Abstract
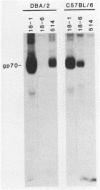
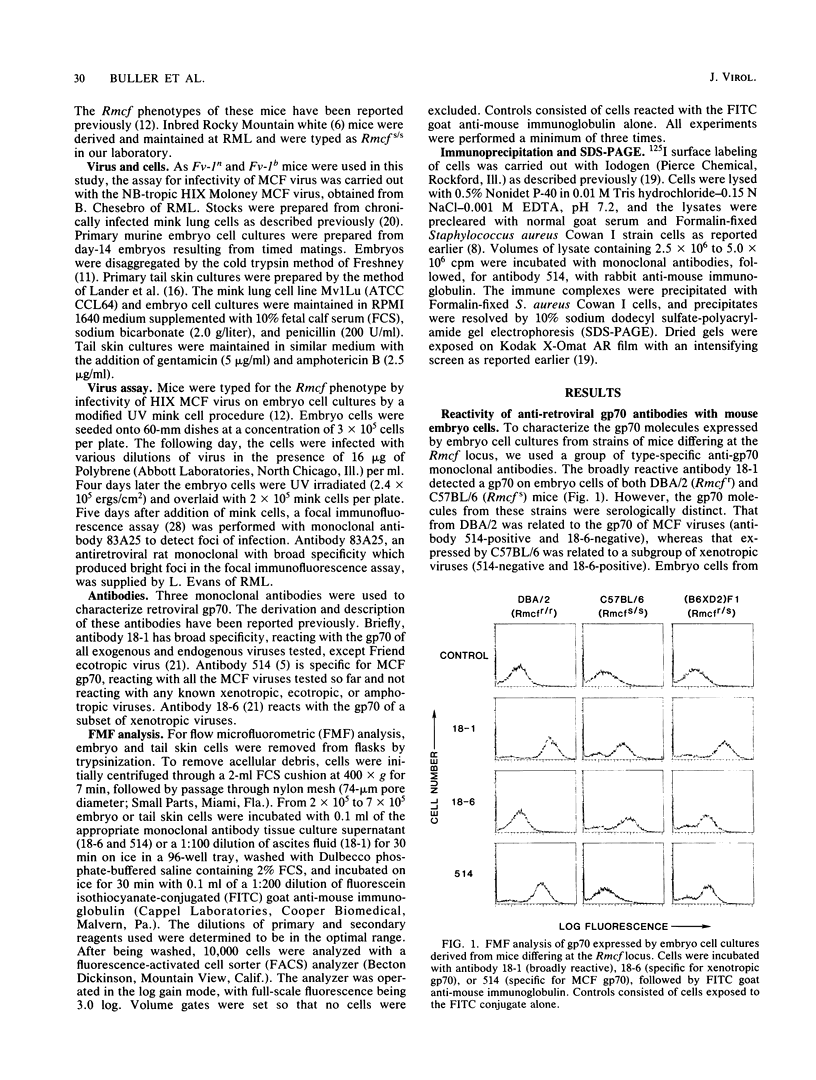
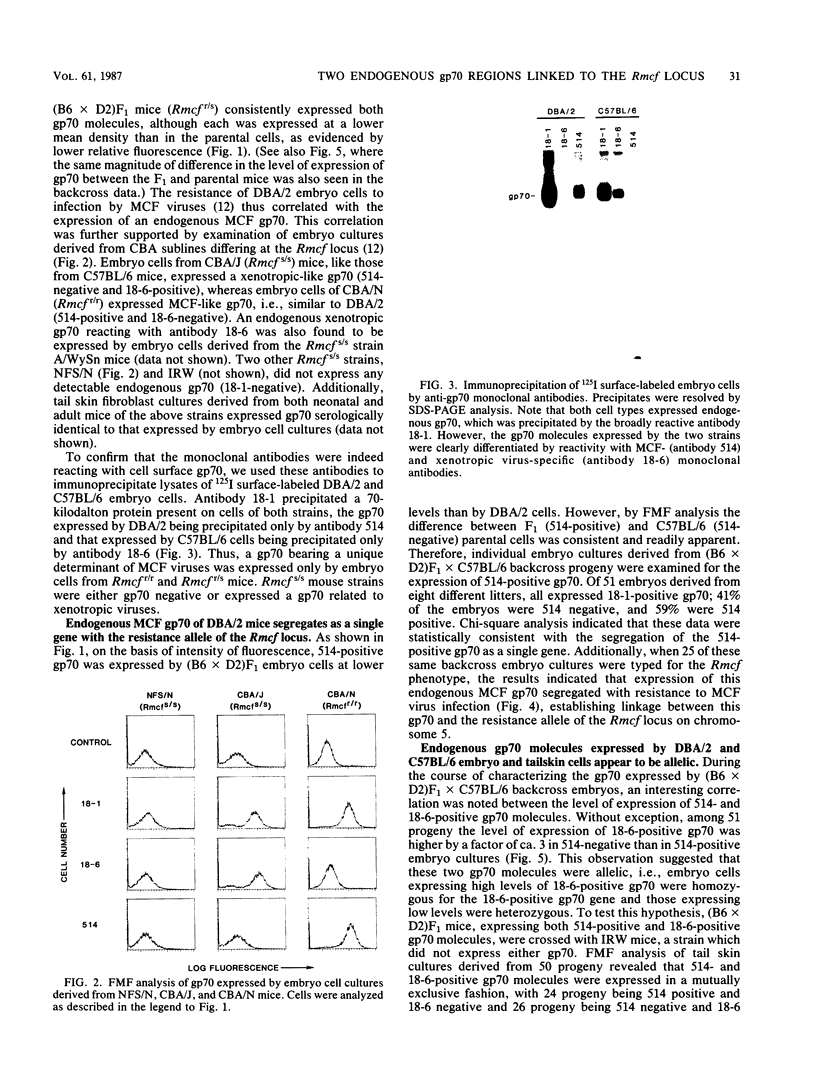
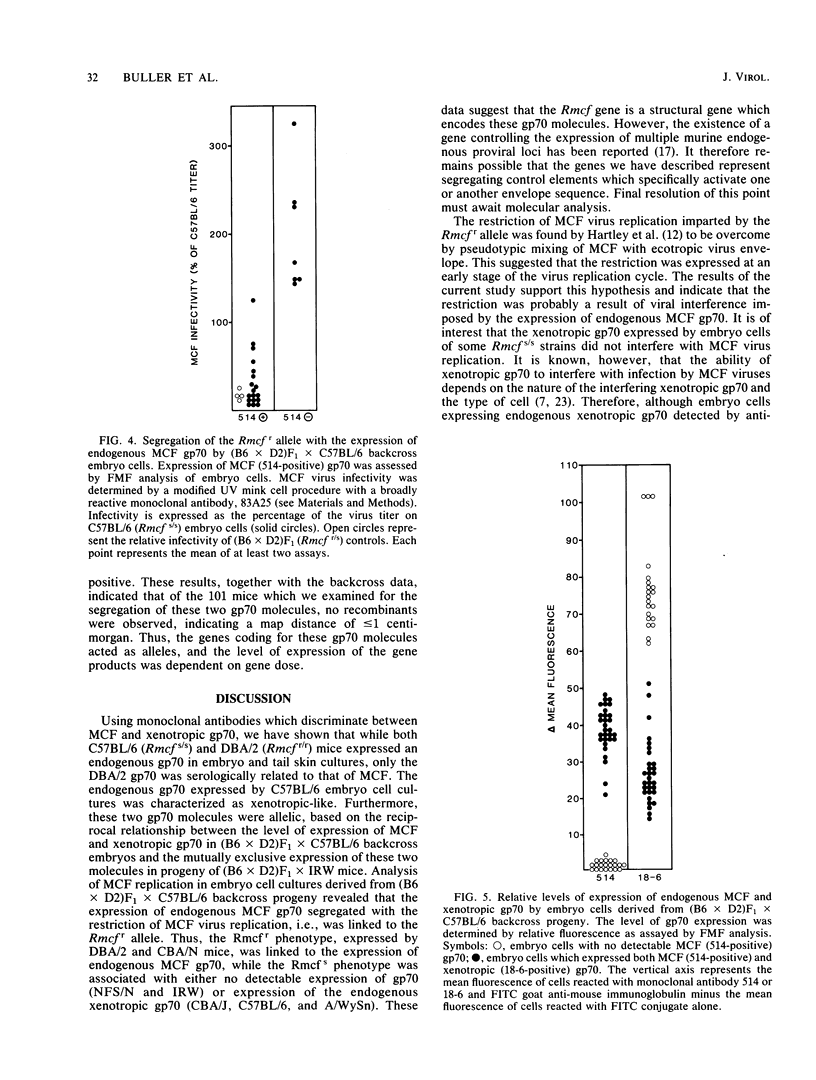
The Rmcf gene restricts the replication of recombinant murine mink cell focus-inducing (MCF) viruses in cell cultures derived from mice carrying the resistance allele (Rmcfr) and may play a role in resistance to retrovirus-induced leukemias in vivo. We have characterized the endogenous gp70 expressed by Rmcfr and Rmcfs mice with a panel of type-specific monoclonal antibodies which discriminate xenotropic and MCF gp70. Embryo and tail skin cultures derived from Rmcfr mice (DBA/2 and CBA/N) expressed gp70 bearing a determinant unique to MCF viruses, whereas cultures from Rmcfs mice expressed either no detectable gp70 (NFS/N and IRW) or a gp70 serologically related to a subgroup of xenotropic viruses (C57BL/6, CBA/J, and A/WySn). Studies of progeny embryos derived from a (C57BL/6 X DBA/2) X C57BL/6 backcross established that the Rmcf resistance allele was linked to the expression of the MCF gp70 and that the gene encoding the xenotropic gp70 expressed by C57BL/6 Rmcfs mice was allelic with the MCF gp70 from Rmcfr mice. These data indicate that the Rmcf locus contains an endogenous gp70 gene having two allelic forms, one of which inhibits exogenous MCF infection in vitro by a mechanism of viral interference.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Ruscetti S., Ali I., Haapala D. K., Rein A. Normal DBA/2 mouse cells synthesize a glycoprotein which interferes with MCF virus infection. Virology. 1982 Nov;123(1):139–151. doi: 10.1016/0042-6822(82)90301-4. [DOI] [PubMed] [Google Scholar]
- Chambers J. A., Cywinski A., Chen P. J., Taylor J. M. Characterization of Rous sarcoma virus-related sequences in the Japanese quail. J Virol. 1986 Aug;59(2):354–362. doi: 10.1128/jvi.59.2.354-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Britt W., Evans L., Wehrly K., Nishio J., Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983 May;127(1):134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Portis J. L., Wehrly K., Nishio J. Effect of murine host genotype on MCF virus expression, latency, and leukemia cell type of leukemias induced by Friend murine leukemia helper virus. Virology. 1983 Jul 15;128(1):221–233. doi: 10.1016/0042-6822(83)90332-x. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Different murine cell lines manifest unique patterns of interference to superinfection by murine leukemia viruses. Virology. 1985 Feb;141(1):119–129. doi: 10.1016/0042-6822(85)90188-6. [DOI] [PubMed] [Google Scholar]
- Dunwiddie C., Faras A. J. Presence of retrovirus reverse transcriptase-related gene sequences in avian cells lacking endogenous avian leukosis viruses. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5097–5101. doi: 10.1073/pnas.82.15.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Yetter R. A., Morse H. C., 3rd A mouse gene on chromosome 5 that restricts infectivity of mink cell focus-forming recombinant murine leukemia viruses. J Exp Med. 1983 Jul 1;158(1):16–24. doi: 10.1084/jem.158.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Kelloff G. J., Sarma P. S., Lane W. T., Turner H. C., Gilden R. V., Oroszlan S., Meier H., Myers D. D., Peters R. L. Group-specific antigen expression during embryogenesis of the genome of the C-type RNA tumor virus: implications for ontogenesis and oncogenesis. Proc Natl Acad Sci U S A. 1970 Sep;67(1):366–376. doi: 10.1073/pnas.67.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Odaka T. Cellular expression of murine leukemia virus gp70-related antigen on thymocytes of uninfected mice correlates with Fv-4 gene-controlled resistance to Friend leukemia virus infection. Virology. 1983 Jul 15;128(1):127–139. doi: 10.1016/0042-6822(83)90324-0. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Gromet N. J., Ikeda H., Buckler C. E. A unique sequence related to the ecotropic murine leukemia virus is associated with the Fv-4 resistance gene. Proc Natl Acad Sci U S A. 1984 Feb;81(3):834–837. doi: 10.1073/pnas.81.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander M. R., Moll B., Rowe W. P. A procedure for culture of cells from mouse tail biopsies: brief communication. J Natl Cancer Inst. 1978 Feb;60(2):477–478. [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. A genetic locus regulates the expression of tissue-specific mRNAs from multiple transcription units. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5823–5827. doi: 10.1073/pnas.79.19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Endogenous C-type viruses in normal and "abnormal" cell development. Cancer Res. 1977 Aug;37(8 Pt 2):2957–2968. [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J. Dissociation of H-2 recognition by antibody and cytotoxic T cells of a cloned murine leukemia cell line. Immunogenetics. 1981;12(1-2):101–115. doi: 10.1007/BF01561654. [DOI] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J. Monoclonal antibodies derived during graft-versus-host reaction. II. Antibodies detect unique determinants common to many MCF viruses. Virology. 1983 Apr 15;126(1):96–105. doi: 10.1016/0042-6822(83)90464-6. [DOI] [PubMed] [Google Scholar]
- Rein A. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982 Jul 15;120(1):251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Rein A., Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984 Jul 15;136(1):144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Senior A. M., Salazar F. H. Host Susceptibility to endogenous viruses: defective, glycoprotein-expressing proviruses interfere with infections. J Virol. 1981 Dec;40(3):745–751. doi: 10.1128/jvi.40.3.745-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Matthai R., Potter M. Susceptibility of BALB/c mice carrying various DBA/2 genes to development of Friend murine leukemia virus-induced erythroleukemia. J Exp Med. 1985 Nov 1;162(5):1579–1587. doi: 10.1084/jem.162.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J. Role of mink cell focus-inducing virus in leukemias induced by Friend ecotropic virus. J Virol. 1984 Jun;50(3):872–877. doi: 10.1128/jvi.50.3.872-877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Nishio J., Wehrly K., Lodmell D., Chesebro B. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology. 1985 Feb;141(1):110–118. doi: 10.1016/0042-6822(85)90187-4. [DOI] [PubMed] [Google Scholar]