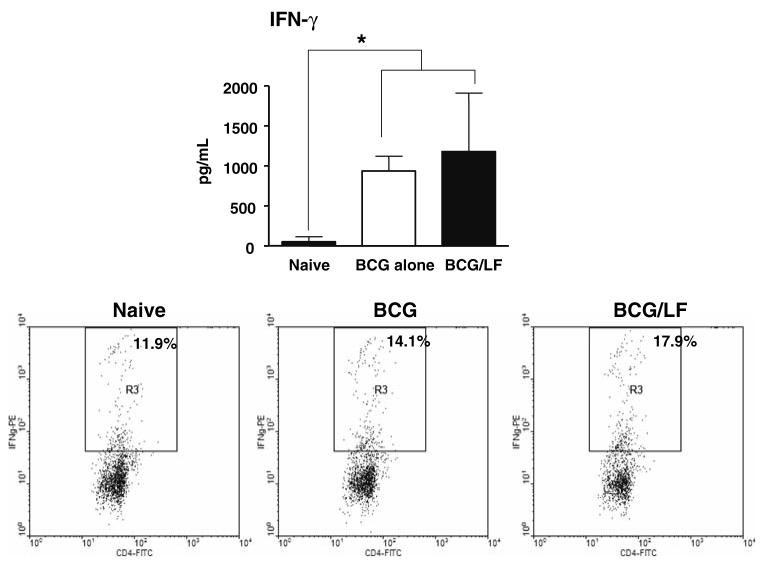
Immunization with BCG and Lactoferrin generates increased IFN-γ producing BCG antigen-speciWc CD4+ cells
The biological significance of increased IL-12-IL-10 ratios was examined through immunization experiments to determine if immunization in the presence of Lactoferrin would alter numbers of CD4+ cells generated that produce IFN-γin specific response to BCG antigens. Mice were immunized with BCG, or BCG admixed with Lactoferrin (100 μg/mL), and boosted after 2 weeks. Eight weeks later, recall responses to BCG antigens were examined. Both the BCG and the BCG/Lactoferrin immunized mice demonstrated high IFN-γ production (Fig. 4, top) compared to non-immunized control splenocytes. No significant IL-4 production above that of the naïve control group was seen (not shown). Splenocyte populations were also subjected to analysis by flow cytometry and intracellular staining (example for one animal shown in Fig 4, bottom) revealing significantly increased numbers of CD4+ cells producing IFN-γ in the Lactoferrin adjuvant group in response to antigenic stimulation (group average,18.36% ± 0.67) compared to BCG alone immunized cells (group average, 11.99% ± 2.93) (P < 0.05). These experiments demonstrate that Lactoferrin included in the vaccination formulation was capable of augmenting the generation of antigen-specific CD4+ TH1 phenotypic cells.
Fig. 4.
Immunization with BCG and Lactoferrin promotes increased IFN-γ production and high numbers of BCG antigen-specific CD4+ cells. Splenocytes from naïve mice, or from mice 8 weeks after immunization with BCG or BCG admixed with Lactoferrin, were stimulated in vitro with BCG antigens. IFN-γ production was measured by ELISA (top), expressed as average values with standard deviation for 6 mice (* P < 0.05). The same populations were restimulated in vitro with BCG antigens; flow cytometric analysis and intracellular staining (bottom) revealed increases in numbers of IFN-γ producing CD4+ cells in the BCG-Lactoferrin immunized population (right), compared to naïve (left) or BCG alone (middle) immunized groups. Representative flow data shown for splenocytes from four stimulated mice per group
Discussion
Lactoferrin is an integral part of the innate immune system [30] which is recognized as an immunomodulator of leukocyte populations, including neutrophils [32], peritoneal macrophages [14], natural killer cells [11, 41], T cells [12], and B cells [51]. More importantly, Lactoferrin as an adjuvant is capable of enhancing the T cell mediated DTH response to a variety of antigens including Mycobacterium bovis Calmette Guerin (BCG) [27, 49, 50]. The primary goal of these studies was to examine, in vitro, the effect of bovine Lactoferrin on cytokine production from activated and non-activated leukocyte populations [42], with focus on defining responsive phenotypes and identifying cytokines that can drive T cell differentiation, in particular IL-12 and IL-10. The secondary goal was to establish that Lactoferrin administered with a complex vaccine antigen would result in increased production of CD4+ T helper antigen-specific responses, a subject under active investigation [18].
Receptors for Lactoferrin are present on both lymphocytes and macrophages [43], thus evaluation of Lactoferrin activity was initially examined on splenocyte. Whole splenocytes stimulated with LPS in the presence of Lactoferrin increased the ratio of IL-12:IL-10 production. The activity was found in adherent splenocytes enriched for F4/80+ (a pan macrophage marker) cells, suggesting that the cell population responding to LPS and Lactoferrin stimulation was macrophages. Indeed, direct stimulation of CD3+ lymphocytes by Lactoferrin does not occur (unpublished observations). Addition of Lactoferrin to naïve macrophages increased TNF-α production, a stimulatory effect that has also been reported elsewhere [42]. The effect of Lactoferrin on activated macrophages is a more complex phenomenon. Addition of Lactoferrin to macrophages stimulated with suboptimal LPS (1 ng/mL) increased production of IL-12 and decreased production of IL-10. This stimulatory activity may be attributed to Lactoferrin forming a complex with LPS to facilitate macrophage activation through CD14 (LPS co-stimulatory molecule) or the TLR-4 (LPS receptor) [36]. Previous studies on LPS activated macrophages and models of sepsis also reported this inhibitory effect of Lactoferrin to moderate other proinflammatory mediators [9, 37]. Certainly, the ability of Lactoferrin to inhibit LPS-induced production of TNF-α and IL-6 is present when Lactoferrin is administered prophylactically (and even therapeutically) [29], or even when given therapeutically. The anti-inflammatory effects of Lactoferrin may simply be attributed to its ability to disrupt LPS binding of CD14 [3, 15]. Other studies indicate that Lactoferrin's anti-inflammatory effect may be more complex, and is regulated through modulation of nitric oxide mediators [28]. Of interest to this report, Lactoferrin decreased inflammatory responses while increasing macrophage mediators to potentially direct development of TH1 immunity.
Increasing IL-12 is an important step in directing development of the TH1 response [39, 40], critical for protection against MTB infection [10, 21]. Lactoferrin-induced increased relative IL-12 would address the mechanisms by which Lactoferrin acts as an adjuvant to augment BCG vaccine efficacy against subsequent mycobacterial challenge [25]. In the studies outlined here, addition of Lactoferrin to BCG infected macrophages enhanced the IL-12:IL-10 ratio, with J774A.1 macrophages and BMM infected with BCG producing significant amounts of IL-12. More critical was the finding that immunization in the presence of Lactoferrin led to antigen-specific responses to BCG that were of the TH1 phenotype. The increased IFN-γ production was further identified as derived in large part from CD4+ T cells, thus indicating a functional link to observations seen in the tissue culture experiments. At this time, it is unknown how Lactoferrin causes macrophages to influence T cell responses in vivo. It may be that Lactoferrin functions through modification of proinflammatory response affecting how macrophages communicate with other cells in a local environment. Just as likely, Lactoferrin may modify surface molecule expression on APC, allowing for direct regulation of responding lymphocytes. These areas require further research.
Lactoferrin can also increase production of TNF-α and IL-6 from stimulated macrophages. During MTB infection, TNF-α and IL-6 work in concert to initiate and influence the development of the granulomatous response, an organization of cellular infiltrate at the site of infection important in containment of bacteria [2, 4, 20, 33, 38]. However, TNF-α and IL-6 are also involved in directing the generation of the TH1 response, but unlike IL-12, they are indirectly involved in suppressing TH1 responses. TNF-α inhibits IL-12 and the subsequent production of IFN-γ, limiting IL-12 through both IL-10 dependent and independent means [23, 31, 48]. IL-6 suppresses TH1 development by boosting the TH2 activity of IL-4 and interfering with IFN-γ signaling [13], and redirecting T helper responses [24]. At this time, the role for Lactoferrin in modifying TNF-α and IL-6 production in infected cells is less clear.
The experiments presented here suggested that Lactoferrin modulates the cytokine environment of LPS stimulated and BCG infected macrophages to allow, potentially, promotion of a substantial TH1 development, in vivo. It also suggests that the activity of Lactoferrin is dependent on the activation status of the macrophage. The direct molecular mechanisms for Lactoferrin mediation of immune activation and cytokine production still remain unresolved. However, the results from this report identify Lactoferrin as an ideal adjuvant candidate, and provide one possible molecular explanation to support its use to boost efficacy of the BCG vaccine to promote host protective responses during virulent MTB infection.
Acknowledgments
This work was accomplished with support from NIH grant R41AI51050-01 and R42-AI051050-02. We thank Michal Zimecki Ph.D. (Department of Experimental Therapy, Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland) and Robert L. Hunter, M.D., Ph.D. (University of Texas-Houston Medical School, Department of Pathology) for helpful conversations and data discussion, and Margaret Olsen for assistance and technical expertise.
References
- 1.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002;2:475–486. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 2.Actor JK, Olsen M, Jagannath C, Hunter RL. Relationship of survival, organism containment, and granuloma formation in acute murine tuberculosis. J Interferon Cytokine Res. 1999;19:1183–1193. doi: 10.1089/107999099313136. [DOI] [PubMed] [Google Scholar]
- 3.Baveye S, Elass E, Fernig DG, Blanquart C, Mazurier J, Legrand D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect Immun. 2000;68:6519–6525. doi: 10.1128/iai.68.12.6519-6525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 5.Behr MA. BCG-different strains, different vaccines? Lancet Infect Dis. 2002;2:86–92. doi: 10.1016/s1473-3099(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 6.Brewer TF. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31(Suppl 3):S64–67. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Boylan MT, Evans CA, Whetton AD, Wright EG. Application of two-dimensional difference gel electrophoresis to studying bone marrow macrophages and their in vivo responses to ionizing radiation. J Proteome Res. 2005;4:1371–1380. doi: 10.1021/pr050067r. [DOI] [PubMed] [Google Scholar]
- 8.Chodaczek G, Zimecki M, Lukasiewicz J, Lugowski C. A complex of lactoferrin with monophosphoryl lipid A is an efficient adjuvant of the humoral and cellular immune response in mice. Med Microbiol Immunol (Berl) 2006;195:207–216. doi: 10.1007/s00430-006-0020-3. [DOI] [PubMed] [Google Scholar]
- 9.Choe YH, Lee SW. Effect of lactoferrin on the production of tumor necrosis factor-alpha and nitric oxide. J Cell Biochem. 1999;76:30–36. doi: 10.1002/(sici)1097-4644(20000101)76:1<30::aid-jcb4>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiens E, Mazurier J, Yazidi I el, Masson M, Duthille I, Spik G, Boilly-Marer Y. Effects of human lactoferrin on NK cell cytotoxicity against haematopoietic and epithelial tumour cells. Biochim Biophys Acta. 1998;1402:277–287. doi: 10.1016/s0167-4889(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 12.Dhennin-Duthille I, Masson M, Damiens E, Fillebeen C, Spik G, Mazurier J. Lactoferrin upregulates the expression of CD4 antigen through the stimulation of the mitogen-activated protein kinase in the human lymphoblastic T Jurkat cell line. J Cell Biochem. 2000;79:583–593. [PubMed] [Google Scholar]
- 13.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 14.Edde L, Hipolito RB, Hwang FF, Headon DR, Shalwitz RA, Sherman MP. Lactoferrin protects neonatal rats from gut-related systemic infection. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1140–1150. doi: 10.1152/ajpgi.2001.281.5.G1140. [DOI] [PubMed] [Google Scholar]
- 15.Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias PS, Mazurier J, Spik G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun. 1998;66:486–491. doi: 10.1128/iai.66.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 17.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 18.Fischer R, Debbabi H, Dubarry M, Boyaka P, Tome D. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem Cell Biol. 2006;84:303–311. doi: 10.1139/o06-058. [DOI] [PubMed] [Google Scholar]
- 19.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 20.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 21.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 22.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 23.Hodge-Dufour J, Marino MW, Horton MR, Jungbluth A, Burdick MD, Strieter RM, Noble PW, Hunter CA, Pure E. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:13806–13811. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hovav AH, Mullerad J, Davidovitch L, Fishman Y, Bigi F, Cataldi A, Bercovier H. The Mycobacterium tuberculosis recombinant 27-kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protection. Infect Immun. 2003;71:3146–3154. doi: 10.1128/IAI.71.6.3146-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol. 2005;5:591–599. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Indrigo J, Hunter RL, Jr, Actor JK. Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology. 2003;149:2049–2059. doi: 10.1099/mic.0.26226-0. [DOI] [PubMed] [Google Scholar]
- 27.Kocieba M, Zimecki M, Kruzel M, Actor J. The adjuvant activity of lactoferrin in the generation of DTH to ovalbumin can be inhibited by bovine serum albumin bearing alpha-d-mannopyranosyl residues. Cell Mol Biol Lett. 2002;7:1131–1136. [PubMed] [Google Scholar]
- 28.Kruzel ML, Bacsi A, Choudhury B, Sur S, Boldogh I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology. 2006 doi: 10.1111/j.1365-2567.2006.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruzel ML, Harari Y, Mailman D, Actor JK, Zimecki M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin Exp Immunol. 2002;130:25–31. doi: 10.1046/j.1365-2249.2002.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruzel ML, Zimecki M. Lactoferrin and immunologic dissonance: clinical implications. Arch Immunol Ther Exp (Warsz) 2002;50:399–410. [PubMed] [Google Scholar]
- 31.Ma X, Sun J, Papasavvas E, Riemann H, Robertson S, Marshall J, Bailer RT, Moore A, Donnelly RP, Trinchieri G, Montaner LJ. Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J Immunol. 2000;164:1722–1729. doi: 10.4049/jimmunol.164.4.1722. [DOI] [PubMed] [Google Scholar]
- 32.Miyauchi H, Hashimoto S, Nakajima M, Shinoda I, Fukuwatari Y, Hayasawa H. Bovine lactoferrin stimulates the phagocytic activity of human neutrophils: identification of its active domain. Cell Immunol. 1998;187:34–37. doi: 10.1006/cimm.1997.1246. [DOI] [PubMed] [Google Scholar]
- 33.Moreira AL, Tsenova L, Aman MH, Bekker LG, Freeman S, Mangaliso B, Schroder U, Jagirdar J, Rom WN, Tovey MG, Freedman VH, Kaplan G. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect Immun. 2002;70:2100–2107. doi: 10.1128/IAI.70.4.2100-2107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 35.Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Na YJ, Han SB, Kang JS, Yoon YD, Park SK, Kim HM, Yang KH, Joe CO. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int Immunopharmacol. 2004;4:1187–1199. doi: 10.1016/j.intimp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Roseanu A, Chelu F, Trif M, Motas C, Brock JH. Inhibition of binding of lactoferrin to the human promonocyte cell line THP-1 by heparin: the role of cell surface sulphated molecules. Biochim Biophys Acta. 2000;1475:35–38. doi: 10.1016/s0304-4165(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 38.Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun. 2000;68:3322–3326. doi: 10.1128/iai.68.6.3322-3326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur J Immunol. 1994;24:793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 40.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shau H, Kim A, Golub SH. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. J Leukoc Biol. 1992;51:343–349. [PubMed] [Google Scholar]
- 42.Sorimachi K, Akimoto K, Hattori Y, Ieiri T, Niwa A. Activation of macrophages by lactoferrin: secretion of TNF-alpha, IL-8 and NO. Biochem Mol Biol Int. 1997;43:79–87. doi: 10.1080/15216549700203841. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki YA, Lonnerdal B. Characterization of mammalian receptors for lactoferrin. Biochem Cell Biol. 2002;80:75–80. doi: 10.1139/o01-228. [DOI] [PubMed] [Google Scholar]
- 44.Teraguchi S, Wakabayashi H, Kuwata H, Yamauchi K, Tamura Y. Protection against infections by oral lactoferrin: evaluation in animal models. Biometals. 2004;17:231–234. doi: 10.1023/b:biom.0000027697.83706.32. [DOI] [PubMed] [Google Scholar]
- 45.Umemura M, Nishimura H, Saito K, Yajima T, Matsuzaki G, Mizuno S, Sugawara I, Yoshikai Y. Interleukin-15 as an immune adjuvant to increase the efficacy of Mycobacterium bovis bacillus Calmette-Guerin vaccination. Infect Immun. 2003;71:6045–6048. doi: 10.1128/IAI.71.10.6045-6048.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakabayashi H, Kurokawa M, Shin K, Teraguchi S, Tamura Y, Shiraki K. Oral lactoferrin prevents body weight loss and increases cytokine responses during herpes simplex virus type 1 infection of mice. Biosci Biotechnol Biochem. 2004;68:537–544. doi: 10.1271/bbb.68.537. [DOI] [PubMed] [Google Scholar]
- 47.Wakabayashi H, Takakura N, Yamauchi K, Tamura Y. Modulation of immunity-related gene expression in small intestines of mice by oral administration of lactoferrin. Clin Vaccine Immunol. 2006;13:239–245. doi: 10.1128/CVI.13.2.239-245.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zganiacz A, Santosuosso M, Wang J, Yang T, Chen L, Anzulovic M, Alexander S, Gicquel B, Wan Y, Bramson J, Inman M, Xing Z. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113:401–413. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimecki M, Kocieba M, Kruzel M. Immunoregulatory activities of lactoferrin in the delayed type hypersensitivity in mice are mediated by a receptor with affinity to mannose. Immunobiology. 2002;205:120–131. doi: 10.1078/0171-2985-00115. [DOI] [PubMed] [Google Scholar]
- 50.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett. 2000;74:183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 51.Zimecki M, Mazurier J, Spik G, Kapp JA. Human lactoferrin induces phenotypic and functional changes in murine splenic B cells. Immunology. 1995;86:122–127. [PMC free article] [PubMed] [Google Scholar]