Abstract
Calanquinone A (1) was isolated from an EtOAc-soluble extract of Calanthe arisanensis through bioassay-guided fractionation. Its structure was identified by spectroscopic methods. Compound 1 showed potent cytotoxicity (EC50 < 0.5 µg/mL) against lung (A549), prostate (PC-3 and DU145), colon (HCT-8), breast (MCF7), nasopharyngeal (KB), and vincristine-resistant nasopharyngeal (KB-VIN) cancer cell lines, and interestingly, showed an improved drug resistance profile compared to paclitaxel. The total synthesis of 1 was also achieved and reported herein.
The Calanthe genus in the Orchidaceae family contains terrestrial perennial herbs that are widely distributed from tropical Africa and Madagascar to tropical and subtropical Asia, China, Japan, southward through Malaysia and Indonesia to the Pacific islands and Australia. This genus includes more than 150 species, but only nineteen are found in Taiwan. Among them Calanthe arisanensis Hayata is endemic to Taiwan and grows in forests from 1000 to 2000 m throughout the island.1
A phytochemical study of this plant has not been reported to date. In cytotoxicity screening of extracts of Formosan plants, an EtOAc extract of C. arisanensis was found to be active against various human cancer cell lines with IC50< 20 µg/mL. Bioassay-directed chromatographic fractionation of this extract produced a new phenanthraquinone calanquinone A (1). Compound 1 showed significant in vitro cytotoxic activicity against seven human cancer cell lines, as described below.
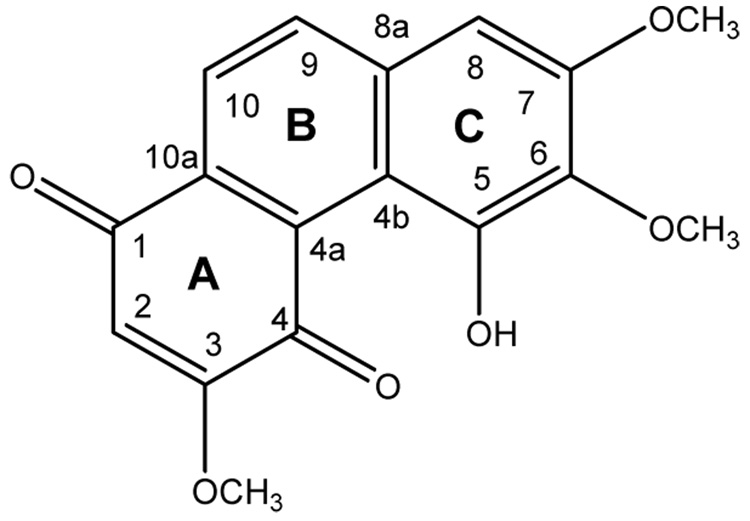
The active MeOH extract (225 g) of dry roots of C. arisanensis (5.42 kg) was partitioned between EtOAc and water (1:1, v/v). Further fractionation of the active EtOAc extract (32.7 g) by repeated liquid chromatography on silica gel gave calanquinone A (1). HRESIMS of 1 showed a [M-H]− ion at m/z 313.0705 (C17H14O6 -H), indicating 11 degrees of unsaturation. The IR spectrum showed absorptions for hydroxyl (3348 cm−1), carbonyl (1642 cm−1), and aromatic ring (1626, 1514, 1460, 1411, and 844 cm−1) functional groups. UV absorptions at 242, 308, and 426 nm also indicated an aromatic system. Seventeen carbon signals, including three methoxy, four methine, and ten quaternary carbons, were observed in the NMR spectra of 1 (Table 1). Among the ten quaternary carbons, two were identified as carbonyl carbons on the basis of chemical shifts δC 184.7 and 186.2. Therefore, the data supported the presence of two carbonyls, six olefins, and three ring moieties to fulfill the 11 degrees of unsaturation, and 1 was postulated to be an phenanthrenedione or anthrenedione.2 1D NMR and HSQC data indicated the presence of three methoxy groups at δH 3.96, 4.01, and 4.02 (δC 57.1, 56.2, and 61.0), one pair of ortho-coupled aromatic protons at δH 8.05 (d, J=8.7 Hz, δC 137.1) and 8.10 (d, J=8.7 Hz, δC 122.0), and two olefinic protons at δH 6.15 (s, δC 107.4) and 6.86 (s, δC 101.4). In the HMBC spectra, the olefinic proton at δH 6.86 exhibited 2J interactions with a carbon at δC 155.2 (C-7), as well as 3J interactions with carbons at δC 118.7 (C-4b), 140.4 (C-6), and 137.1 (C-9). The other olefinic proton at δH 6.15 exhibited 2J interactions with a carbon at δC 161.7 (C-3), as well as 3J interactions with carbons at δC 186.2 (C-4) and 133.0 (C-10a). Locations of methoxy groups at C-3, C-6, and C-7 were confirmed by the following NOESY correlations: δH 6.15 (H-2)/3.96 (3-OMe), δH 6.86 (H-8)/4.01 (7-OMe) and 8.05 (H-9), δH 4.01 (7-OMe)/4.02 (6-OMe), and δH 8.05 (H-9)/8.10 (H-10). Thus, compound 1 was identified as 5-hydroxy-3,6,7-trimethoxy-1,4-phenanthrenequinone and has been named as calanquinone A (1).
Table 1.
NMR data of calanquinone A (1)a
| Proton | 13C (δC) | 1H (δH) | HMBC (13C no) | NOESY (1H no) |
|---|---|---|---|---|
| 1 | 184.7 | |||
| 2 | 107.4 | 6.15 s | 3, 4, 10a | C3-OCH3 |
| 3 | 161.7 | |||
| 4 | 186.2 | |||
| 4a | 128.3 | |||
| 4b | 118.7 | |||
| 5 | 148.3 | |||
| 6 | 140.4 | |||
| 7 | 155.2 | |||
| 8 | 101.4 | 6.86 s | 4b, 6, 7, 9 | 9, C7-OCH3 |
| 8a | 135.1 | |||
| 9 | 137.1 | 8.05 d (8.7) | 4b, 8, 10a | 8, 10 |
| 10 | 122.0 | 8.10 d (8.7) | 1, 4a, 8a | 9 |
| 10a | 133.0 | |||
| −OCH3 | C3-OCH3 57.1 | C3-OCH3 3.96 s | 3 | 2 |
| C6-OCH3 61.0 | C6-OCH3 4.02 s | 6 | C7-OCH3 | |
| C7-OCH3 56.2 | C7-OCH3 4.02 s | 7 | 8, C6-OCH3 | |
| C5-OH | 10.73 |
Measured in CDCl3 (300 and 500 MHz, δ in ppm, J in Hz).
Compound 1 is related in structure to other naturally occurring phenanthrenequinones, including the des-oxy analog sphenone (lacking the C-5 OH group),3 cymbinodin A (lacking the two methoxy groups at C-6 and C-7),4 and annoquinone A (lacking any substituents on ring C).5,6 In prior studies, sphenone and annoquinone A showed cytotoxic activity against the KB cell line (reported EC50 2.73 and 1.65 µg/mL, respectively).
Compound 1 exhibited potent cytotoxicity (EC50 0.03–0.45 µg/mL) against human lung (A549), prostate (PC-3 and DU145), colon (HCT-8), breast (MCF7), nasopharyngeal (KB), and vincristine-resistant nasopharyngeal (KB-VIN) cancer cell lines. Paclitaxel was used as a positive control (data shown in Table 2). Interestingly, 1 exhibited comparble potency against both KB and its drug-resistant KB-VIN subline, and thus, showed an improved drug resistance profile compared to paclitaxel. The cytotoxic values demonstrate the strong potential of 1 as a promising lead compound and C. arisanensis as a promising plant source of new agents for cancer chemotherapy.
Table 2.
Cytotoxicity of calanquinone A (1) isolated from C. arisanensis
| EC50 (µg/mL) / Cell line | |||||||
|---|---|---|---|---|---|---|---|
| Compound | A549 | PC-3 | DU145 | HCT-8 | MCF-7 | KB | KB-VIN |
| Calanquinone A (1) | 0.19 | 0.16 | 0.34 | 0.20 | 0.03 | 0.32 | 0.45 |
| Paclitaxela | <0.005 | 0.0097 | <0.005 | 0.21 | 0.0072 | <0.005 | 2.16 |
positive control
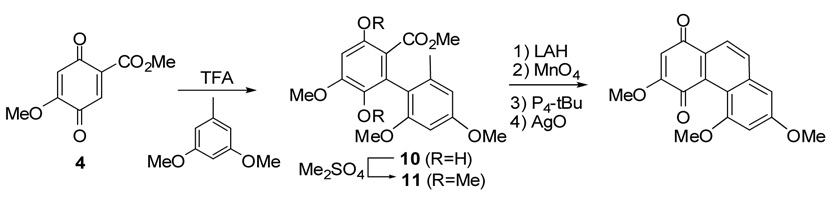
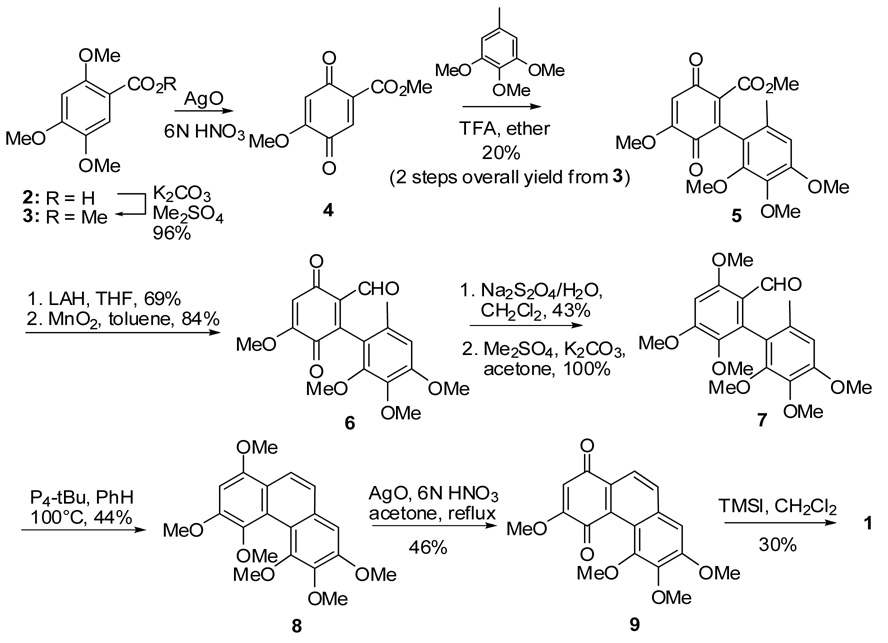
In order to make sufficient quantities of 1 for extensive biological evaluation, we modified the synthetic procedure of Kraus and co-workers7,8 (Scheme 1) to synthesize 1. As shown in Scheme 2, we prepared 2-methoxy-5-carboxylic acid methyl ester-1,4-quinone (4)9 by AgO oxidation of the methyl ester (3) of commercially available 2,4,5-trimethoxybenzoic acid (2). Compound 4 was coupled with 3,4,5-trimethoxytoluene in the presence of 1 equiv. of trifluoroacetic acid to produce quinone 5, although Kraus reported the production of hydroquinone 10 under these reaction conditions (Scheme 1). The quinone skeleton of 5 was confirmed from 13C-NMR data, which showed two carbonyl groups at 180.6 and 183.6 ppm. In addition, reaction of 5 with Me2SO4 did not give a methoxylated product (i.e., 11 in Scheme 1). We attempted to reduce 5 with Na2S2O4 to obtain the corresponding hydroquinone, which could be transformed to the Kraus type of intermediate 11 (R = OMe) after methylation. Despite extending the reaction time and adding more reducing agent, only starting material was recovered.
Scheme 1.
Synthetic procedure of Kraus.
Scheme 2.
Our total synthesis of calanquinone A (1).
Therefore, 5 was reduced with LAH (THF, reflux, 1 h) to alcohol, which was oxidized selectively to aldehyde 6 with activated MnO2 (toluene, 110 °C, overnight) (Scheme 2). After an unsuccessful attempt to obtain the phenanthraquinone 9 directly from 6 using P4-tBu, quinone 6 was first reduced to the hydroquinone using aq. Na2S2O4 (CH2Cl2, rt., overnight),10 and then methylated with Me2SO4 in the presence of K2CO3(acetone, 60 °C, 1.5 h) to give the desired compound 7 (Scheme 2). Cyclization of 7 with P4-t Bu (benzene, 100 °C, 63 h) gave 8, which was oxidized with AgO (6N HNO3, acetone, 50 °C, 2–3 min) to phenanthraquinone 9. Compound 9 was converted to calanquinone A (1) by selective demethylation with TMSI in CH2Cl2 at r.t. (Scheme 2).
Synthesized 1 and intermediates 5–9 were screened in an in vitro cytotoxicity assay (data shown in Table 3). Compound 1 exhibited the highest potency (EC50 0.15–0.75 µg/mL) against all seven tested cancer cell lines. The remaining compounds showed no (6–8) or only weak (5 and 9) activity. Clearly, the potency of 1 merits further study and our synthetic route can efficiently produce sufficient quantities of 1 for future extensive biological evaluation and SAR investigation.
Table 3.
Cytotoxicity of synthesized 1 and related intermediates
| EC50 (µg/mL) / Cell line | |||||||
|---|---|---|---|---|---|---|---|
| Compound | A549 | PC3 | DU145 | HCT8 | MCF7 | KB | KB-VIN |
| 1 | 0.31 | 0.75 | 0.48 | 0.29 | 0.15 | 0.30 | 0.24 |
| 5 | 6.12 | 6.09 | 4.74 | 5.85 | 6.40 | 4.02 | 5.48 |
| 6 | NA | NA | NA | NA | NA | NA | NA |
| 7 | NA | NA | NA | NA | NA | NA | NA |
| 8 | NA | NA | NA | NA | NA | NA | NA |
| 9 | 7.06 | 7.81 | 4.40 | 15.25 | 5.33 | 8.08 | 9.14 |
NA: no activity up to 20 µg/mL
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at
Figure 1.
Structure of calanquinone A (1).
Acknowledgments
This work was supported by grants from National Cancer Institute, NIH, USA (CA 17625, K. H. Lee), National Science Council, Taiwan (Y.-C. Wu) and KMU-EM-97-2.1.b (Y.-C. Wu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Boufford DE, Hsieh C-F, Huang T-C, Kuoh C-S, Ohashi H, Su H-J, Yeh H-Y, Hsiao J-L, Hsiao S-H. Flora of Taiwan. Vol. 5. Taipei, Taiwan: Editorial Committee of the Flora of Taiwan; 2000. pp. 776–777.pp. 782–783. [Google Scholar]
- 2.Fan C, Wang W, Wang Y, Qin G, Zhao W. Phytochemistry. 2001;57:1255. doi: 10.1016/s0031-9422(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 3.Wu TS, Huang SC, Chen MT, Jong TT. Phytochemistry. 1989;28:1280. [Google Scholar]
- 4.Barula AK, Ghosh BB, Ray S, Patra A. Phytochemistry. 1990;29:3056. [Google Scholar]
- 5.Wu TS, Jong TT, Tien HJ, Kuoh CS, Furukawa H, Lee KH. Phytochemistry. 1987;26:1623. [Google Scholar]
- 6.Krohn K, Loock U, Paavilainen K, Hausen B, Schmalle HW, Kiesele H. ARKIVOC. 2001;2:88. [Google Scholar]
- 7.Kraus GA, Hoover K, Zhang N. Tetrahedron Lett. 2002;43:5319. [Google Scholar]
- 8.Kraus GA, Zhang N. Tetrahedron Lett. 2002;43:9597. [Google Scholar]
- 9.Parker KA, Spero DM, Koziski KA. J. Org. Chem. 1987;52:183. [Google Scholar]
- 10.Hormi OEO, Moilanen AM. Tetrahedron. 1998;54:1943. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at