Abstract
Host cell involvement in Sindbis virus (SB) RNA synthesis was examined in cells which had been treated before infection with actinomycin D or alpha-amanitin (alpha-A). Overall synthesis of SB RNA was reduced significantly in CHO cells treated for 18 h before infection with alpha-A. However, SB RNA was produced at near normal levels in CHOama-1 cells, a line which contains an alpha-A-resistant RNA polymerase II. In BHK or CHO cells infected with SBamr, a mutant which replicates normally in cells pretreated with either actinomycin D or alpha-A, viral RNA synthesis was not decreased. The levels of negative strand RNA and of replicative forms I, II, and III in SB-infected cells were progressively reduced with increasing times of pretreatment with host transcription inhibitors, indicating fewer functional replicative intermediates in treated cells. Replicative events after replicative intermediate formation also were inhibited but only to the extent predicted by the reduction in replicative intermediates. Similarly, events preceding negative strand synthesis, adsorption, penetration, uncoating, and translation of nonstructural proteins, apparently were not impeded in treated cells. Therefore, our results are consistent with the involvement of a host component after translation of the nonstructural proteins but before or during the synthesis of SB negative strand RNA.
Full text
PDF

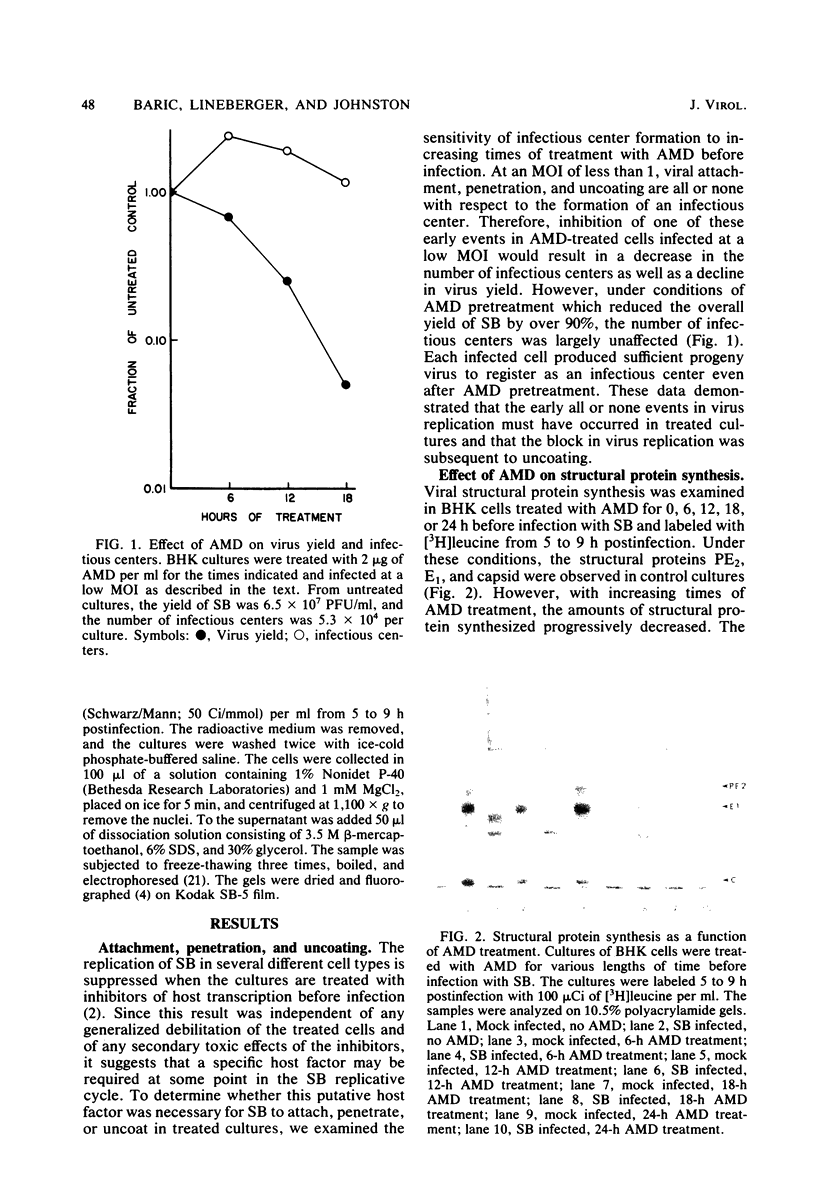

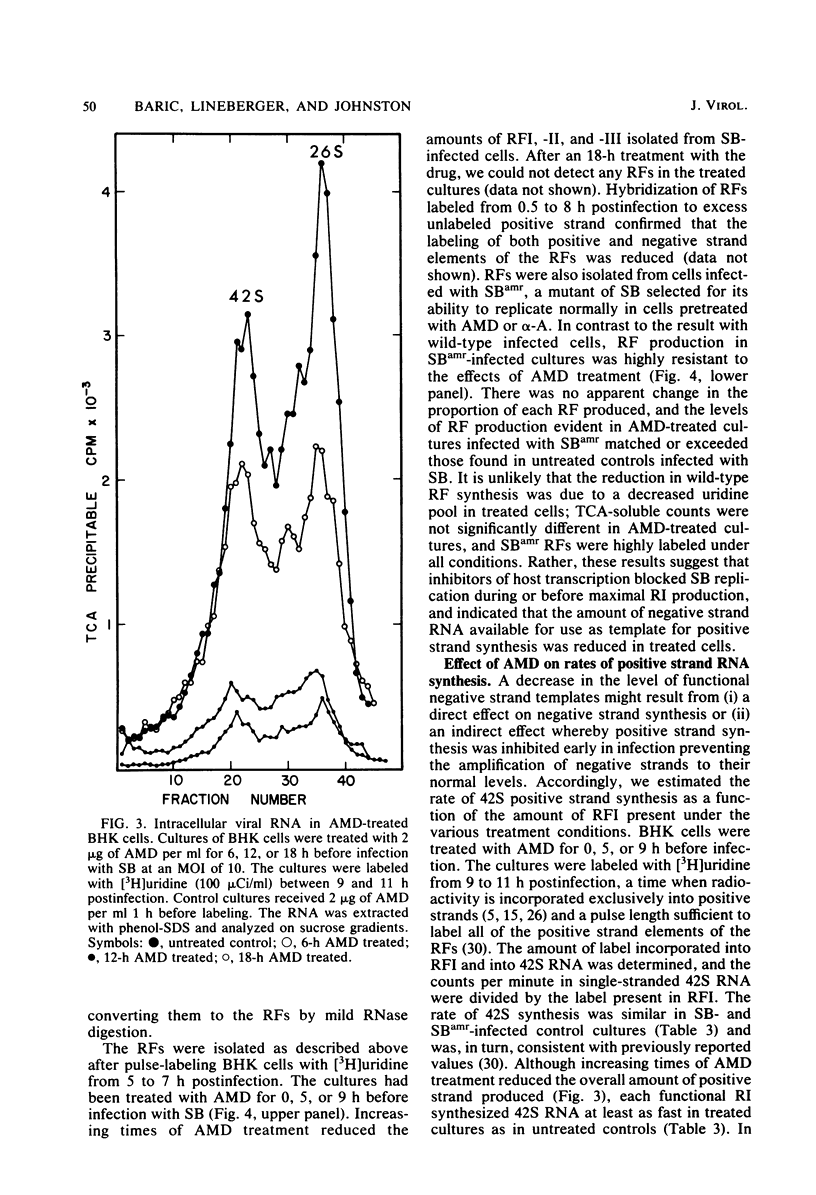
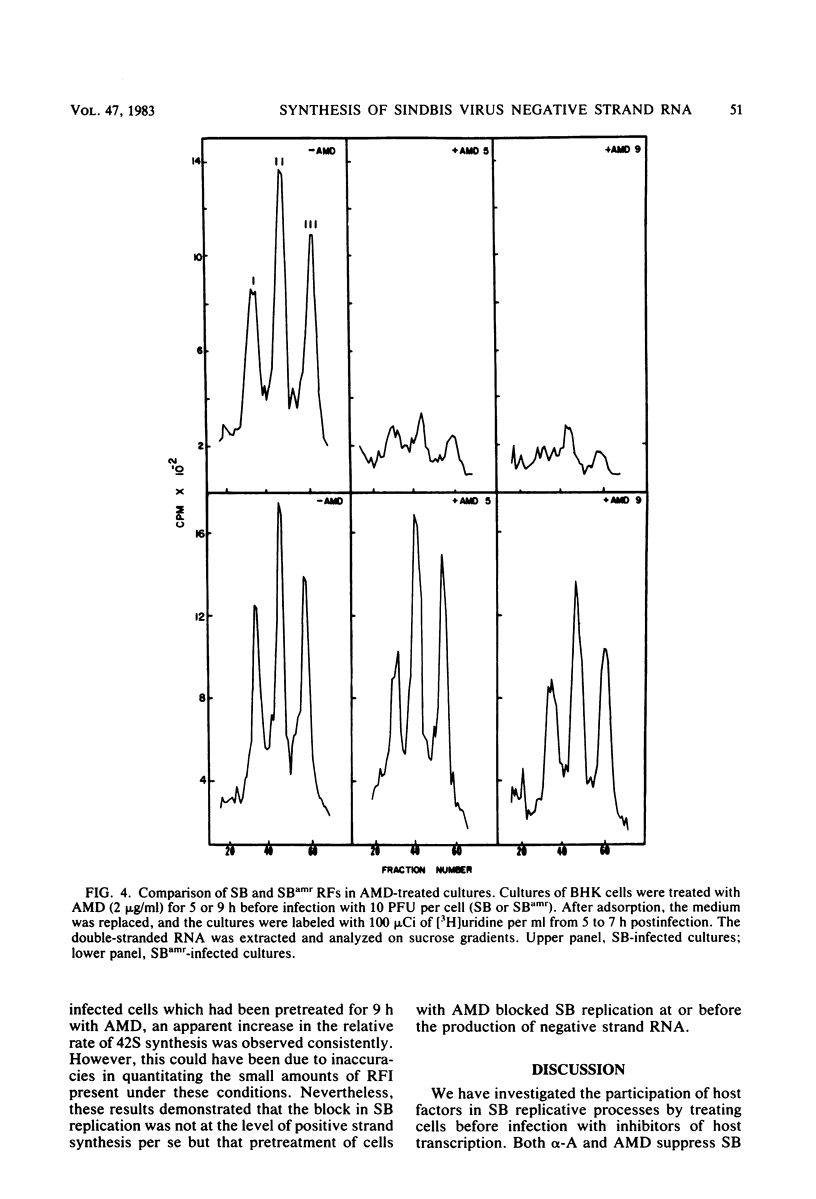



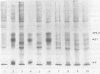
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baric R. S., Carlin L. J., Johnston R. E. Requirement for host transcription in the replication of Sindbis virus. J Virol. 1983 Jan;45(1):200–205. doi: 10.1128/jvi.45.1.200-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baric R. S., Trent D. W., Johnston R. E. A Sindbis virus variant with a cell-determined latent period. Virology. 1981 Apr 15;110(1):237–242. doi: 10.1016/0042-6822(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Kennedy S. I. Semliki Forest virus intracellular RNA: properties of the multi-stranded RNA species and kinetics of positive and negative strand synthesis. J Gen Virol. 1975 Jul;28(1):111–127. doi: 10.1099/0022-1317-28-1-111. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Villa-Komaroff L., Lodish H. F., Schlesinger M. Initiation sites for translation of sindbis virus 42S and 26S messenger RNAs. Cell. 1975 Oct;6(2):215–222. doi: 10.1016/0092-8674(75)90012-4. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Kennedy S. I. Purification and polypeptide composition of Semliki Forest virus RNA polymerase. J Gen Virol. 1976 Sep;32(3):395–411. doi: 10.1099/0022-1317-32-3-395. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Fuller F. J., Marcus P. I., Hightower L. E., Ball L. A. Synthesis and processing of Sindbis virus nonstructural proteins in vitro. Virology. 1982 Apr 30;118(2):363–379. doi: 10.1016/0042-6822(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Davey M. W., Dalgarno L. Semliki Forest virus replication in cultured Aedes albopictus cells: studies on the establishment of persistence. J Gen Virol. 1974 Sep;24(3):453–463. doi: 10.1099/0022-1317-24-3-453. [DOI] [PubMed] [Google Scholar]
- Erwin C., Brown D. T. Requirement of cell nucleus for Sindbis virus replication in cultured Aedes albopictus cells. J Virol. 1983 Feb;45(2):792–799. doi: 10.1128/jvi.45.2.792-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Koo R., Stollar V. Evolution and properties of Aedes albopictus cell cultures persistently infected with sindbis virus. Virology. 1977 Oct 1;82(1):69–83. doi: 10.1016/0042-6822(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Ingles C. J., Guialis A., Lam J., Siminovitch L. Alpha-Amanitin resistance of RNA polymerase II in mutant Chinese hamster ovary cell lines. J Biol Chem. 1976 May 10;251(9):2729–2734. [PubMed] [Google Scholar]
- Johnston R. E., Bose H. R. Correlation of messenger RNA function with adenylate-rich segments in the genomes of single-stranded RNA viruses. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1514–1516. doi: 10.1073/pnas.69.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal K. J., Stollar V. Temperature-sensitive host-dependent mutants of Sindbis virus. Virology. 1981 Oct 15;114(1):140–148. doi: 10.1016/0042-6822(81)90260-9. [DOI] [PubMed] [Google Scholar]
- Lachmi B. E., Käriäinen L. Sequential translation of nonstructural proteins in cells infected with a Semliki Forest virus mutant. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1936–1940. doi: 10.1073/pnas.73.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovaara P., Ulmanen I., Käriäinen L., Keränen S., Philipson L. Synthesis and processing of Semliki Forest virus-specific nonstructural proteins in vivo and in vitro. Eur J Biochem. 1980 Dec;112(3):461–468. doi: 10.1111/j.1432-1033.1980.tb06108.x. [DOI] [PubMed] [Google Scholar]
- Mento S. J., Siminovitch L. Isolation and preliminary characterization of Sindbis virus-resistant Chinese hamster ovary cells. Virology. 1981 Jun;111(2):320–330. doi: 10.1016/0042-6822(81)90336-6. [DOI] [PubMed] [Google Scholar]
- Mowshowitz D. Identification of polysomal RNA in BHK cells infected by sindbis virus. J Virol. 1973 Apr;11(4):535–543. doi: 10.1128/jvi.11.4.535-543.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Role of extracellular virus on the maintenance of the persistent infection induced in Aedes albopictus (mosquito) cells by Sindbis virus. J Virol. 1977 Sep;23(3):554–561. doi: 10.1128/jvi.23.3.554-561.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977 Jul 15;80(2):390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G., Keränen S., Käriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981 Aug;39(2):348–358. doi: 10.1128/jvi.39.2.348-358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980 Apr;34(1):108–118. doi: 10.1128/jvi.34.1.108-118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L., Käriäinen L., Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Scheefers-Borchel U., Scheefers H., Edwards J., Brown D. T. Sindbis virus maturation in cultured mosquito cells is sensitive to actinomycin D. Virology. 1981 Apr 30;110(2):292–301. doi: 10.1016/0042-6822(81)90061-1. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. II. Multiple forms of double-stranded RNA isolated from infected cells. J Mol Biol. 1972 Nov 28;71(3):615–631. doi: 10.1016/s0022-2836(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. V. Polyribosomes and mRNA in infected cells. J Virol. 1974 Sep;14(3):552–559. doi: 10.1128/jvi.14.3.552-559.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Sonnabend J. A., Martin E. M., Mécs E. Viral specific RNAs in infected cells. Nature. 1967 Jan 28;213(5074):365–367. doi: 10.1038/213365a0. [DOI] [PubMed] [Google Scholar]
- Tooker P., Kennedy S. I. Semliki Forest virus multiplication in clones of Aedes albopictus cells. J Virol. 1981 Feb;37(2):589–600. doi: 10.1128/jvi.37.2.589-600.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WECKER E. The extraction of infectious virus nucleic acid with hot phenol. Virology. 1959 Feb;7(2):241–243. doi: 10.1016/0042-6822(59)90191-6. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Katz F., Small B., Lodish H. F. How a single Sindbis virus mRNA directs the synthesis of one soluble protein and two integral membrane glycoproteins. Cell. 1977 Feb;10(2):253–263. doi: 10.1016/0092-8674(77)90219-7. [DOI] [PubMed] [Google Scholar]