Abstract
To explore the role of nonmuscle myosin II isoforms during mouse gametogenesis, fertilization, and early development, localization and microinjection studies were performed using monospecific antibodies to myosin IIA and IIB isotypes. Each myosin II antibody recognizes a 205-kDa protein in oocytes, but not mature sperm. Myosin IIA and IIB demonstrate differential expression during meiotic maturation and following fertilization: only the IIA isoform detects metaphase spindles or accumulates in the mitotic cleavage furrow. In the unfertilized oocyte, both myosin isoforms are polarized in the cortex directly overlying the metaphase-arrested second meiotic spindle. Cortical polarization is altered after spindle disassembly with Colcemid: the scattered meiotic chromosomes initiate myosin IIA and microfilament assemble in the vicinity of each chromosome mass. During sperm incorporation, both myosin II isotypes concentrate in the second polar body cleavage furrow and the sperm incorporation cone. In functional experiments, the microinjection of myosin IIA antibody disrupts meiotic maturation to metaphase II arrest, probably through depletion of spindle-associated myosin IIA protein and antibody binding to chromosome surfaces. Conversely, the microinjection of myosin IIB antibody blocks microfilament-directed chromosome scattering in Colcemid-treated mature oocytes, suggesting a role in mediating chromosome–cortical actomyosin interactions. Neither myosin II antibody, alone or coinjected, blocks second polar body formation, in vitro fertilization, or cytokinesis. Finally, microinjection of a nonphosphorylatable 20-kDa regulatory myosin light chain specifically blocks sperm incorporation cone disassembly and impedes cell cycle progression, suggesting that interference with myosin II phosphorylation influences fertilization. Thus, conventional myosins break cortical symmetry in oocytes by participating in eccentric meiotic spindle positioning, sperm incorporation cone dynamics, and cytokinesis. Although murine sperm do not express myosin II, different myosin II isotypes may have distinct roles during early embryonic development.
INTRODUCTION
The cortex of mature mouse oocytes is polarized: the area adjacent to the eccentrically positioned second meiotic spindle is devoid of cortical granules and surface microvilli, diminished in concanavalin A lectin binding and demonstrates an increase in cortical actin filaments (reviewed by Longo, 1989). Similar events are observed in the cortex and plasma membrane in the vicinity of the incorporating sperm head (Nicosia et al., 1977, 1978; Shalgi et al., 1978). The induction of these cortical and cell surface modifications are strongly correlated to the presence of meiotic chromosomes or demembranated sperm DNA (Longo and Chen, 1985; Maro et al., 1986; Schatten et al., 1986a; Van Blerkom and Bell, 1986). Actin filaments and actin-binding proteins like fodrin have been implicated with the dynamic changes observed in cortical structure and function during murine development (Reimer and Lehtonen, 1985; Sobel and Allegro, 1985; Damjanov et al., 1986; Schatten et al., 1986b). Cytoskeletal inhibitors like cytochalasin B and latrunculin, in combination with antiactin antibodies and phalloidin analogues, have shown that microfilaments are crucial for cortical meiotic spindle positioning and maintenance, formation of the first and second polar bodies, incorporation cone formation, pronuclear apposition, and cytokinesis (Longo and Chen, 1985; Maro et al., 1986; Schatten et al., 1986a; Webb et al., 1986). Actin assembly is not required for sperm head penetration in the mouse (Simerly et al., 1993).
Nonmuscle myosin II heavy chain isozymes are encoded by at least two genes whose products have been designated myosin heavy chains A and B (Kelley et al., 1995; Phillips et al., 1995; Rochlin et al., 1995). Although little is known regarding the distribution and functions of these myosin II isotypes, the cDNAs encoding mammalian myosin heavy chains A and B isoforms have been cloned and sequenced and antibodies raised to isoform-specific regions (Phillips et al., 1995). Analysis has demonstrated that the mRNAs encoding each nonmuscle isoform is expressed in many tissues and cell lines (Katsuragawa et al., 1989; Saez et al., 1990, Toothaker et al., 1991). Furthermore, they are differentially expressed in neurons and cultured cells. Each nonmuscle isoform may perform different cellular tasks, e.g., growth cone protrusion versus retraction (Sun and Chantler, 1991; Cheng et al., 1992; Miller et al., 1992; Murakami and Elzinga, 1992; Maupin et al., 1994; Rochlin et al., 1995).
Notwithstanding the literature on microfilaments in mammalian oocytes and embryos, little is known about myosin II distribution or functioning in mammalian gametes. Myosin II is reported to be homogeneously distributed in the cortex of immature rat oocytes (Amsterdam et al., 1976). In preimplantation mouse embryos and blastocysts, myosin II is found in the apical surfaces of blastomeres, restricted to these cortical regions by the continuous basolateral cell contacts formed in the early embryo (Sobel, 1983a,b; 1984; Slager et al., 1992). Myosins have also been detected in the nuclei of rat testicular primary spermatocytes (Campanella et al., 1979; De Martino et al., 1980; Walt et al., 1982), in the neck and subacrosomal region of mature human spermatozoa (Campanella et al., 1979; Virtanen et al., 1984), and detected biochemically in ejaculated bull sperm (Tamblyn, 1981). In this report, we use affinity-purified, isoform-specific antibodies to myosin IIA and myosin IIB to detect both proteins in mouse gametes. Both myosin isoforms demonstrate coincidental as well as differential expression during key developmental stages of meiotic maturation, fertilization, and first mitosis. Furthermore, antibody microinjection studies with the IIA and IIB isoforms suggest different functional roles for these proteins during meiosis. Finally, myosin light chain phosphorylation may play a crucial role in the completion of sperm incorporation, as suggested from microinjection experiments with a mutant 20-kDa myosin regulatory light chain (mMLC20) which cannot be phosphorylated in vivo by myosin light chain kinase. These studies suggest that conventional myosins play an active role in meiosis, sperm incorporation, and early development in mammals.
MATERIALS AND METHODS
Antisera and Bacterially expressed MLC20 Probes
The production, affinity purification, and characterization of rabbit polyclonal antibodies to myosins I and IIA, their inhibition of actin-activated ATPase activity in vitro, and cross-reactivity with a variety of mammalian nonmuscle cell myosins have been described (de Lanerolle et al., 1993; Nowak et al., 1997). Myosin IIB antibody is an affinity-purified antipeptide antibody raised against the carboxyl-terminal end of T cell myosin heavy chain B and does not cross-react with myosin A on Western blots (Rochlin et al., 1995). No immunolabeling was detected with myosin preimmune sera in oocytes or sperm.
Microfilaments were colocalized with myosins using either rhodamine phalloidin (Molecular Probes, Eugene, OR) or a mAb to actin (clone 4B; ICN, Costa Mesa, CA). Microtubules were detected with either a mouse monoclonal β-tubulin (E-7; Hybridoma Bank, IA) or an acetylated α-tubulin antibody (clone 6–11B-1; Sigma, St. Louis, MO; Schatten et al., 1988).
A murine leukemia retroviral vector was engineered to incorporate the DNA encoding either wild-type, rat aorta MLC20, or a mutant form in which threonine 18 and serine 19 sites were mutated into alanines, rendering a MLC20 form that cannot be phosphorylated by myosin light chain kinase; both were cloned, bacterially expressed, and purified as described by Gandhi et al. (1997) for microinjection into mouse unfertilized oocytes.
Gamete Collection, Microinjection, and Fertilization In Vitro
The collection of immature oocytes as well as superovulation, in vitro fertilization, and the collection of oviductal zygotes have been described (Simerly et al. 1990; Simerly and Schatten, 1994). All gametes were collected in TALP-HEPES (Tyrodes medium with bovine albumin, sodium lactate, and sodium pyruvate; Bavister, 1989) and maintained in TALP culture media. Cumulus cells were removed by pipetting or a brief treatment with 1 mg/ml hyaluronidase (Sigma).
For microinjection experiments, myosin antibodies or the MLC20 protein were front-loaded into 1-μm beveled micropipettes and pressure injected (Simerly et al., 1990). Sham or microinjection of protein A-purified IgG antibodies were performed as controls. The starting concentrations for microinjection of myosin antibodies or MLC20 was between 1 and 15 mg/ml and all oocytes were microinjected with about 5% of the egg volume. In vitro fertilization of microinjected oocytes using 1 × 106 capacitated sperm/ml was accomplished after zona pellucida removal by acidified Tyrodes and a brief recovery in TALP medium. Oocytes were removed from sperm after a 1-h incubation at 37°C in 5% CO2 and cultured in TALP until processed for immunocytochemistry as described below.
Cytoskeletal Drug Treatment and Parthenogenetic Activation
Colcemid (20 μM), Taxol (1 μM), and cytochalasin B (10 μM) were prepared as 10 mM stocks in DMSO and diluted to the final concentration indicated in TALP. Controls oocytes were exposed to DMSO alone, which never exceeded 0.2%. Artificial activation of unfertilized oocytes was accomplished using 7% ethanol in TALP for 7 min (Kaufman, 1983).
Immunocytochemistry
Zona-free oocytes and zygotes were permeablized, fixed, and processed for the detection of myosin, actin, and microtubules using either methanol or formaldehyde fixation (Simerly and Schatten, 1994). For methanol fixation, oocytes and zygotes were affixed to polylysine-coated coverslips (Mazia et al., 1975) and permeabilized for 10 min in a glycerol-based buffer containing 1% Triton X-100 detergent with 10% methanol. After absolute methanol fixation (−10°C, 10 min), oocytes were rinsed in PBS with 0.1% Triton X-100 (PBS-TX) before immunostaining as described below. Gametes, fixed 24 h in 2% formaldehyde, were permeabilized in 10 mM PBS with 1% Triton X-100 for 40 min and then incubated for 30 min in PBS blocking solution (PBS, 50 mM glycine, 3 mg/ml BSA) to reduce nonspecific background labeling. Myosin localization in sperm was performed after fixation in absolute methanol for 6 min or after fixation in 2% formaldehyde for 30 min. Fixed sperm were incubated in 10% normal goat serum for 30 min to retard nonspecific antibody binding prior to immunostaining for myosin detection.
Oocytes microinjected with myosin antibodies were processed without further addition of primary antibody. Localization of myosins I, IIA, or IIB in noninjected oocytes or sperm was accomplished using 10 μg/ml affinity-purified antibody diluted in PBS and applied for 60 min at 37°C. After PBS-TX rinses, myosins were detected in injected or noninjected cells with a 1:40 dilution of fluorescein goat anti-rabbit IgG secondary antibody (Sigma). Microfilaments were simultaneously detected in methanol-fixed cells with clone 4B antiactin antibody diluted 1:100 in PBS and applied under identical conditions or, for formaldehyde fixed material, by using 15 U/ml rhodamine phalloidin for 30 min (Molecular Probes). Clone 4B-labeled actin filaments were detected with rhodamine goat anti-mouse IgG secondary antibody (1:40, 60 min).
Microtubules were detected with mouse mAbs to either acetylated α-tubulin (1:100; Schatten et al., 1988) or E-7 anti-β-tubulin (1:5). DNA was detected using 10 μg/ml Hoechst 33342 added to the penultimate PBS-TX rinse. Conventional epifluorescent microscopy and laser scanning confocal microscopy were performed using a Zeiss Axiphot or a Bio-Rad MRC 600 microscope, respectively. All images were archived on magneto optical disks and printed on a Sony dye sublimation printer (UP8800; Sony Corp., New York, NY) using Adobe Photoshop software (Adobe Systems, Mountain View, CA).
PAGE and Immunoblotting
SDS-PAGE and immunoblotting for the detection of myosin antigens were performed as described by Wilson et al. (1991). Myosin II immunoblots were performed with about 2000 unfertilized oocytes.
Statistical Analysis
Statistical comparisons between the means of sham controls and microinjected myosin I, IIA, or IIB oocytes were performed with Student’s t test. Three trials were performed with each myosin antibody and differences were considered statistically significant when p value <0.05.
RESULTS
Affinity-purified Myosin II Antibodies Detect Myosin II Heavy Chains A and B in Unfertilized Mouse Oocytes
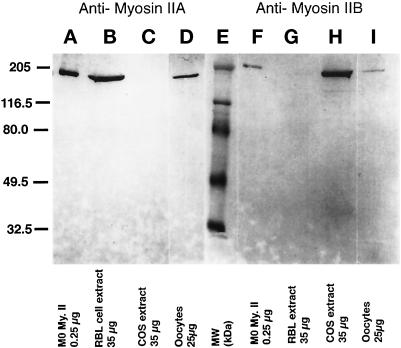
The specificity of the antibodies to myosin IIA and myosin IIB was demonstrated by determining the reactivity of the antibodies with extracts from RBL and COS cells (Figure 1). RBL cells have myosin IIA but not IIB whereas COS cells have myosin IIB but not IIA (R.S. Adelstein, personal communication). Figure 1 shows that the myosin IIA antibodies only recognize myosin II in RBL cells (Figure 1, lane B) and that the myosin IIB antibodies recognize myosin II in COS cells, demonstrating their specificity for myosin IIA or IIB. Both antibodies react with purified macrophage myosin II (Figure 1, lanes A and F), which contains a mixture of both myosin IIA and IIB isoforms. Similarly, affinity-purified myosin II antibodies detect myosin II heavy chains A and B in unfertilized mouse oocytes. Both antibodies recognize a 205-kDa protein in unfertilized oocytes (Figure 1, lanes D and I).
Figure 1.
Antibodies prepared against isoform-specific regions of myosin heavy chains A and B are monospecific and detect 205-kDa proteins in unfertilized mouse oocytes. Lanes A–D, antimyosin IIA antibody. (A) 0.25 μg of purified macrophage (Mφ) myosin II protein; (B) 35 μg of RBL cell extract; (C) 35 μg of COS cell extract; and (D) 25 μg of mouse unfertilized oocyte extract. Lanes F–I, antimyosin IIB antibody. (F) 0.25 μg of purified (Mφ) myosin II protein; (G) 35 μg of RBL cell extract; (H) 35 μg of COS cell extract; and (I) 25 μg of mouse unfertilized oocytes extract. Lane E, molecular mass standards, in kDa. Myosin IIA antibody recognizes a 205-kDa myosin IIA isoform in RBL cell extracts, but not extracts from COS cells which lack myosin IIA. In contrast, the 205-kDa myosin IIB isoform is detected in COS cell extracts, but is absent in RBL cell extracts which lack the IIB protein. Myosin IIA and IIB antibodies detect 205-kDa proteins in mature oocytes. The IIB isoform appears to be less prevalent in oocytes than myosin IIA.
Detection of Myosin IIA and IIB in the Maturing Oocyte
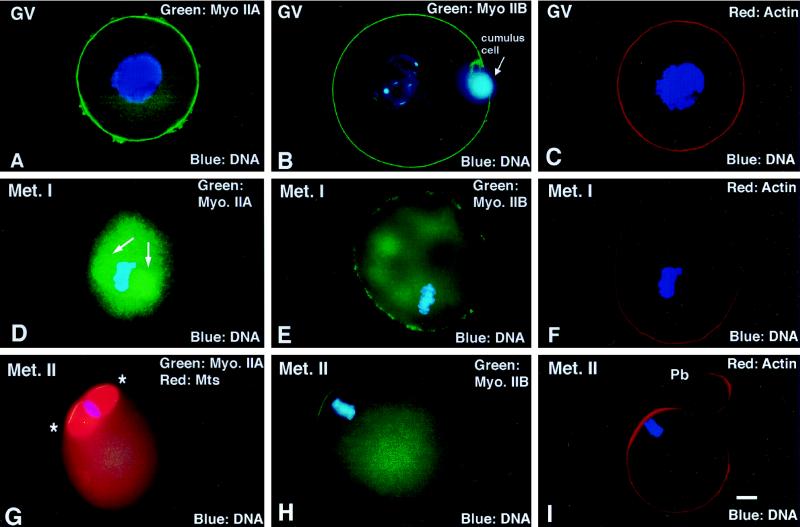
Germinal vesicle (GV) stage oocytes demonstrate a uniform cortical staining of myosin IIA and IIB (Figure 2,A and B), colocalizing with actin (Figure 2C). Following GV breakdown (GVBD), the cortical detection of both isozymes diminishes while the cytoplasmic detection of myosin IIA and IIB increases. Only myosin IIA isotype associates with the first meiotic spindle (Figure 2D, arrow); myosin IIB appears nonuniform in the cortex (Figure 2, D and E; actin, Figure 2F). By second meiotic metaphase arrest, actin filaments and both myosin II isoforms are restricted to the region overlying the metaphase-arrested second meiotic spindle (Figure 2, G-I, ∗, area of increased myosin staining). Neither preimmune antibodies nor a myosin I antibody immunostained the cortex or first meiotic spindles in maturing mouse oocytes.
Figure 2.
Detection of myosin IIA and IIB isotypes during meiotic maturation and in the unfertilized oocyte arrested at second meiotic metaphase. (A–C) In immature GV stage oocytes, myosin IIA, myosin IIB, and actin are uniform in the cortex. (D–F) By the first meiotic metaphase, myosin IIA decreases in the cortical region and increases in the cytoplasm, associating prominently with the metaphase spindle (D, arrows). Myosin IIB is discontinuous in the cortex and detected within the cytoplasm, but no association with the meiotic spindle or chromosomes is observed. Cortical actin filament detection remains uniform. (G–I). In the unfertilized oocyte, myosins IIA and IIB as well as actin filaments are enhanced in the region overlying the metaphase-arrested second meiotic spindle (G, ∗, second meiotic spindle region). (A, C, D, and F) Triple-labeled images for myosin IIA (green), actin (red), and DNA (blue). (B, E, and H) Double labeled for myosin IIB (green) and DNA (blue). (G) Triple labeled for myosin IIA (green), microtubules (red), and DNA (blue). (I) Double labeled for actin (red) and DNA (blue). Bar, 10 μm.
Cytoskeletal inhibitors and dbcAMP arrest meiotic maturation at specific stages (Wassarman et al., 1976; Alexandre et al., 1989), useful methods for exploring the influences of meiotic chromosomes on cortical myosin II. When oocytes are prevented from undergoing GVBD with 100 μg/ml dbcAMP, no increase in cortical myosin occurs, regardless of whether the intact GV remains at the cell center or is eccentrically displaced. Incubation in 10 μM nocodazole permits bivalent chromosome condensation at the cell cortex following GVBD, although no meiotic spindle formation is observed. An increase in myosin IIA in the cortical region overlying the bivalent chromosomes is observed (our unpublished results). Finally, incubation of GV stage oocytes in 10 μM cytochalasin B blocks cell cycle progression at first meiotic metaphase and the observed spindles remain in the cell center (Wassarman et al., 1976). No increase in cortical myosin II is observed (our unpublished results). Collectively, these results demonstrate that cortical myosin II organization in the maturing oocyte is influenced by the proximity of peripheral meiotic chromosomes, as previously shown for cortical microfilament arrangements (reviewed in Longo, 1989).
Microinjected Myosin IIA Antibody Depletes Cortical and Spindle-associated Myosin: Association with Chromosome Surfaces and Effects on Meiotic Maturation In Vitro
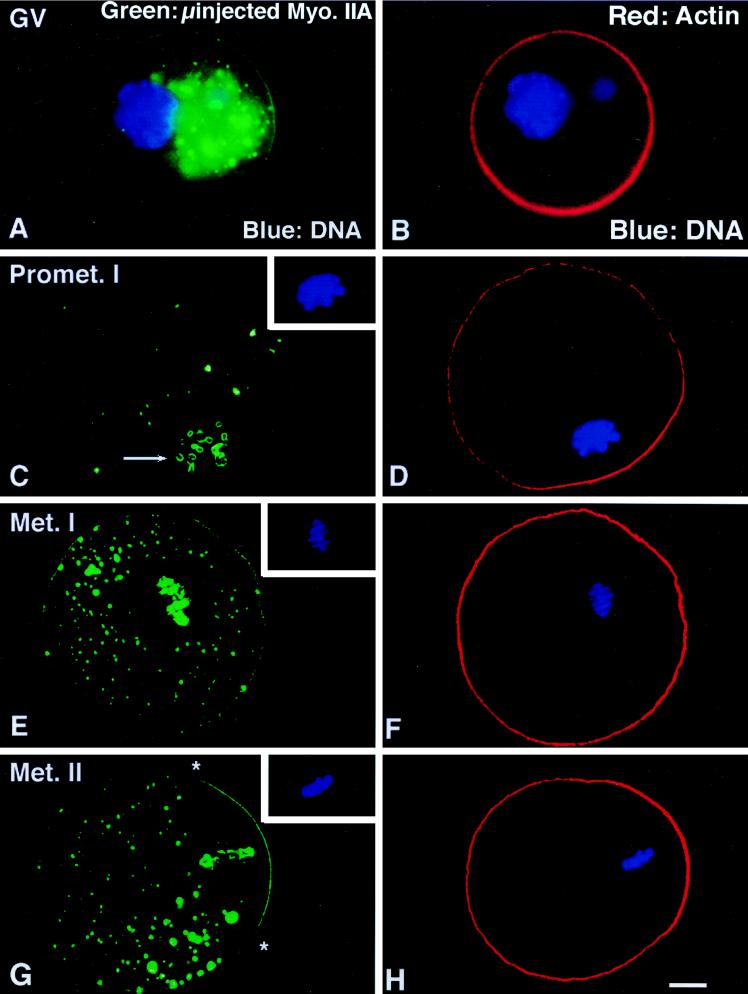
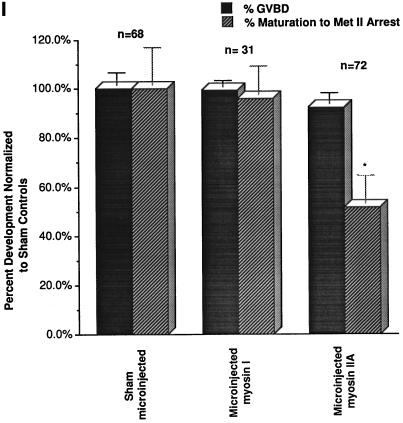
Microinjection of GV stage oocytes with myosin antibodies and their developmental effects are shown in Figure 3. In GV stage oocytes, microinjection of the myosin IIA antibody results in large cytoplasmic aggregates and reduced cortical staining (Figure 3A). Neither the localization pattern nor the intensity of actin labeling is influenced (Figure 3B). After GVBD, microinjected myosin IIA antibody significantly reduces the cytoplasmic localization of the IIA isotype in both the first or second meiotic spindles (Figure 3, C, E, and G). In addition, the peripheries of the meiotic chromosomes bind myosin IIA antibody (Figure 3C, arrow; inset, DNA). However, neither chromosome congression nor metaphase alignment at the spindle equator is blocked by the microinjection of myosin IIA antibody (Figure 3E, inset, DNA). Furthermore, microfilament polarization overlying cortical-positioned chromosomes is unaffected (Figure 3, D and H). Nearly half of the myosin IIA-microinjected GV stage oocytes do not complete first meiosis (Figure 3I) but stall at metaphase I in the cell center, indicating that meiotic spindle migration to the cortex has been interrupted. Sham injections, microinjected myosin I (Figure 3I) or IIB antibodies (our unpublished observations) do not retard completion of meiotic maturation from GVBD to metaphase II arrest.
Figure 3.
Microinjection of myosin IIA antibody blocks the eccentric positioning of the metaphase I spindle during meiotic maturation. (A and B) Microinjection of myosin IIA antibody into GV-arrested oocytes reduces myosin IIA, but not actin filaments, in the cortex and results in the formation of large immunofluorescent aggregates within the cytoplasm. (C and D) By 16 h after microinjection of myosin IIA antibody, oocytes arrested at prometaphase I (C, inset) demonstrate cytoplasmic myosin aggregates, a reduced cortical intensity of myosin IIA, and peripheral staining of chromosomal surfaces (C, arrow). Cortical actin enhancement overlying the peripheral chromosomes is not influenced after microinjection of the myosin IIA antibody (D). (E and F) In E, a GV stage oocyte microinjected with myosin IIA antibody has arrested at metaphase I (inset, DNA). Myosin IIA is not detected in the meiotic spindle region, except at surfaces of the aligned equatorial chromosomes. The large cytoplasmic immunofluorescent aggregates which form after microinjection of myosin IIA antibody are also excluded from the meiotic spindle region. Actin filaments remain uniform in the cortex of metaphase I-blocked oocytes. (G and H) In G, a GV stage oocyte microinjected with myosin IIA antibody has arrested at metaphase of second meiosis (inset, DNA). Both cortical myosin IIA and actin filaments are enhanced in the region overlying the spindle (∗, spindle region). Microinjection of myosin IIA antibody eliminates myosin IIA detection in the meiotic spindle but labels the peripheries of the meiotic chromosomes. (I) An analysis of the impact of microinjected myosin antibodies on meiotic maturation in vitro demonstrates that GVBD is not prevented in the presence of either myosin I or myosin IIA antibodies (solid bars), but completion of meiotic maturation to metaphase II arrest is significantly reduced by the IIA antibody (stippled bars). ∗, significant difference compared with microinjected sham controls (p < 0.001). All images triple labeled for myosin IIA (green), actin (red), and DNA (blue). (A and B) epifluorescence. (C–H) Laser scanning confocal microscopy. (I) Graph of the mean ± SD. Bar, 10 μm.
Microinjected Myosin IIB Antibody Affects Microfilament-mediated Chromosome Scattering following Meiotic Spindle Disassembly with Colcemid
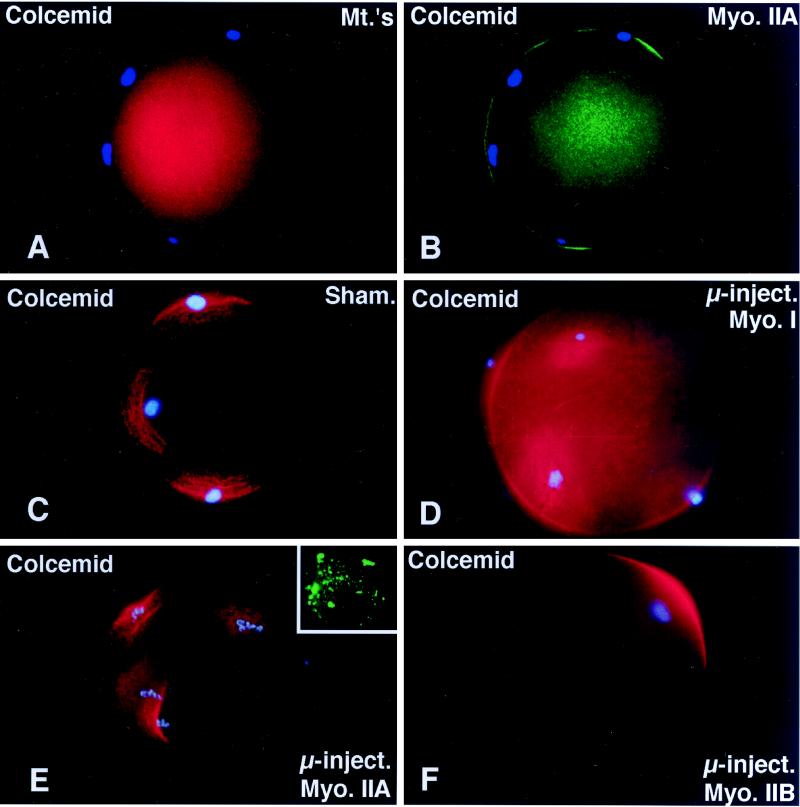
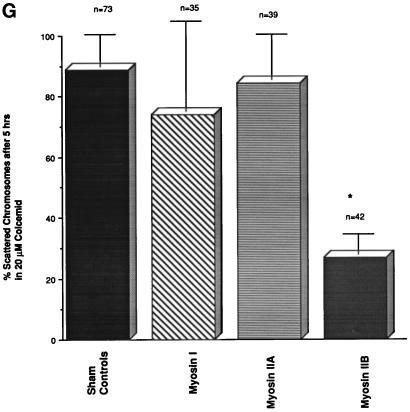
Disassemble of the second meiotic spindle by treating unfertilized oocytes with microtubule inhibitors results in meiotic chromosome scattering along the cortex (Figure 4A) in a microfilament-dependent manner (Maro et al., 1986; Schatten et al., 1986a). Both cortical microfilaments (Maro et al., 1986; Schatten et al., 1986a) and myosin II (Figure 4B) increase in the regions directly overlying each scattered chromosome mass. To investigate whether either myosin II isozyme is involved with this novel chromosome–cortical microfilament motility event, unfertilized oocytes were first microinjected with myosins I, IIA, or IIB antibodies and then treated for 5 h with 10 μM Colcemid. In Figure 4C, a sham-microinjected oocyte fixed for 5 h after Colcemid treatment demonstrates meiotic chromosome scattering and increased cortical microfilament organization overlying each chromosome mass. Identical chromosome dispersion and cortical microfilament reorganization was observed in Colcemid-treated unfertilized oocytes microinjected with either myosin I or IIA antibodies (Figure 4, D and E). However, in unfertilized oocytes microinjected with the IIB antibody, nearly 70% of the oocytes demonstrated intact meiotic metaphase chromosomes at the original metaphase II equator after spindle dissolution by Colcemid treatment (Figure 4, F and G). Interestingly, the extent of chromosome decondensation in myosin IIA microinjected oocytes was less pronounced than in oocytes microinjected with myosin I or IIB, perhaps due to the association of the IIA antibody with the peripheries of the meiotic chromosomes.
Figure 4.
Microinjected myosin IIB antibody blocks microfilament-mediated chromosome scattering following Colcemid-induced disassembly of the second meiotic spindle. (A and B) Unfertilized oocytes incubated in 20 μM Colcemid for 5 h disassemble the meiotic spindle (A, red), resulting in meiotic chromosome scattering (A, blue). Meiotic chromosome scattering induces an increase in cortical myosin IIA (B, green) at sites adjacent to, but often not over, each set of chromosomes (B, blue). (C–E) Mature unfertilized oocytes which were sham-treated (C) and microinjected with myosin I (D) or myosin IIA (E) antibodies. After 5 h in 20 μM Colcemid, all three sets of oocytes demonstrate dispersed cortical chromosomes and an increase in actin accumulation (red) in the plasma membrane regions adjacent to the scattered chromosome masses (blue). Inset in E, microinjected myosin IIA labeling of the scattered meiotic chromosomes. (F) Microinjection of myosin IIB antibody blocks chromosomal dispersion in Colcemid-treated unfertilized oocytes. A single region of enhanced cortical actin overlies the intact chromosomes. (G) To quantify the effects of myosin antibodies on meiotic chromosome scattering following Colcemid-induced spindle disassembly, unfertilized oocytes were microinjected with myosin I, myosin IIA, or myosin IIB antibodies before placement into Colcemid for 5 h. The percentage of scattered chromosomes with overlying enhanced cortical actin was then recorded. The graph demonstrates that chromosome scattering is significantly reduced in the presence of myosin IIB antibody but not by the microinjection of myosin I or IIA antibodies. ∗, significant difference with sham-microinjected oocytes (p < 0.01). (A and B) Triple labeled for myosin IIA (green), microtubules (red), and DNA (blue). (C–F) Double labeled images for actin (red) and DNA (blue). Bar, 10 μm.
Taxol, which augments microtubule assembly at the second meiotic spindle and in the cytoplasmic asters (Schatten et al., 1988), did not modify the patterns of cortical myosin IIA or IIB nor microfilament organization in the unfertilized oocyte (our unpublished results).
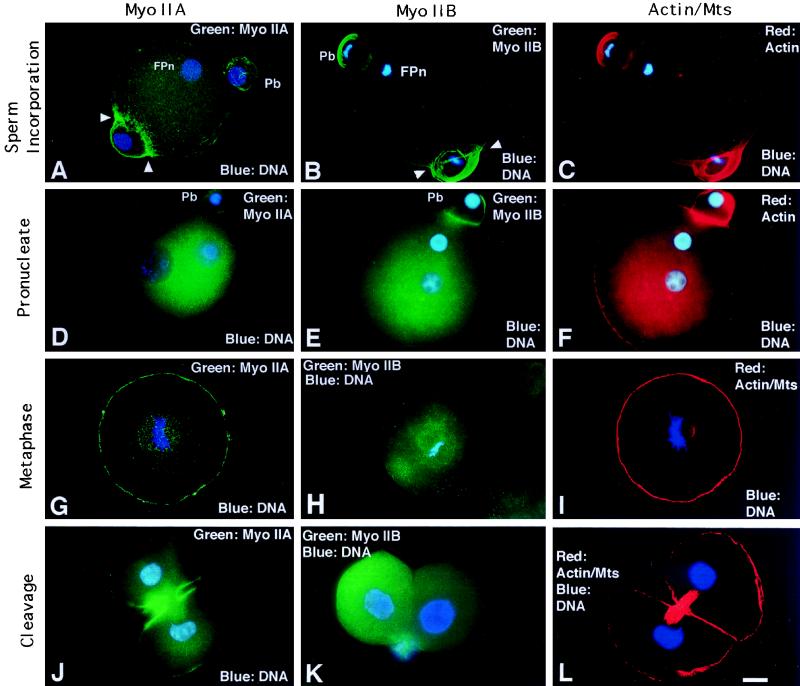
Myosin IIA and IIB Concentrate at the Site of Sperm Incorporation and in the Cleavage Furrow during Second Polar Body Formation, but Only Myosin IIA is Prevalent during First Interphase and Mitosis: Neither Myosin II Isozyme is Detected in Mature Spermatozoa
Both myosin II isozymes, as well as microfilaments, are observed in the cleavage furrow of the second polar body following resumption of second meiosis (Figures 5 and 7A). The IIB isoform is strongly detected in the distal portion of the second polar body (Figure 5B, Pb). In addition, a dramatic assembly of both the IIA and IIB isoforms encircles the decondensing sperm nucleus at the incorporation cone during sperm penetration (Figure 5, A and B, arrowheads). As the incorporation cone disassembles, the cortical detection of both myosin II isozymes is reduced except near the second polar body (Figure 5, D and E; actin, Figure 5F). This pattern for both myosin IIA and IIB is observed throughout interphase. However, by mitotic metaphase, only the IIA isoform reappears at the cortex and associates with the mitotic apparatus (Figure 5G; corresponding actin/acetylated α-tubulin image, Figure 5I). The myosin IIB isoform is weakly detected in the cytoplasm and cortical regions (Figure 5H). During first mitotic telophase, myosin IIA dramatically concentrates in the cleavage furrow while the IIB isoform remains diffuse within the cytoplasm even after daughter cell formation (Figure 5, J and K; actin/acetylated α-tubulin microtubules in similar stage zygote, Figure 5L).
Figure 5.
Myosin IIA and IIB localization during sperm incorporation and first mitosis. (A–C) Myosin IIA (A), IIB (B), and actin (C) assemble in the second polar bodies (Pb) and the sperm incorporation cones, where both isoforms ensheathe the sperm penetration site (A and B, arrowheads). (D–F) In early interphase oocytes, cytoplasmic myosin IIA (D) and IIB (E) increases as cortical detection decreases, except in the region of the second polar body (Pb). Cortical actin staining remains uniform (F). (G–I) At first mitotic metaphase, cortical and spindle-associated myosin IIA staining is prominent (G). Myosin IIB appears diffuse in the cytoplasm, is absent cortically, and is not detected in the mitotic spindle (H). Actin filaments remain uniform in the cortex (I, spindle poles colabeled with acetylated α-tubulin antibody). (J) In telophase zygotes, myosin IIA strongly localizes to the cleavage furrow and opposing plasma membranes of daughter blastomeres; the midbody is weakly detected. (K) Assembly of myosin IIB is not detected in cleaving zygotes or daughter cells following division. (L) A newly formed two-cell embryo double labeled for microfilaments with antiactin antibody and for midbody microtubules with acetylated α-tubulin antibody. (A, D, G, I, J, and L) Quadrupled labeled for myosin IIA (green), actin, acetylated α-tubulin (red), and DNA (blue). (B, C, E, F, and H) Double labeled for myosin IIB (green) and DNA (blue). (K) Double labeled for myosin IIB (green) and DNA (blue). Fpn, female pronucleus; Pb, second polar body. Confocal images: A, B, C, G, I, and L. Epifluorescence: D, E, F, H, J, and K. Bar, 10 μm.
Figure 7.
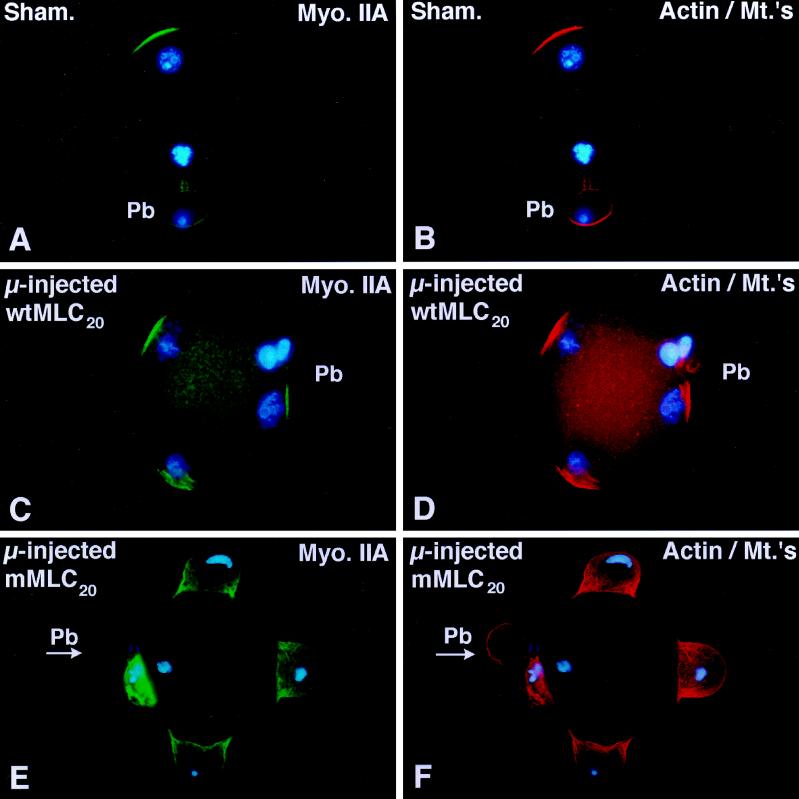
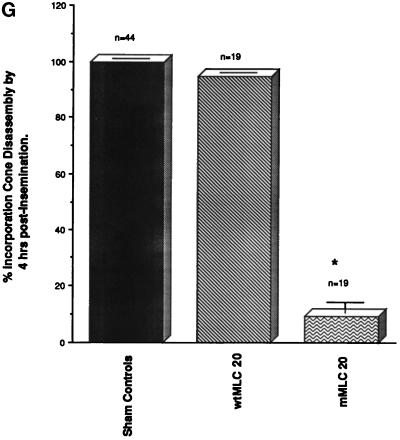
Microinjection of a mutated 20-kDa myosin light chain which cannot be phosphorylated delays the timing of fertilization cone disassembly. (A and B) Sham-microinjected oocytes disassemble the fertilization cone 5 h after insemination in vitro. As male and female pronuclei form, myosin IIA (A) and actin (B) are detected in the second polar body and at the cortex overlying the site of insemination cone disassembly. (C and D) Microinjection of unfertilized oocytes with bacterially expressed wtMLC20 has no effects on second polar body formation, sperm penetration, or fertilization cone disassembly 5 h after insemination. Myosin IIA (C) and actin filament (D) costain similar cortical regions overlying the developing male and female pronuclei. (E and F) A polyspermic zygote derived from an oocyte microinjected with the mMLC20 and fertilized in vitro demonstrates the failure to disassemble the fertilization cone by 5 h after insemination. Second polar body formation is not prevented (E, arrow, PB) and extensive myosin IIA (E) and actin (F) organization is detected within each fully formed insemination cone. The paternal and maternal DNA remains condensed. (G) Analysis of unfertilized oocytes microinjected with either wtMLC20 or mMLC20 protein and subsequently fertilized in vitro demonstrates that fertilization cone disassembly is not affected in the presence of wtMLC20 protein (middle column), but is significantly impaired in the presence of mMLC20 (right column). Sham controls, left column. p < 0.01. (G) mean ± SD of three independent trials. Confocal images are triple labeled for myosin IIA (green), actin (red), and DNA (blue). Bar, 10 μm.
Neither myosin isotype was detected in mature mouse sperm following immunostaining or after Western blotting (our unpublished results).
Microinjected Myosin IIA or IIB Antibodies Do Not Prevent Sperm Incorporation or Cytokinesis
The effects of microinjecting myosin IIA on sperm incorporation and cell division are shown in Figure 6. The completion of meiosis, the formation of the second polar body, and sperm incorporation are not prevented by microinjection of myosin IIA antibody into the unfertilized oocyte before in vitro fertilization. Seventy-three percent (25/34) of oocytes microinjected with myosin IIA, 82% (22/27) of oocytes microinjected with myosin I, and 85% (36/42) of the sham controls extrude a second polar body within the first 3 h after insemination. Similarly, 89% (26/29) of oocytes microinjected with the myosin IIA antibody underwent sperm incorporation following in vitro fertilization compared with 95% (58/61) for sham controls. Immunofluorescent analysis of in vitro fertilized oocytes microinjected with myosin IIA demonstrated diminished cortical myosin IIA staining and a strong labeling of both the male DNA and female chromosomes (Figure 6A, arrows denote sperm nuclei; actin/acetylated α-tubulin, Figure 6B; insets, DNA).
Figure 6.
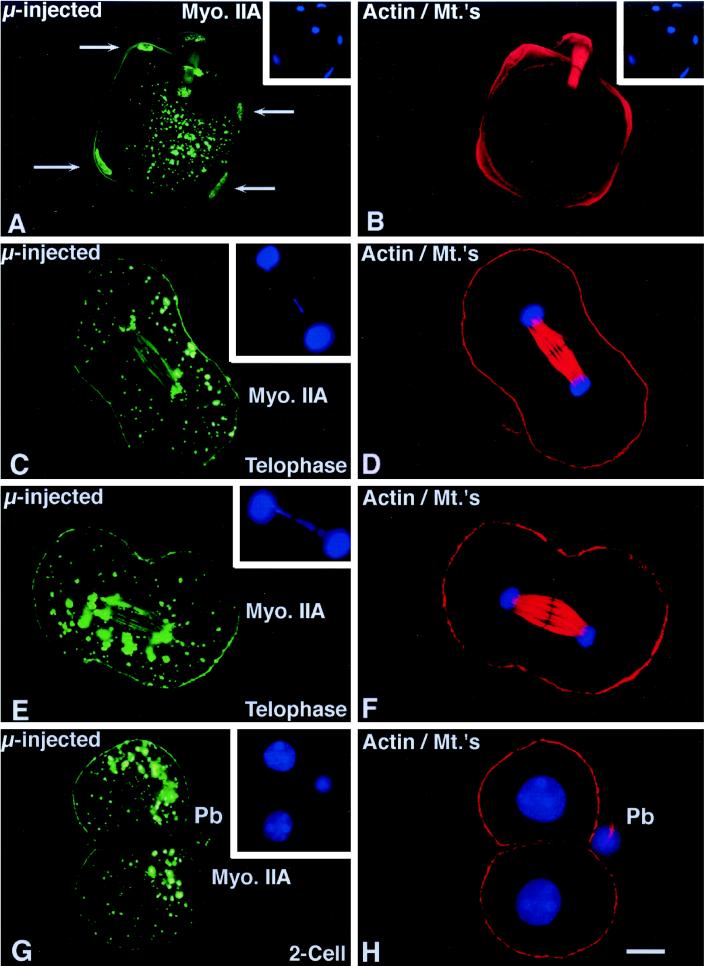
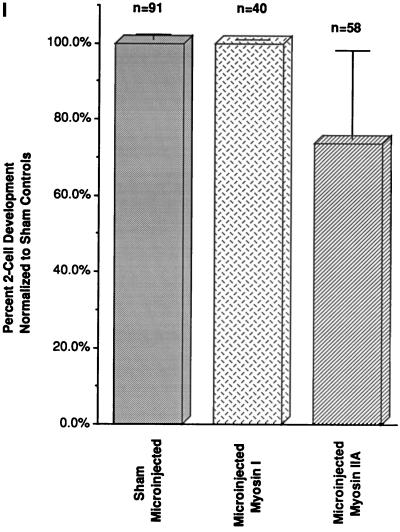
Effects of microinjected myosin IIA antibody on first mitosis. (A and B) Sperm incorporation in zona-free in vitro fertilized mature oocytes is not blocked in the presence of microinjected myosin IIA antibody. The assembly of cortical myosin IIA at the sperm incorporation cones appears reduced (A, arrows; compare with Figure 5A). Immunoreactive cytoplasmic aggregates are observed and both the paternal (A, arrows) as well as maternal chromatin bind the microinjected myosin IIA antibody. (C–F) Microinjection of myosin IIA into interphase zygotes does not impair chromosome congression, segregation, and cleavage furrow formation during mitosis. Cortical myosin IIA is not uniform and the intensity of antibody staining is reduced, especially within the forming cleavage furrow. Numerous cytoplasmic aggregates are observed and the labeling of mitotic chromosome is also evident. An occasional lagging chromosome is observed during late anaphase within the midbody region (insets). (D and F) Simultaneous antiactin and acetylated α-tubulin labeling demonstrates midbody and cortical microfilaments labeling in the same zygotes. (G and H) Cleavage of zygotes microinjected with myosin IIA appears to be normal and at the correct time for division. Detection of cortical myosin IIA in sister blastomeres is reduced in the cell–cell contact regions. No myosin IIA is found within daughter cell nuclei after cytokinesis, suggesting a transient association of myosin IIA with the condensed chromosomal surfaces during mitosis. H is the corresponding antiactin and acetylated α-tubulin image of the oocyte in G. (I) To quantify the effects of myosin II antibodies on cell division, pronucleate stage oocytes were microinjected with myosin I (I, middle column) or myosin IIA antibody (I, right column) and allowed to develop in vitro. Neither myosin antibody significantly impacts cytokinesis and two-cell formation following mitosis. Similar observations were made following microinjection of myosin IIB antibody, either injected alone or coinjection with the IIA antibody (our unpublished results). Left column, sham controls. Confocal images quadruple labeled for microinjected myosin IIA (green), actin, and acetylated α-tubulin (red) and DNA (blue). Bar, 10 μm.
To investigate the role of myosin II isozymes during mitosis and cytokinesis, in vivo fertilized zygotes (20–22 h after human chorionic gonadotropin treatment) were collected, microinjected with antibodies to myosin IIA and IIB or coinjected with both antibodies, and subsequently cultured through first cleavage (40 h after human chorionic gonadotrophin treatment). Microinjection of myosin IIA antibody results in the formation of large immunoreactive cytoplasmic aggregates and a “patchy” cortical myosin and actin filament pattern. However, no effects on pronuclear formation, pronuclear migration, nuclear envelope breakdown, or chromosome congression at the mitotic spindle equator was observed (our unpublished results). At telophase, chromosome segregation and cleavage furrow formation occurred in the presence of myosin IIA antibody (Figure 6, C and E). Infrequently, the microinjected myosin II antibody labeled lagging mitotic chromosomes during chromosome separation (Figure 6C, inset; corresponding actin/acetylated α-tubulin images, Figure 6, D and F). Following cytokinesis, cortical myosin IIA remains disorganized in daughter cells, particularly at the regions where sister cells are closely apposed (Figure 6G; actin/acetylated α-tubulin, Figure 6H). A majority of zygotes microinjected with the myosin IIA antibody complete cell division (Figure 6I). Similar results were observed in embryos microinjected with myosin I antibody (Figure 6I), myosin IIB antibody (our unpublished observations), or coinjection of antibodies to both myosin II isozymes (our unpublished observations).
The 20-kDa Myosin Light Chain, Which Cannot Be Phosphorylated, Retards Insemination Cone Disassembly In Vivo
Myosin II actin-mediated ATPase activity and the assembly of filaments is regulated by the phosphorylation status of MLC20 (reviewed by Sellers, 1991). Both myosin isotypes undergo dynamic assembly/disassembly in the incorporation cone within a few hours of insemination (see Figure 5), although microinjection experiments with antibodies against either myosin heavy chain A or myosin heavy chain B does not interfere with this structure (Figure 6). To further investigate the role of myosin II in the formation of the insemination cone, a wild-type and a mutant form of MLC20 were microinjected into unfertilized oocytes and then fertilized in vitro (Figure 7). In sham-microinjected oocytes, the incorporation cone disassembles by 5 h after insemination. Myosin IIA (Figure 7A) and microfilaments (Figure 7B) are organized overlying the developing male pronucleus and in the cleavage furrow region of the second polar body. A similar pattern for myosin IIA and microfilaments was observed following the fertilization of zona-free oocytes microinjected with the phosphorylatable form of MLC20 [wild-type MLC (wtMLC20)]. The formation of the second polar body and the disassembly of the incorporation cone occurs in a temporally correct period, with cortically organized myosin IIA and microfilaments overlying each developing male pronucleus (Figure 7, C and D). However, microinjection of mMLC20, which cannot be phosphorylated at a site crucial for actin-mediated ATPase activity, affects the disassembly of the incorporation cone, pronuclear development, and normal cell cycle progression. Formation of the second polar body is not blocked after microinjection of mMLC20 (Figure 7E, arrow, Pb). The percentage of oocytes which can disassemble the sperm incorporation cone within 4 h of insemination is significantly reduced compared with sham-injected and wtMLC20-microinjected oocytes (Figure 7G).
DISCUSSION
The mouse oocyte is an excellent model for exploring myosin structure and function. The immature germinal vesicle stage oocyte matures spontaneously in vitro and can be arrested at specific cell cycle stages during first meiosis. This permits observations on dynamic motility events like peripheral spindle migration, cortical spindle anchoring, and cell surface modifications (Wassarman et al., 1976; Longo and Chen, 1985; Alexandre et al., 1989). Furthermore, the ovulated oocyte is arrested at metaphase of the second meiosis and can easily be artificially activated or fertilized in vitro, permitting detailed investigations on spindle rotation and cytokinesis during second polar body formation. Finally, the events during sperm incorporation, pronuclear formation, and migration, as well as mitosis, can be investigated.
This study demonstrates myosin II participation in the cortical polarization events which occur during mouse meiotic maturation. During meiosis, myosin II organization undergoes dynamic changes in the cortex that mirror the events described for the reorganization of microfilaments and other cortical organelles (Longo and Chen, 1985; Ducibella et al., 1990). Immature GV stage oocytes have a uniform distribution of both myosin II isotypes (Figure 2) as well as surface microvilli, microfilaments, and cortical granules (reviewed by Longo, 1989). However, after maturation of the immature oocyte, myosin IIA, IIB, and microfilaments are restricted to a region directly overlying the arrested second meiotic spindle. This cortical region is also free of surface microvilli, devoid of underlying cortical granules, and demonstrates a reduced affinity for the plant lectin, concanavalin A (reviewed by Longo, 1989). Cortical polarization during meiosis is important for the completion of the first nuclear reductional event, accomplished by the extrusion of the first polar body. In addition, cortical polarization establishes a nonfusogenic plasma membrane region overlying the spindle region that prevents sperm binding at the site where second polar body formation will occur, thus averting the potential loss of the paternal genome during the final maternal nuclear reductional division.
The dramatic cortical and cell surface modifications which occur during meiosis are closely linked to the peripheral migration of the first meiotic spindle, an event mediated by actin filaments and blocked by microfilament inhibitors (Wassarman et al., 1976; Shalgi et al., 1978; Longo and Chen, 1985; Longo, 1989; Maro et al., 1984; Battaglia and Gaddum-Rose, 1986). Our data suggest that myosin IIA, but not myosin IIB, is involved in meiotic spindle migration (Figure 3). This is based on the observation that myosin IIA resides in the region of the first meiotic spindle apparatus (Figure 2D). Moreover, microinjection of antibodies to myosin IIA blocks nearly half of the oocytes in metaphase I (Figure 3I). Interestingly, only the cortical positioning of the metaphase I spindle is prevented, not its formation or chromosome congression. Immunocytochemistry and laser-scanning confocal microscopy demonstrate that microinjected myosin IIA antibody depletes spindle-associated IIA protein and ensheathes each bivalent chromosome (Figure 3, C, E, and G), perhaps providing a mechanism for interfering with cytoplasmic microfilaments which are involved in spindle migration. Microinjection of either nonimmune protein A-purified IgGs, myosin I antibody, or myosin IIB antibody (Nowak et al., 1997) did not reproduce these events, indicating the specificity of the myosin IIA protein for spindle positioning during meiosis. Myosin II localization in mitotic spindles has also been reported in other systems but without a known function (Fujiwara and Pollard, 1976; Sanger et al., 1989).
The presence of meiotic chromosomes at the cortex impacts cortical myosin II organization in the mature oocyte (Figure 5), as previously described for microfilaments (reviewed by Longo, 1989). Spindle dissolution with microtubule inhibitors like Colcemid results in the scattering of meiotic chromosomes along the cortex and a dramatic reorganization of myosin II (Figure 4B) and microfilaments (Longo and Chen, 1985; Van Blerkom and Bell, 1986; Maro et al., 1984, 1986). This uncommon motility event appears to be mediated by overlying cortical actin since microfilament inhibitors like cytochalasin and latrunculin block chromosome dispersion after microtubule disassembly (Maro et al., 1986; Schatten et al., 1986a). Our study suggests that the myosin IIB isoform may mediate chromosome interaction with the overlying cortical actomyosin cytoskeleton. We demonstrate that microinjection of unfertilized oocytes with the myosin IIB antibody, but not myosin I or myosin IIA, interferes with chromosome scattering after Colcemid treatment. The exact mechanism of this interaction between the chromosomes and cortex is not known. Recently, filamentous actin association with meiotic chromosomes has been reported in Xenopus embryos and Drosophila salivary gland squashes, suggesting that microfilaments may be an integral part of the chromosomal scaffold (Sauman and Berry, 1994).
A number of cortical microfilament-mediated events are initiated during sperm penetration, including meiotic spindle rotation, second polar body formation, and the creation of the sperm incorporation cone (reviewed by Simerly et al., 1995). A dramatic assembly of both myosin II isotypes occurs during the formation of these structures, as shown previously for microfilaments (Maro et al., 1984). Despite the indication that myosin II may participate during events leading to sperm incorporation, microinjection of the myosin IIA or IIB antibody into unfertilized oocytes did not block sperm incorporation following in vitro fertilization. Upstream events like meiotic spindle rotation, second polar body formation, and cortical microfilament assembly were also unaffected by microinjected myosin IIA antibody. These results demonstrate that the recruitment of cortical actin is independent of myosin II, as suggested from a previous report (Zurek et al., 1990). Furthermore, our results showing the lack of a myosin II requirement for sperm incorporation agrees with the data obtained in starfish oocytes microinjected with myosin II antibody (Kiehart et al., 1982).
Although myosin II microinjection studies did not demonstrate an inhibition of incorporation cone formation, the microinjection of a mutated regulatory myosin light chain (mMLC20) which cannot be phosphorylated (Gandhi et al., 1997) did block sperm incorporation cone disassembly and disrupt the timing of fertilization in vitro. Surprisingly, unfertilized oocytes microinjected with mMLC20 formed normal second polar bodies and sperm insemination cones following in vitro fertilization, events which involve cortical myosin assembly dynamics. Indeed, the microinjection of mMLC20 did not appear to affect the assembly of myosin IIA or microfilaments in the sperm incorporation cones. Nevertheless, significant delays in insemination cone resorption, sperm decondensation, and pronuclear formation were observed compared with sham and wild-type MLC20-microinjected oocytes.
The presence of both actin filaments and myosin II in the sperm incorporation cone suggest that sperm penetration into the cytoplasm is an active process involving actomyosin interactions. The demonstration that microinjection of a nonphosphorylatable MLC20 interferes with sperm incorporation supports this notion. Many myosin II-mediated motility events in vertebrate cells are regulated by the phosphorylation of MLC20 on serine 19 by myosin light chain kinase (Craig et al., 1983; Holzapfel et al., 1983; Fishkind et al., 1991; Wilson et al., 1991; Satterwhite et al., 1992; Yoshihiko et al., 1994; Gandhi et al., 1996; Yumura and Uyeda, 1997). Whereas bacterially expressed wild-type MLC20 are phosphorylated in vitro by purified smooth muscle myosin light chain kinase, mMLC20 is not phosphorylated under identical conditions (Gandhi et al., 1997). Moreover, expression of wtMLC20 or mMLC20 in epithelial cells results in the hybridization of the exogenous MLC20 with the endogenous myosin II heavy chains and appropriate changes in actin-activated ATPase activity (Gandhi et al., 1997). Thus, microinjection of mMLC20 may affect the timing of insemination cone disassembly by altering the biochemical properties of myosin II.
The involvement of conventional myosin II in cytokinesis is well documented and has benefited tremendously from studies employing fluorescent antibody staining and the microinjection of myosin II antibodies into living eggs (Mabuchi, 1986; reviewed by Kiehart et al., 1990). Similarly, microinjection of antibodies prepared against vertebrate myosin IIA and IIB might be expected to impair the in vivo functions of these proteins. Both affinity-purified polyclonal antibodies are monospecific and biochemical analyses have shown that they inhibit the actin-activated ATPase activities of myosin IIA and IIB in vitro (Kelley et al., 1995; Nowak et al., 1997). In mouse oocytes, both myosin II isoforms are present in the polar body cleavage furrows. In addition, myosin IIA is detected within the mitotic cleavage furrow during cytokinesis. Despite these observations, however, the microinjection of myosin IIA or IIB antibodies, whether alone or in combination, did not inhibit polar body formations or cell division. Plausible explanations for these observations include: 1) insufficient concentration of microinjected myosin II antibodies in the cytoplasm; 2) the failure of microinjected antibodies to completely remove all myosin II assembled in the cleavage furrows, perhaps caused by the steric hindrance of antibody binding to myosin II protein in the living oocyte; 3) the presence of other, unidentified myosin heavy chain isoforms which might be required for cytokinesis; or 4) that cytokinesis in mouse oocytes does not require myosin II activity. It is interesting that similar findings on the failure of myosin antibodies to interfere with cell division has been observed in higher mammalian cells (Zurek et al., 1990). In this report, the microinjection of myosin IIA antibody transiently interacts with the surfaces of condensed chromosomes and results in large cytoplasmic aggregates (Figures 3 and 6). In addition, microinjection of myosin IIA antibody inhibits meiotic maturation (Figure 3I) whereas the microinjection of myosin IIB antibody decreases chromosome scattering after meiotic spindle disassembly (Figure 4G). Collectively, these data argue that myosin IIA and IIB proteins are accessible to the microinjected antibodies and show that they inhibit certain cellular functions in oocytes. However, although myosin IIA detection in the cleavage furrow is greatly reduced after microinjection of the myosin IIA antibody prior to cytokinesis, it is not completely eliminated (Figure 6, C and E). These observations suggest that enough myosin II may be present or can assemble at the equatorial regions of microinjected zygotes to initiate and complete cell division. It is interesting to speculate that perhaps cytoplasmic myosin II protein has a different sensitivity to functional inhibition by microinjected myosin II antibody than does myosin II protein which assembles in the cortical region of oocytes.
Antibodies to conventional myosin and actin have detected these proteins in mature sperm of different mammalian species (Clarke and Yanagimachi, 1978; Campanella et al., 1979; Tamblyn, 1981; Virtanen et al., 1984; Flaherty et al., 1986), and a role has been suggested for actomyosin in the organization of specialized domains within the sperm plasma membrane (Olson et al., 1987). However, this study failed to detect either myosin IIA or IIB isoforms in the mature epididymal mouse spermatozoa. These observations are supported by reports on the presence of actin filaments and myosin II in rodent spermatogenic cells (Campanella et al., 1979; De Martino et al., 1980; Walt et al., 1982) which appear to be lost in residual bodies during the latter stages of spermiogenesis (Manandhar, personal communication). Furthermore, experiments do not suggest a role for the assembly of sperm F-actin in either the capacitation or acrosome reaction prior to mouse fertilization, as demonstrated for the acrosomal process in lower animal species like the sea urchin (reviewed by Schatten and Schatten, 1987). Finally, mouse sperm do not contain actin at the equatorial region where sperm-oocyte fusion occurs (Flaherty et al., 1986) and studies of mouse in vitro fertilization do not support a required role for actin filaments in sperm head penetration (Simerly et al., 1993). Collectively, these observations question an active role for either conventional myosin or F-actin in the structural organization of the mature mouse spermatozoa or in murine sperm penetration. Mature mouse sperm, therefore, may represent one of the few differentiated cells which do not express myosin II protein.
Isotype-specific myosin IIA and IIB antibodies detect cortical and cytoplasmic myosins in mouse oocytes. Microinjected myosin IIA and IIB antibodies interfere in specific microfilament-mediated cortical events in the maturing oocyte but not with sperm incorporation or cytokinesis. Regulation of the disassembly of myosin in the sperm incorporation cone appears to depend on the phosphorylation of MLC20. These observations provide a basis for investigating the links between the identification of myosin isotypes, their interactions with microfilaments, and myosin-actin functions during meiosis, fertilization, and early development in mammals.
ACKNOWLEDGMENTS
We thank Dr. R. S. Adelstein for the gracious donation of the myosin IIB antibody. It is our pleasure to acknowledge our colleagues for assistance and stimulating discussions: Drs. B. Bement, N. First, J. Hearn, J. Hardin, L. Hewitson, C. Navara, P. Sutovsky, and M. Tengowski. We thank the Integrated Microscopy Resource at the University of Wisconsin for use of the laser-scanning confocal microscope. This research was supported by grants from the National Institutes of Health and United States Department of Agriculture to G. S. (HD 12912 and HD 32887) and by National Institutes of Health grant HL-02411 and National Science Foundation grant NSF9631833 to P.d.L. This work was performed during the tenure of P.d.L. as the Florence and Arthur Brock Established Investigator of the Chicago Lung Association.
REFERENCES
- Alexandre H, Van Cauwenberge A, Mulnard J. Involvement of microtubules and microfilaments in the control of the nuclear movement during maturation of mouse oocyte. Dev Biol. 1989;136:311–320. doi: 10.1016/0012-1606(89)90258-3. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Lindner AR, Groschel-Stewart U. Localization of actin and myosin in the rat oocyte and follicular wall by immunofluorescence. Anat Rec. 1976;187:311–328. doi: 10.1002/ar.1091870304. [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Gaddum-Rosse P. The distribution of polymerized actin in the rat egg and its sensitivity to cytochalasin B during fertilization. J Exp Zool. 1986;237:97–105. doi: 10.1002/jez.1402370114. [DOI] [PubMed] [Google Scholar]
- Bavister BD. A consistently successful procedure for in vitro fertilization of golden hamster eggs. Gamete Res. 1989;23:139–158. doi: 10.1002/mrd.1120230202. [DOI] [PubMed] [Google Scholar]
- Campanella C, Gabbiani G, Baccetti B, Burrini AG, Pallini V. Actin and myosin in the vertebrate acrosomal region. J Submicrosc Cytol. 1979;11:53–71. [Google Scholar]
- Cheng TP, Murakami N, Elzinga M. Localization of myosin-IIB at the leading-edge of growth cones from rat dorsal-root ganglionic cells. FEBS Lett. 1992;311:91–94. doi: 10.1016/0014-5793(92)81374-u. [DOI] [PubMed] [Google Scholar]
- Craig R, Smith R, Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature. 1983;302:436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- Damjanov I, Damjanov A, Lehto VP, Virtanen I. Spectrin in mouse gametogenesis and embryogenesis. Dev Biol. 1986;114:132–140. doi: 10.1016/0012-1606(86)90389-1. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P, Gogas G, Li X, Schluns K. Myosin light chain phosphorylation does not increase during yeast phagocytosis by macrophages. J Biol Chem. 1993;268:16883–16886. [PubMed] [Google Scholar]
- De Martino C, Capanna E, Nicotra MR, Natali PG. Immunochemical localization of contractile proteins in mammalian meiotic chromosomes. Cell Tissue Res. 1980;213:159–178. doi: 10.1007/BF00236928. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Duffy P, Reindollar R, Su B. Changes in the distribution of mouse oocyte cortical granules and the ability to undergo the cortical reaction during gonadotropin-stimulated meiotic maturation and aging in vivo. Biol Reprod. 1990;43:870–876. doi: 10.1095/biolreprod43.5.870. [DOI] [PubMed] [Google Scholar]
- Fishkind DJ, Cao L-G, Wang Y-L. Microinjection of the catalytic fragment of myosin light chain kinase into dividing cells: effects on mitosis and cytokinesis. J Cell Biol. 1991;114:967–975. doi: 10.1083/jcb.114.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty SP, Winfrey VP, Olson GE. Localization of actin in mammalian spermatozoa: a comparison of eight species. Anat Rec. 1986;216:504–515. doi: 10.1002/ar.1092160407. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Pollard TD. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow and mitotic spindle of human cells. J Cell Biol. 1976;71:848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Lorimer DD, de Lanerolle P. Expression of a mutant myosin light chain that cannot be phosphorylated increases paracellular permeability. Am J Physiol. 1997;272:F214–F221. doi: 10.1152/ajprenal.1997.272.2.F214. [DOI] [PubMed] [Google Scholar]
- Holzapfel G, Wehland J, Weber K. Calcium control of actin-myosin based contraction in Triton models of mouse 3T3 fibroblasts is mediated by the myosin light chain kinase (MLCK)-calmodulin complex. Exp Cell Res. 1983;148:117–126. doi: 10.1016/0014-4827(83)90192-1. [DOI] [PubMed] [Google Scholar]
- Katsuragawa Y, Yanagisawa M, Inoue A, Masaki T. Two distinct nonmuscle myosin-heavy chain mRNA’s are differentially expressed in various chicken tissues: identification of a novel gene family of vertebrate non-sarcomeric myosin heavy chains. Eur J Biochem. 1989;184:611–616. doi: 10.1111/j.1432-1033.1989.tb15057.x. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. Early Mammalian Development: Parthenogenetic Studies. 1st ed. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- Kelley CA, Oberman F, Yisraeli JK, Adelstein RS. A Xenopus nonmuscle myosin heavy chain isoform is phosphorylated by cyclin-P34cdc2 kinase during meiosis. J Biol Chem. 1995;270:1395–1401. doi: 10.1074/jbc.270.3.1395. [DOI] [PubMed] [Google Scholar]
- Kelley CA, Sellers JR, Adelstein RS, Baines IC. Xenopus nonmuscle myosin isoforms have different enzymatic activities and subcellular localizations. Mol Biol Cell. 1995;6:26a. doi: 10.1083/jcb.134.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP, et al. Contractile proteins in Drosophila development. Ann NY Acad Sci. 1990;528:233–251. doi: 10.1111/j.1749-6632.1990.tb21683.x. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Mabuchi I, Inoue S. Evidence that myosin does not contribute to force production in chromosome movement. J Cell Biol. 1982;94:165–178. doi: 10.1083/jcb.94.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo FJ. In: Egg cortical architecture. In:. The Cell Biology of Fertilization. Schatten H, Schatten G, editors. San Diego: Academic Press; 1989. pp. 105–138. [Google Scholar]
- Longo FJ, Chen DY. Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol. 1985;107:382–394. doi: 10.1016/0012-1606(85)90320-3. [DOI] [PubMed] [Google Scholar]
- Mabuchi I. Biochemical aspects of cytokinesis. Int Rev Cytol. 1986;101:175–213. doi: 10.1016/s0074-7696(08)60249-1. [DOI] [PubMed] [Google Scholar]
- Maro B, Johnson MH, Pickering SJ, Flach G. Changes in actin distribution during fertilization of the mouse egg. J Embryol Exp Morphol. 1984;81:211–237. [PubMed] [Google Scholar]
- Maro B, Johnson MH, Webb M, Flach G. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton, and the plasma membrane. J Embryol Exp Morphol. 1986;92:11–32. [PubMed] [Google Scholar]
- Maupin P, Phillips CL, Adelstein RS, Pollard TD. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J Cell Sci. 1994;107:3077–3090. doi: 10.1242/jcs.107.11.3077. [DOI] [PubMed] [Google Scholar]
- Mazia D, Schatten G, Sale W. Adhesion of cells to surfaces coated with polylysine. J Cell Biol. 1975;66:198–200. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Bower E, Levitt P, Li D, Chantler PD. Myosin II distribution in neurons is consistent with a role in growth cone motility but not synaptic vesicle mobilization. Neuron. 1992;8:25–44. doi: 10.1016/0896-6273(92)90106-n. [DOI] [PubMed] [Google Scholar]
- Murakami N, Elzinga M. Immunohistochemical studies on distribution of cellular myosin II isoforms in brain and aorta. Cell Motil Cytoskeleton. 1992;22:281–295. doi: 10.1002/cm.970220408. [DOI] [PubMed] [Google Scholar]
- Nicosia SV, Wolf DP, Inoue M. Cortical granule distribution and cell surface characteristics in mouse eggs. Dev Biol. 1977;57:56–74. doi: 10.1016/0012-1606(77)90354-2. [DOI] [PubMed] [Google Scholar]
- Nicosia SV, Wolf DP, Mastroianni L. Surface topography of mouse eggs before and after insemination. Gamete Res. 1978;1:145–155. [Google Scholar]
- Nowak G, Pestic-Dragovich L, Hozak P, Philimonenko A, Simerly C, Schatten G, de Lanerolle P. Evidence for the presence of myosin I in the nucleus. J Biol Chem. 1997;272:17176–17181. doi: 10.1074/jbc.272.27.17176. [DOI] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP, Flaherty SP. Cytoskeletal assemblies of mammalian spermatozoa. Ann NY Acad Sci. 1987;513:222–246. doi: 10.1111/j.1749-6632.1987.tb25012.x. [DOI] [PubMed] [Google Scholar]
- Phillips CL, Yamakawa K, Adelstein RS. Cloning of the cDNA encoding human nonmuscle myosin heavy chain-B and analysis of human tissues with isoform-specific antibodies. J Muscle Res Cell Motil. 1995;16:379–389. doi: 10.1007/BF00114503. [DOI] [PubMed] [Google Scholar]
- Reima I, Lehtonen E. Localization of nonerythroid spectrin and actin in mouse oocytes and preimplantation embryos. Differentiation. 1985;30:68–75. doi: 10.1111/j.1432-0436.1985.tb00515.x. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin IIA and B isoforms in cultured neurons. J Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Saez CG, Myers JC, Shows TB, Leinwand LA. Human nonmuscle myosin heavy chain mRNA: generation of diversity through alternative polyadenylation. Proc Natl Acad Sci USA. 1990;87:1164–1168. doi: 10.1073/pnas.87.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Mittal B, Dome JS, Sanger JW. Analysis of cell division using fluorescently labeled actin and myosin in living PtK2 cells. Cell Motil Cytoskeleton. 1989;14:201–219. doi: 10.1002/cm.970140207. [DOI] [PubMed] [Google Scholar]
- Satterwhite LL, Lohka MJ, Wilson KL, Scherson TY, Cisek LJ, Corden JL, Pollard TD. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc2: a mechanism for the timing of cytokinesis. J Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauman I, Berry SJ. An actin infrastructure is associated with eukaryotic chromosomes: structural and functional significance. Eur J Cell Biol. 1994;64:348–356. [PubMed] [Google Scholar]
- Schatten G, Schatten H, Spector I, Cline C, Paweletz N, Simerly C, Petzelt C. Latrunculin inhibits the microfilament-mediated processes during fertilization, cleavage, and early development in sea urchins and mice. Exp Cell Res. 1986a;166:191–208. doi: 10.1016/0014-4827(86)90519-7. [DOI] [PubMed] [Google Scholar]
- Schatten G, Simerly C, Asai DJ, Szöke E, Cooke P, Schatten H. Acetylated α-tubulin in microtubules during mouse fertilization and early development. Dev Biol. 1988;130:74–86. doi: 10.1016/0012-1606(88)90415-0. [DOI] [PubMed] [Google Scholar]
- Schatten G, Schatten H. Cytoskeletal alterations and nuclear architectural changes during mammalian fertilization. Curr Top Dev Biol. 1987;23:23–54. doi: 10.1016/s0070-2153(08)60618-3. [DOI] [PubMed] [Google Scholar]
- Schatten H, Cheney R, Balczon R, Willard M, Cline C, Simerly C, Schatten G. Localization of fodrin during fertilization and early development of sea urchins and mice. Dev Biol. 1986b;118:457–466. doi: 10.1016/0012-1606(86)90016-3. [DOI] [PubMed] [Google Scholar]
- Sellers JR. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol. 1991;3:98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- Shalgi R, Phillips DM, Kraicer PF. Observation on the incorporation cone in the rat. Gamete Res. 1978;1:27–37. [Google Scholar]
- Simerly C, Balczon R, Brinkley BR, Schatten G. Microinjected kinetochore antibodies interfere with chromosome movement in meiotic and mitotic mouse oocytes. J Cell Biol. 1990;111:1491–1504. doi: 10.1083/jcb.111.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly C, Hecht N, Goldberg E, Schatten G. Tracing the incorporation of the sperm tail in the mouse zygote and early embryo using an anti-testicular α-tubulin antibody. Dev Biol. 1993;158:536–548. doi: 10.1006/dbio.1993.1211. [DOI] [PubMed] [Google Scholar]
- Simerly C, Navara C, Wu G-J, Schatten G. Cytoskeletal organization and dynamics in mammalian oocytes during maturation and fertilization. In: Grudzinskas JG, Yovich JL, editors. Cambridge Reviews in Human Reproduction. Gametes: The Oocyte. Cambridge: Cambridge University Press; 1995. pp. 54–94. [Google Scholar]
- Simerly C, Schatten G. Techniques for the localization of specific molecules in oocytes and embryos. In: Wassarman PM, DePamphila M L, editors. Methods in Enzymology. Vol. 225. San Diego: Academic Press; 1994. pp. 516–553. [DOI] [PubMed] [Google Scholar]
- Slager HG, Good MJ, Schaart G, Groenewoud JS, Mummery CL. Organization of non-muscle myosin during early murine embryonic differentiation. Differentiation. 1992;50:47–56. doi: 10.1111/j.1432-0436.1992.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sobel JS. Localization of myosin in the preimplantation mouse embryo. Dev Biol. 1983a;95:227–231. doi: 10.1016/0012-1606(83)90021-0. [DOI] [PubMed] [Google Scholar]
- Sobel JS. Cell–cell contact modulation of myosin organization in the early mouse embryo. Dev Biol. 1983b;100:207–213. doi: 10.1016/0012-1606(83)90212-9. [DOI] [PubMed] [Google Scholar]
- Sobel JS. Myosin rings and spreading in mouse blastomeres. J Cell Biol. 1984;99:1145–1150. doi: 10.1083/jcb.99.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel JS, Allegro MA. Changes in the distribution of spectrin-like protein during development of the preimplantation mouse embryo. J Cell Biol. 1985;100:333–336. doi: 10.1083/jcb.100.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Chantler PD. A unique cellular myosin II exhibiting differential expression in the cerebral cortex. Biochem Biophys Res Commun. 1991;175:244–249. doi: 10.1016/s0006-291x(05)81226-4. [DOI] [PubMed] [Google Scholar]
- Tamblyn TM. Evidence for non-muscle myosin in bovine ejaculated spermatozoa. Gamete Res. 1981;4:499–506. [Google Scholar]
- Toothaker LE, Gonzalez DA, Tung N, Lemons RS, Le Beau MM, Arnaout MA, Clayton LK, Tenen DG. Cellular myosin heavy chain in human leukocytes: isolation of 5′ cDNA clones, characterization of the protein, chromosomal localization, and up-regulation during myeloid differentiation. Blood. 1991;78:1826–1833. [PubMed] [Google Scholar]
- Van Blerkom J, Bell H. Regulation of development in the fully grown mouse oocyte: chromosome-mediated temporal and spatial differentiation of the cytoplasm and plasma membrane. J Embryol Exp Morphol. 1986;93:213–238. [PubMed] [Google Scholar]
- Virtanen I, Badley RA, Paasivuo R, Lehto VP. Distinct cytoskeletal domains revealed in sperm cells. J Cell Biol. 1984;99:1083–1091. doi: 10.1083/jcb.99.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walt H, Saremaslani P, Heizmann CW, Hedinger C. Myosin in early spermatids of the rat. Virchows Arch B Cell Pathol Inc Mol Pathol. 1982;38:307–310. doi: 10.1007/BF02892826. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Josefowicz WJ, Letourneau GE. Meiotic maturation of mouse oocytes in vitro: inhibition of maturation at specific stages of nuclear progression. J Cell Sci. 1976;22:531–545. doi: 10.1242/jcs.22.3.531. [DOI] [PubMed] [Google Scholar]
- Webb M, Howlett SK, Maro B. Parthenogenesis and cytoskeleton organization in aging mouse oocytes. J Embryol Exp Morphol. 1986;95:131–145. [PubMed] [Google Scholar]
- Wilson AK, Gongas G, Clampool WD, de Lanerolle P. An increase or a decrease in myosin II phosphorylation inhibits macrophage motility. J Cell Biol. 1991;114:277–283. doi: 10.1083/jcb.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihiko Y, Yamashiro S, Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J Cell Biol. 1994;124:129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumura S, Uyeda TQP. Myosin II can be localized to the cleavage furrow and to the posterior region of Dictyostelium amoebae without control by phosphorylation of myosin heavy and light chains. Cell Motil Cytoskeleton. 1997;36:313–322. doi: 10.1002/(SICI)1097-0169(1997)36:4<313::AID-CM2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Zurek B, Sanger JM, Sanger JW, Jockusch BM. Differential effects of myosin-antibody complexes on contractile rings and circumferential belts in epithelioid cells. J Cell Sci. 1990;97:297–306. doi: 10.1242/jcs.97.2.297. [DOI] [PubMed] [Google Scholar]