Abstract
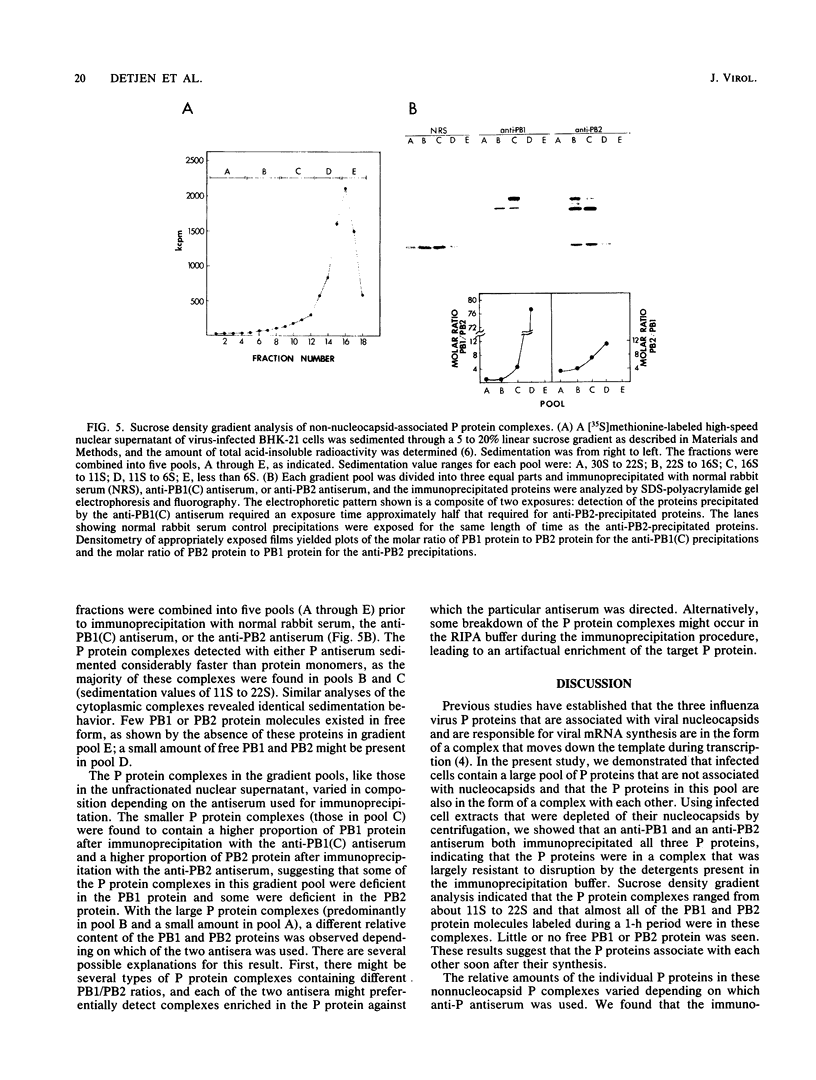
The three influenza virus polymerase, or P, proteins (PB1, PB2, and PA) that are associated with viral nucleocapsids and are responsible for viral mRNA synthesis are in the form of a complex that moves down the template in association with the growing mRNAs during transcription (J. Braam, I. Ulmanen, and R.M. Krug, Cell 34:609-618, 1983). We determined whether infected cells contained a pool of P proteins not associated with viral nucleocapsids and, if so, whether the P proteins in this pool were in the form of a complex with each other. The cytoplasmic and nuclear extracts from infected cells were depleted of nucleocapsids by centrifugation, and the resulting supernatants were subjected to immunoprecipitation with an antiserum specific for either the PB1 protein or the PB2 protein. Both antisera precipitated all three P proteins, indicating that the P proteins were in a complex that was largely resistant to disruption by the detergents present in the immunoprecipitation buffer. Sucrose density gradient analysis showed that the P protein complexes ranged from about 11S to 22S and that almost all of the PB1 and PB2 protein molecules synthesized during a 1-h period (2.5 to 3.5 h postinfection) were in these complexes. Little or no free PB1 or PB2 protein was detected. The possible role of these nonnucleocapsid P protein complexes in the initiation and reinitiation of virus-specific RNA synthesis is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Davis N. L., Wertz G., Schubert M., Lazzarini R. A. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell. 1985 May;41(1):259–267. doi: 10.1016/0092-8674(85)90079-0. [DOI] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Antibodies against the chemically synthesized genome-linked protein of poliovirus react with native virus-specific proteins. Cell. 1982 Feb;28(2):395–404. doi: 10.1016/0092-8674(82)90357-9. [DOI] [PubMed] [Google Scholar]
- Beaton A. R., Krug R. M. Transcription antitermination during influenza viral template RNA synthesis requires the nucleocapsid protein and the absence of a 5' capped end. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6282–6286. doi: 10.1073/pnas.83.17.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam-Markson J., Jaudon C., Krug R. M. Expression of a functional influenza viral cap-recognizing protein by using a bovine papilloma virus vector. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4326–4330. doi: 10.1073/pnas.82.13.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J., Ulmanen I., Krug R. M. Molecular model of a eucaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell. 1983 Sep;34(2):609–618. doi: 10.1016/0092-8674(83)90393-8. [DOI] [PubMed] [Google Scholar]
- Brendler T., Godefroy-Colburn T., Carlill R. D., Thach R. E. The role of mRNA competition in regulating translation. II. Development of a quantitative in vitro assay. J Biol Chem. 1981 Nov 25;256(22):11747–11754. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlsen S. R., Peluso R. W., Moyer S. A. In vitro replication of Sendai virus wild-type and defective interfering particle genome RNAs. J Virol. 1985 May;54(2):493–500. doi: 10.1128/jvi.54.2.493-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide sequences of influenza virus segments 1 and 3 reveal mosaic structure of a small viral RNA segment. Cell. 1982 Feb;28(2):303–313. doi: 10.1016/0092-8674(82)90348-8. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Abraham G., Skehel J. J., Smith J. C., Fellner P. Influenza virus messenger RNAs are incomplete transcripts of the genome RNAs. Nucleic Acids Res. 1977 Dec;4(12):4197–4209. doi: 10.1093/nar/4.12.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J., McCauley J. Characterization of influenza virus RNA complete transcripts. Virology. 1982 Jan 30;116(2):517–522. doi: 10.1016/0042-6822(82)90144-1. [DOI] [PubMed] [Google Scholar]
- Herz C., Stavnezer E., Krug R., Gurney T., Jr Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981 Nov;26(3 Pt 1):391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Kaptein J. S., Nayak D. P. Complete nucleotide sequence of the polymerase 3 gene of human influenza virus A/WSN/33. J Virol. 1982 Apr;42(1):55–63. doi: 10.1128/jvi.42.1.55-63.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Mizumoto K., Ishihama A. Purification and enzymatic properties of an RNA polymerase-RNA complex from influenza virus. Virus Res. 1985 Sep;3(2):115–127. doi: 10.1016/0168-1702(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Ishihama A. RNA polymerase of influenza virus. III. Isolation of RNA polymerase-RNA complexes from influenza virus PR8. J Biochem. 1983 Apr;93(4):989–996. doi: 10.1093/oxfordjournals.jbchem.a134254. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Cytoplasmic and nucleoplasmic viral RNPs in influenza virus-infected MDCK cells. Virology. 1972 Oct;50(1):103–113. doi: 10.1016/0042-6822(72)90350-9. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Etkind P. R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973 Nov;56(1):334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Influenza viral RNPs newly synthesized during the latent period of viral growth in MDCK cells. Virology. 1971 Apr;44(1):125–136. doi: 10.1016/0042-6822(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus proteins in infected cells: translation of viral polypeptides, including three P polypeptides, from RNA produced by primary transcription. Virology. 1976 Oct 15;74(2):504–519. doi: 10.1016/0042-6822(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Ulmanen I., Krug R. M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981 Mar;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasubramanian N., Nayak D. P. Sequence analysis of the polymerase 1 gene and the secondary structure prediction of polymerase 1 protein of human influenza virus A/WSN/33. J Virol. 1982 Oct;44(1):321–329. doi: 10.1128/jvi.44.1.321-329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Broni B. A., Krug R. M. Role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7355–7359. doi: 10.1073/pnas.78.12.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Broni B., Krug R. M. Influenza virus temperature-sensitive cap (m7GpppNm)-dependent endonuclease. J Virol. 1983 Jan;45(1):27–35. doi: 10.1128/jvi.45.1.27-35.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Scheidtmann K. H., Carbone A., Laudano A. P., Doolittle R. F. Antibodies specific for the carboxy- and amino-terminal regions of simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5197–5200. doi: 10.1073/pnas.77.9.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Fields S. Nucleotide sequence of human influenza A/PR/8/34 segment 2. Nucleic Acids Res. 1982 Mar 25;10(6):2135–2143. doi: 10.1093/nar/10.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. J., Content J. 5'-terminus of influenza virus RNA. Nat New Biol. 1971 Mar 31;230(13):140–142. doi: 10.1038/newbio230140a0. [DOI] [PubMed] [Google Scholar]