Dietary polyphenols (Fig. 1) are a main source of antioxidants for humans1. These polyphenols have a variety of biological activities, ranging from anti-ageing, anticancer, to lowering of blood cholesterol level and improving bone strength1–6. Millions of women also take a subclass of polyphenols called phytoestrogens to relieve symptoms associated with menopause7, 8.
Fig. 1.
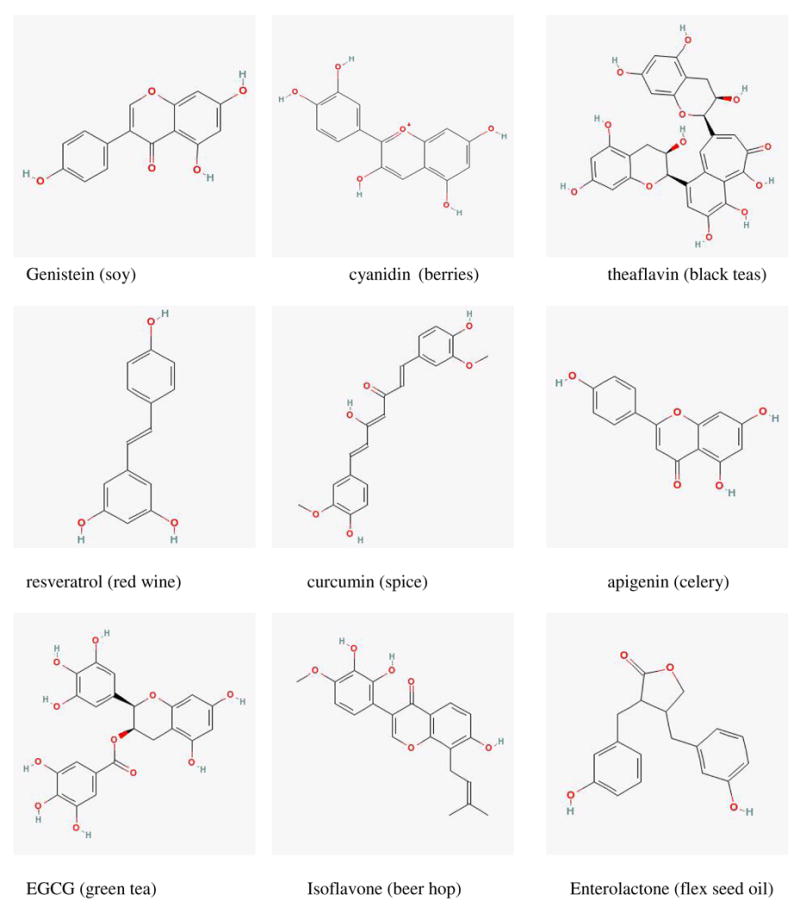
Structures of Representative Dietary Polyphenols.
Dietary polyphenols are derived from plants, and are consumed in the forms of fruits, vegetables, species and herbs. Dietary intakes of polyphenols widely fluctuate between cultures, ethnic groups, and even within a narrow geological location. Large percentages of dietary polyphenols are consumed in the form of flavonoids, although cultural and dietary habit will dictate which forms of polyphenols are taken up. For example, in northeastern Asian countries such as China and Japan, isoflavones are main source of polyphenols along with other flavonoids derived from teas, vegetables and fruits9, 10. In southeastern Asian countries such as India, a significant percentage of population takes large quantities of curcumin as result of ingesting the turmeric spices11. In European countries, a large population of people consumes lignans as the result of ingesting cereal bran or whole grain breads or flex seed oils9, 10. Finally, in population worldwide, many people consume teas, which also contains large amounts of polyphenols and have a variety of effects including anticancer3.
A majority of the population takes sufficient amounts of dietary polyphenols and enjoys the beneficial their effects. However, a large percentage of adults living in Western and developed countries and a smaller but growing adult population living in the developing countries such as India and China are not taking sufficient quantities of dietary polyphenols12. This selected population appears to have distastes for fruits and vegetables that are rich in flavonoids and other polyphenols. The reason for this lack of interest in healthy food probably varies greatly, but some researchers have attributed this to the fast-paced life style and fast food restaurants that will not serve tasteful fruits and vegetables.
Many people are hoping that they can one day take pills that will provide the same benefit effects of dietary polyphenols without eating or eating minimal amounts of fruits and vegetables. A large population of people (e.g., prostate cancer patients13) also believes that they should take additional pills with flavonoids and other polyphenols even though they are taking recommended quantities of fruits and vegetables in hopes of achieving more beneficial effects, as evidenced by ever increasing amounts of dietary supplements consumed in developed countries. They are motivated by scientific research that is widely carried in the news media, which indicated these flavonoids and polyphenols could prevent cancer, ageing, and cardiovascular diseases3, 12, 14–16. However, these research are often carried out in animals and their effects in humans remain uncertain17.
A critically important scientific question is then: are these flavonoids and polyphenols as effective as people believed? Many researchers have devoted significant efforts to the study of dietary polyphenols and hundreds of grants have been funded by a variety of funding agency to determine if the polyphenols are indeed active. A large body of evidence, mainly derived from preclinical studies in animals, has concluded that dietary polyphenols, when given in large quantities, can have desirable outcomes12, 14, 15. Although a few government sponsored trials are ongoing, it is too complex or too costly to demonstrate the effectiveness in humans, because these agents have limited if any intellectual property protection. In addition, poor bioavailability of polyphenols makes it even more difficult to conduct relevant but smaller clinical trials because large exposure differences are expected among the participants. Typical polyphenols have oral bioavailability (mostly in animals) of 10% of less, and range of 2–20% is quite common. Assuming exposure in humans, which are more genetically diverse than experimental animals, have similar differences, a very large population is needed to demonstrate efficacy which are often not affordable. Therefore, an urgent issue in the development of polyphenols as disease prevention agents is find a way to increase their bioavailability so we can use a smaller population to conduct relevant trials.
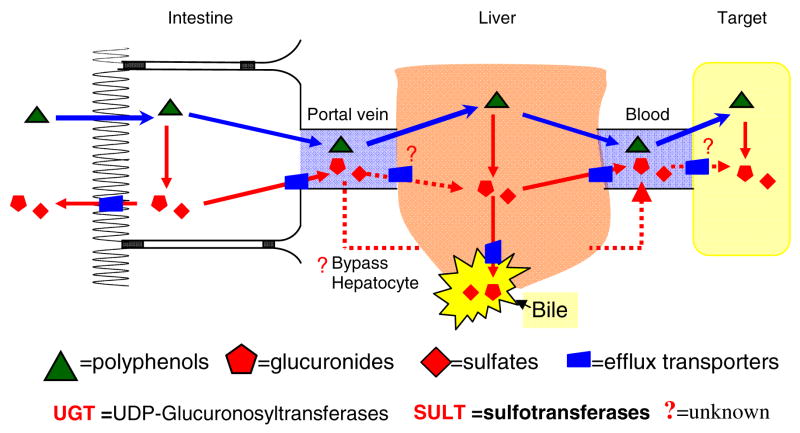
In order to increase the bioavailability of polyphenols, we must overcome multiple challenges associated with their development. Because these agents are targeted for disease prevention, oral route of administration is the only viable route, except for topical application on external organs such as skin. For polyphenols to become bioavailable, the following barriers must be overcome: solubility, permeability, metabolism, excretion, target tissue uptake and disposition (Fig. 2). If we deliver hydrophilic polyphenols, which are typically glycosides, they are usually too polar or sometimes are too large to rapidly penetrate the intestinal membrane18. Instead, these glycosides often need the action of intestinal or more likely the microfloral enzymes to release sugar so the polyphenols are available as the more absorbable aglycone forms18, 19. Aglycones forms are highly permeable in Caco-2 and perfused rat intestinal models and are expected to be rapidly absorbed20–22. However, the bioavailability is not high because pure aglycone forms have very poor solubility, often less than 20 μg/ml in water. This low solubility can cause slow dissolution rates, which can slow down the absorption. Coupled with the fact that absorbed aglycones are rapidly conjugated to glucuronides via UGT and sulfates via ST in intestine and liver23, 24, solubility can becomes a critical factor as higher aglycone concentration can overwhelm the metabolic enzymes allowing more drugs to reach the systemic circulation intact. Therefore, there is an urgent need to perform systematic studies to demonstrate how changes in polyphenol structures affect solubility and dissolution rates, and how various pharmaceutical excipients may be used to improve their dissolution rate.
Fig. 2.
Organ Bioavailability Barriers to Polyphenol Bioavailability. We depicted a organ bioavailability that ultimately determines the bioavailability of polyphenols.
The bioavailability issue is important since we only know that the aglycones are active. Very few studies have attempted to determine if the metabolites are active. Historically, conjugates of polyphenols are considered to be inactive even though studies are not published to demonstrate that this is the case, primarily because it is difficult to purchase these metabolites from commercial sources. Although many phase II conjugates are shown pharmacologically inactive, some are more pharmacologically active than parent compound, including morphine-glucuronide and ezetimibe-glucuronide25. Therefore, we believe that it is necessary to conduct more mechanistic studies to determine the activities or functions of these phase II conjugates.
How metabolites move across different biological membrane is critically important, assuming some metabolites are active or can be converted into active parent compounds at target organs. Transport of lipophilic conjugates out of the main metabolic organs such as liver and intestine has only been studied recently, and available evidence suggests that a variety of organic transporters may be involved in the transport of these conjugates in and out of cells. However, these studies have mainly been conducted in liver and intestine and almost none in other vital organs (e.g., heart) or target organs (e.g., mammary glands). The latter is partially because we are unable or incapable of determining the concentrations of metabolites as we lack standards to do so. We believe that more studies in this area of research would help us understand how active metabolites may work in vivo.
Lastly, use of large amounts of concentrated flavonoids may post as public health concerns, as limited in vivo information are known about their adverse effects and their ability to interact with other drugs. Among the hot debated subject is the use of soy isoflavones in women26. Limited study has shown that soy isoflavone genistein can stimulate the growth of MCF-7 cells in the absence of hormone27, but this remains a debatable point since genistein inhibits estradiol or estrone sulfate stimulated MCF-7 cell growth. Another important point is that flavones and isoflavones may interact with broad spectrum efflux inhibitors such as breast cancer resistance protein28.
In this special issue of Molecular Pharmaceutics, we have assembled a group of active and experienced researchers. They each provided their own research results or survey current research landscape in the form of review articles. Taken together, our collection serve to make a broad assessment of current knowledge base and state-of-the-art techniques. Additional papers represented current or new methodologies that can be used to assess the bioavailability of flavonoids and polyphenols. As you can find, the lack of ability to improve bioavailable other than the use of methylated prodrugs29 suggests the need to further our research into this field since methylation often can change the activities of polyphenols. More bioavailable or highly bioavailable polyphenol formulations or derivatives are very desirable because they will be easier to develop and less costly to test. Therefore, we hope that this Special Molecular Pharmaceutics Issue will also serve the purpose of stimulating more discussion and research of the bioavailability problems among molecular and pharmaceutical scientists. With their help, the successful development of polyphenols as chemopreventive agents in the future will soon be within our reach.
Acknowledgments
Work supported by NIH GM070737.
References
- 1.Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food. 2005;8(3):281–90. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- 2.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 3.Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol Carcinog. 2006;45(6):431–5. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- 4.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387(6633):561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 6.Krzystyniak KL. Current strategies for anticancer chemoprevention and chemoprotection. Acta Pol Pharm. 2002;59(6):473–8. [PubMed] [Google Scholar]
- 7.Tempfer CB, Bentz EK, Leodolter S, Tscherne G, Reuss F, Cross HS, Huber JC. Phytoestrogens in clinical practice: a review of the literature. Fertil Steril. 2007;87(6):1243–9. doi: 10.1016/j.fertnstert.2007.01.120. [DOI] [PubMed] [Google Scholar]
- 8.Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–81. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher RJ. Food sources of phyto-oestrogens and their precursors in Europe. Br J Nutr. 2003;89(Suppl 1):S39–43. doi: 10.1079/BJN2002795. [DOI] [PubMed] [Google Scholar]
- 10.Slavin J. Why whole grains are protective: biological mechanisms. Proc Nutr Soc. 2003;62(1):129–34. doi: 10.1079/PNS2002221. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 12.Adhami VM, Mukhtar H. Polyphenols from green tea and pomegranate for prevention of prostate cancer. Free Radic Res. 2006;40(10):1095–104. doi: 10.1080/10715760600796498. [DOI] [PubMed] [Google Scholar]
- 13.Bemis DL, Capodice JL, Costello JE, Vorys GC, Katz AE, Buttyan R. The use of herbal and over-the-counter dietary supplements for the prevention of prostate cancer. Curr Urol Rep. 2006;7(3):166–74. doi: 10.1007/s11934-006-0017-x. [DOI] [PubMed] [Google Scholar]
- 14.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18(9):567–79. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120(3):451–8. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 16.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50(2):170–5. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc Res. 2007;73(2):341–7. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30(4):370–7. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Liu Y, Dai Y, Xun L, Hu M. Enteric disposition and recycling of flavonoids and ginkgo flavonoids. J Altern Complement Med. 2003;9(5):631–40. doi: 10.1089/107555303322524481. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55(2):159–69. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang SW, Chen J, Jia X, Tam VH, Hu M. Disposition of flavonoids via enteric recycling: structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab Dispos. 2006;34(11):1837–48. doi: 10.1124/dmd.106.009910. [DOI] [PubMed] [Google Scholar]
- 22.Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med. 2004;36(7):829–37. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Ng SP, Wong KY, Zhang L, Zuo Z, Lin G. Evaluation of the first-pass glucuronidation of selected flavones in gut by Caco-2 monolayer model. J Pharm Pharm Sci. 2004;8(1):1–9. [PubMed] [Google Scholar]
- 24.Zhang L, Lin G, Chang Q, Zuo Z. Role of intestinal first-pass metabolism of baicalein in its absorption process. Pharm Res. 2005;22(7):1050–8. doi: 10.1007/s11095-005-5303-7. [DOI] [PubMed] [Google Scholar]
- 25.Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44(5):467–94. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 26.Barnes S. Soy isoflavones--phytoestrogens and what else? J Nutr. 2004;134(5):1225S–1228S. doi: 10.1093/jn/134.5.1225S. [DOI] [PubMed] [Google Scholar]
- 27.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131(11 Suppl):3095S–108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 28.Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78(18):2116–30. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Walle T, Wen X, Walle UK. Improving metabolic stability of cancer chemoprotective polyphenols. Expert Opin Drug Metab Toxicol. 2007;3(3):379–88. doi: 10.1517/17425255.3.3.379. [DOI] [PubMed] [Google Scholar]