Abstract
Contact of cultured mammary epithelial cells with the basement membrane protein laminin induces multiple responses, including cell shape changes, growth arrest, and, in the presence of prolactin, transcription of the milk protein β-casein. We sought to identify the specific laminin receptor(s) mediating the multiple cell responses to laminin. Using assays with clonal mammary epithelial cells, we reveal distinct functions for the α6β4 integrin, β1 integrins, and an E3 laminin receptor. Signals from laminin for β-casein expression were inhibited in the presence of function-blocking antibodies against both the α6 and β1 integrin subunits and by the laminin E3 fragment. The α6-blocking antibody perturbed signals mediated by the α6β4 integrin, and the β1-blocking antibody perturbed signals mediated by another integrin, the α subunit(s) of which remains to be determined. Neither α6- nor β1-blocking antibodies perturbed the cell shape changes resulting from cell exposure to laminin. However, the E3 laminin fragment and heparin both inhibited cell shape changes induced by laminin, thereby implicating an E3 laminin receptor in this function. These results elucidate the multiplicity of cell-extracellular matrix interactions required to integrate cell structure and signaling and ultimately permit normal cell function.
INTRODUCTION
Cell contact with the extracellular matrix (ECM) serves as a dominant regulator of cellular structure and function (for reviews, see Roskelley et al., 1995; Giancotti, 1997). The ECM functions both as a scaffold for cell attachment and cytoskeletal organization and as an array of signaling molecules. Cell surface receptors for ECM molecules integrate the three cellular responses of attachment, cytoskeletal organization, and signaling. Consequently, cellular structure and signaling events are coupled within these receptors, as shown by adhesion dependence of cell growth and cell shape dependence of some signaling pathways leading to cell survival and tissue-specific gene expression (Petersen et al., 1992; Roskelley et al., 1994; Boudreau et al., 1996; Chen et al., 1997a; Kheradmand et al., 1998; Wang et al., 1998). Although the multiple ECM receptors on the cell surface are presumed to play different roles in signaling and morphogenesis, their distinct functions are not well characterized in the same cell system.
Our laboratory has been dissecting the mechanism by which the ECM regulates epithelial cell behavior using assays of normal cell function in both primary mammary epithelial cells and cell lines. Cells of mammary epithelial origin comprise the myoepithelial and milk-secreting cells of the mammary gland. Like all epithelial cells, they contact the basement membrane, and signaling from the basement membrane is important in all stages of mammary gland development (for review, see Roskelley et al., 1995). Removing mammary epithelial cells from contact with the basement membrane and placing them on tissue culture plastic leads to altered cellular structure and growth, increased apoptosis, and a loss of function, the latter being measured by the cell’s inability to respond to lactogenic hormones by producing milk proteins (Emerman and Pitelka, 1977; Barcellos-Hoff et al., 1989; Boudreau et al., 1995; Lin et al., 1995). However, many of these functions can be recovered by culturing cells in the presence of either a reconstituted basement membrane (Matrigel) or the purified basement membrane glycoprotein laminin. Primary mammary epithelial cells, and certain cell lines, cultured in the presence of laminin will arrest growth and reorganize to form rounded cell clusters that regain the ability to respond to lactogenic hormones and to express β-casein mRNA and protein (Roskelley et al., 1994; Streuli et al., 1995).
The specific receptors mediating the signaling responses to laminin in mammary epithelial cells have not been identified. Laminin, which exists in many isoforms, has in excess of 12 reported cell surface receptors. The best characterized laminin receptors belong to the integrin receptor family; these include the α1β1, α2β1, α3β1, α6β1, α7β1, α9β1, and α6β4 integrins (Mercurio, 1995). In addition to the integrins, several other cell surface molecules have been implicated in cell-laminin interactions, including dystroglycan, the 67-kDa laminin receptor, an isoform of syndecan-1, and potentially others (Salmivirta et al., 1994; Henry and Campbell, 1996; Hinek, 1996; Chen et al., 1997b). Nearly all of the laminin receptors listed above have been implicated in linkages to the cytoskeleton and may transmit distinct signals via their unique cytoplasmic domains (Sastry and Horwitz, 1993; Henry and Campbell, 1996; Carey, 1997).
Previous studies from our laboratory showed that receptor binding to the E3 domain of laminin is required for β-casein expression and that antibodies blocking β1 integrins inhibit β-casein production (Streuli et al., 1995). These studies utilized primary cell cultures and the mammary epithelial cell line CID-9 (Schmidhauser et al., 1990), both of which contain a mixture of epithelial and mesenchymal-like cells. Because primary and mixed cell cultures have the potential to produce an endogenous basement membrane, we have more recently employed a clonal mammary epithelial cell line, SCp2, which has lost the ability to assemble a functional basement membrane and, therefore, circumvents the interference from endogenous laminin deposition (Desprez et al., 1993). This cell line nevertheless responds to reconstituted basement membrane or laminin by making β-casein. Using SCp2 cells, we previously reported two distinct signaling pathways for β-casein expression in response to ECM, a morphogenic and a biochemical pathway (Roskelley et al., 1994). The morphogenic signal is the induction of a rounded morphology in cells exposed to laminin. This signal is a prerequisite for subsequent biochemical signals leading to transcription and translation of the β-casein gene. β-Casein expression was perturbed by a tyrosine kinase inhibitor, whereas the morphological changes were unaffected (Roskelley et al., 1994; Roskelley and Bissell, 1995). Therefore, the morphogenic and biochemical signaling pathways induced by laminin were separated, yet the precise receptor(s) initiating these signals were still to be determined.
In the present study, we have used reagents that block receptor-ligand interactions at the cell surface to dissect the function(s) of the laminin receptors operating in mammary epithelial cells. We demonstrate distinct but cooperative roles for the α6β4 integrin, β1 integrins, and an E3 laminin receptor in the functional differentiation of mammary epithelial cells. We also show that some of these signaling functions can be masked when the population in the cell culture is heterogeneous.
MATERIALS AND METHODS
Antibodies and Reagents
The function-blocking integrin antibodies against α1 (Ha31/8), α5 (5H10-27), α6 (GoH3), αv (H9.2B8), and β1 (Ha2/5 and 9EG7) subunits were purchased as azide- and endotoxin-free reagents from PharMingen (San Diego, CA). The anti-integrin β4 subunit (clone 346-11A) was also purchased from PharMingen. Polyclonal anti-β-casein antibody was generated against whole mouse milk in our laboratory as described (Lee et al., 1984). The monoclonal anti-rat β-casein antibody was a gift from Dr. C. Kaetzel (Kaetzel and Ray, 1984). The anti-E-cadherin antibody (C20820) was purchased from Transduction Laboratories (Lexington, KY). Laminin fragments were prepared as previously described (Schittny and Yurchenco, 1990; Sung et al., 1993) and dialyzed against PBS. Heparin and heparan sulfate were purchased from Sigma Chemical (St. Louis, MO), product numbers H3393 and H9902, respectively.
Cell Culture and β-Casein Assays
The SCp2 cell line (Desprez et al., 1993) is a functionally normal murine mammary epithelial line cloned from the heterogeneous cell strain CID-9 (Schmidhauser et al., 1990). SCp2, NIH3T3, and primary mammary epithelial cells were cultured in DMEM/F12 medium (1:1) supplemented with insulin (5 μg/ml) (Sigma Chemical) and 2% fetal bovine serum (Atlanta Biologicals, Norcross, GA). Primary mammary epithelial cells were isolated from midpregnant CD-1 mice, as described (Lee et al., 1984).
To assay β-casein expression in mammary epithelial cells treated on tissue culture plastic, cells were plated at subconfluence in serum-free DMEM/F12 medium supplemented with insulin (5 μg/ml) and hydrocortisone (1 μg/ml) (Sigma Chemical) at a density of ∼50,000 cells/cm2. Cells were allowed to attach and spread for 2 d before treatment. Once completely spread, they were treated with fresh serum-free medium, insulin, hydrocortisone, and prolactin (3 μg/ml) with or without laminin or Matrigel. Laminin or Matrigel diluted in the culture medium rapidly fall out of solution, forming a precipitate covering the cultured cells and thereby producing a high concentration of laminin at the cell surface. Cells were treated for 5 d, with one change of medium after 3 d, and then extracted for protein analysis. For extraction, cells were rinsed once with PBS, frozen and thawed in 100 μl of protein extraction buffer (50 mM Tris-HCl, pH 7.4, 30 mM NaCl, 1% [vol/vol] NP-40, 1% [wt/vol] deoxycholate, 0.1% [wt/vol] SDS, and protease inhibitor cocktail [Calbiochem, La Jolla, CA]), and cleared by centrifugation for 5 min at 12,000 × g. The resulting supernatant was added to reducing protein sample buffer and separated by SDS-PAGE as described below. Ovine prolactin-20 (AFP 10677C) was a gift from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Bethesda, MD). Purified Engelbreth-Holm-Swarm laminin was purchased from Sigma Chemical and included in the assays at 150 μg/ml. Matrigel was purchased from Collaborative Biomedical Products (Bedford, MA) and used at a 1.5% dilution (∼150–200 μg protein/ml).
For assays of β-casein expression and survival in prerounded cells, cells were first cultured in suspension by placing 4.0 × 106 cells in a 10-cm culture dish coated with the nonadhesive substratum poly(2-hydroxyethyl methacrylate) (polyHEMA) (Sigma Chemical) in 10 ml of serum-free medium, plus insulin and hydrocortisone. Cells were allowed to aggregate in suspension for 2 d and then divided into either 48- or 96-well culture dishes coated with polyHEMA (2.0 × 105 or 1.2 × 105 cells per well, respectively) in serum-free medium plus insulin, hydrocortisone, and prolactin, with or without laminin. Cells were incubated for 3 d before extraction for protein analysis. For extraction, cells were transferred to Eppendorf tubes, centrifuged at 3000 × g for 5 min, and lysed in protein extraction buffer, as described above. Viability of treated cells in suspension was assayed after 4 d using the Alamar Blue vital dye assay (Accumed International, Westlake, OH) according to the manufacturer’s instructions. PolyHEMA-coated dishes were prepared using a solution of 6 mg/ml polyHEMA in 95% ethanol added to culture plates at 0.05 ml/cm2 and allowed to evaporate to dryness.
Immunoblotting and Immunoprecipitations
SDS-PAGE was performed as previously described (Laemmli, 1970). For β-casein immunoblots, cell extracts equivalent to ∼50,000 cells per sample were separated on 13% acrylamide gels and transferred to an Immobilon-P membrane (Amersham, Arlington Heights, IL). Filters were blocked with 5% (wt/vol) BSA in TBST (50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 0.1% [vol/vol] Tween 20) and probed with either an anti-mouse milk polyclonal antisera or an anti-rat β-casein monoclonal antibody, diluted in TBST plus 1.0% (wt/vol) BSA. Antibody binding was detected by a horseradish peroxidase-conjugated secondary antibody and an ECL reagent (Amersham), according to the manufacturer’s instructions.
For integrin immunoprecipitations, SCp2 cells were metabolically labeled for 16 h with 200 μCi of [35S]methionine (Amersham) per milliliter of culture medium. Labeled cells were washed several times with cold medium and extracted in NP-40 lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1.0% [vol/vol] NP-40). Antibodies were added to aliquots of the extract at a final concentration of 10 μg/ml and incubated overnight at 4°C. Simultaneously, protein G-agarose (Sigma Chemical) was blocked by incubation overnight with a nonradioactive SCp2 cell extract at 4°C, then rinsed several times with NP-40 extraction buffer. Subsequently, the protein G-agarose was incubated with the antibody/extract mixture for 1 h at 4°C, washed three times with NP-40 extraction buffer, once with 1 M sucrose in NP-40 extraction buffer, and twice with 50 mM Tris-HCl, pH 7.5. The precipitated proteins were recovered from the beads in nonreducing SDS-PAGE sample buffer and separated on 7% SDS-polyacrylamide gels. The gels were dried and exposed to X-Omat AR film (Eastman Kodak, Rochester, NY).
RESULTS
Laminin-induced β-Casein Expression Is Perturbed by Function-blocking Antibodies against the β1 and α6 Integrins without Perturbing the Induction of Cell Shape Changes
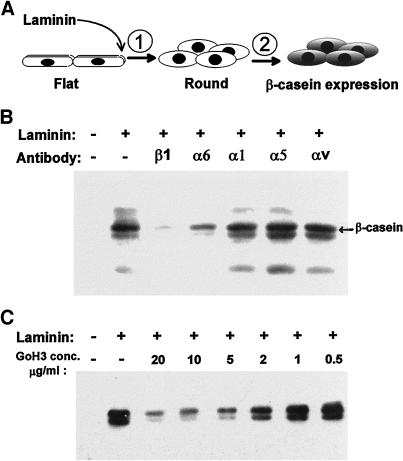
Signals induced by laminin in mammary epithelial cells include a two-step process leading to induction of tissue-specific gene expression as measured by β-casein production (Figure 1A). To identify the laminin receptor(s) mediating these distinct signals, assays for both cell rounding and β-casein expression were performed in the presence of available function-perturbing antibodies against murine integrins. These included antibodies against the β1, α1, α5, α6, and αv subunits. Assays were performed using the cell line SCp2, a clonal murine mammary epithelial cell line that, like primary mammary epithelial cells, responds to contact with laminin by producing β-casein in the presence of lactogenic hormones (Desprez et al., 1993).
Figure 1.
Inhibition of β-casein expression by function-blocking integrin antibodies. Assays for the induction of β-casein expression in SCp2 mammary epithelial cells were performed on cells initially spread on plastic and subsequently exposed to medium containing the lactogenic hormone prolactin, plus or minus laminin, and function-blocking antibodies against the β1, α6, α1, α5, and αv integrin subunits. (A) Schematic representation of the assay. A two-step signaling process leads to β-casein expression after cell contact with laminin, beginning with a prerequisite cell shape change (rounding), followed by subsequent biochemical signaling events. (B) β-Casein expression was assayed by immunoblots of cell extracts and appears as a doublet migrating at ∼34 kDa. Laminin-induced β-casein expression was inhibited in the presence of β1 and α6 integrin-blocking antibodies. (C) Titration of the α6-blocking (GoH3) antibody shows maximal inhibition in the range of 2–5 μg/ml.
The treated cells were tested for the ability to signal β-casein expression when exposed to laminin in the presence of function-perturbing anti-integrin antibodies. Assays for β-casein expression were performed on cells initially attached and spread on cell culture plastic. Spread cells were treated with serum-free medium containing soluble laminin, lactogenic hormones, and function-perturbing antibodies against integrin receptors. Both pure laminin and the laminin-rich reconstituted basement membrane (Matrigel) were used in these studies, and both led to expression of β-casein, as previously demonstrated (Roskelley et al., 1994; Streuli et al., 1995). After 5 d of exposure to laminin, hormones, and antibodies, the treated cells were extracted and assayed for β-casein expression by immunoblotting. Treatment with the α1-, α5-, and αv-blocking antibodies had no inhibitory effect (Figure 1B). In contrast, treatment with the function-blocking antibody against β1 integrins inhibited β-casein expression almost completely, as shown previously for primary cultures and CID-9 cells (Streuli et al., 1995). Contrary to previous observations in primary cultures (Streuli et al., 1991), the GoH3 antibody, directed against the integrin α6 subunit, also blocked the expression of β-casein. Titration of the GoH3 antibody showed a significant blockage of β-casein expression at concentrations between 2 and 5 μg/ml (Figure 1C).
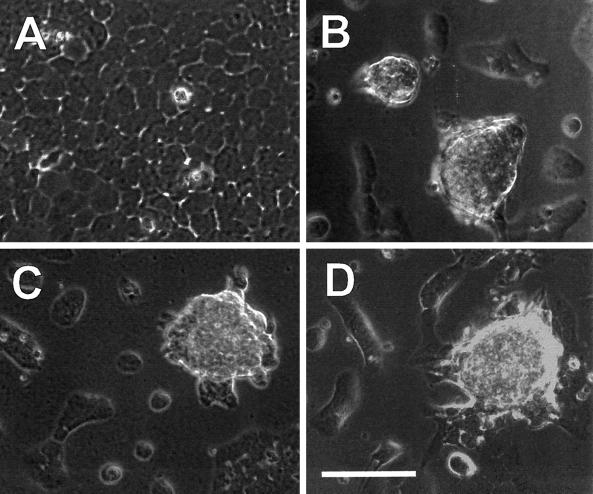
The ability of laminin to induce the rounded cell morphology was not impaired by any of the function-blocking anti-integrin antibodies (Figure 2 and data not shown). The cells exposed to laminin in the presence of β1- and α6-blocking antibodies were indistinguishable in morphology from those exposed to laminin alone, as were those exposed to laminin in the presence of both antibodies in combination (data not shown). Therefore, the inhibition of casein expression by the two integrin antibodies did not appear to occur by the inhibition of prerequisite cell shape changes. To confirm this, β-casein was assayed in cells forced to adopt a rounded conformation by culturing on a nonadhesive substratum (polyHEMA). Under these conditions, the cells were rounded and aggregated before laminin exposure and remained so throughout the assay (Figure 3A). Cells were assayed after just 3 d of laminin exposure because the induction of β-casein was more rapid in prerounded cells than in flat cells, permitting the correspondingly shorter assay duration (Roskelley et al., 1994). In prerounded cells, β-casein expression was still inhibited by both the β1- and α6-blocking antibodies (Figure 3B), demonstrating that the inhibition of β-casein expression by these antibodies was not caused by effects on cell shape. These results also indicate that yet another laminin receptor, distinct from the β1 and α6 integrins, is required to mediate the cell shape changes induced by laminin.
Figure 2.
Morphogenic changes induced by laminin are unaltered by the presence of α6- and β1-blocking antibodies. Assays for the induction of β-casein expression in SCp2 mammary epithelial cells were performed on cells initially spread on plastic and subsequently exposed to medium containing the lactogenic hormone prolactin, plus or minus laminin, and function-blocking antibodies against the β1 and α6 integrin subunits. In the absence of added laminin, cells remained attached and spread on the plastic and continued to grow to confluence (A). In contrast, cells exposed to laminin underwent cell rounding, and those in contact with other cells clustered into multicellular aggregates, leaving much of the plastic culture dish exposed (B). Cells exposed to laminin in the presence of function-blocking antibodies against the α6 (C) and β1 (D) integrin subunits continued to undergo the cell shape changes induced by laminin even though these same antibody treatments perturbed signals for β-casein expression.
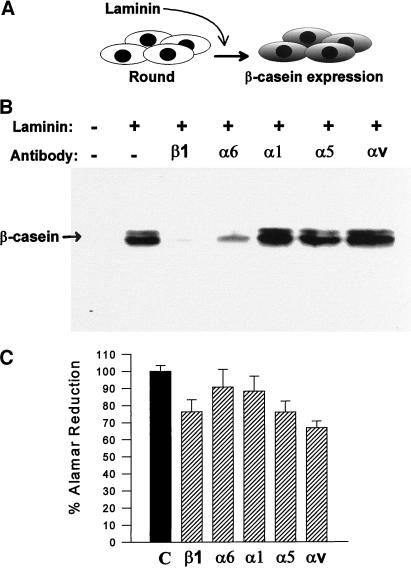
Figure 3.
β-Casein expression and cell survival in the presence of function-blocking antibodies against β1 and α6 integrins in prerounded cells. SCp2 cells cultured in suspension were treated with prolactin, plus or minus laminin, and function-blocking antibodies against the β1, α6, α1, α5, and αv integrin subunits. (A) Assays of β-casein expression in suspension cultures measure signaling events subsequent to, and independent of, the required cell shape changes. (B) Immunoblots of cell extracts after 3 d of laminin exposure show that laminin-induced β-casein expression in prerounded cells was still inhibited in the presence of β1 and α6 integrin-blocking antibodies. (C) Cell viability was assayed by Alamar Blue dye reduction in duplicate wells under β-casein assay conditions identical to those described for B, but culture was for 4 d (1 d longer) to measure any cell death that may have been initiated during the course of the β-casein assay.
Blocking of β1 integrin function has been demonstrated previously to initiate programmed cell death in mammary epithelial cells under specific conditions (Boudreau et al., 1995; Pullan et al., 1996), and enhanced cell death alone could have caused the observed loss of β-casein expression. However, no obvious signs of cell death were apparent under our culture conditions. This is likely due to the fact that rounded and clustered mammary epithelial cells are more resistant to apoptosis than single cells or cells spread on plastic (Boudreau et al., 1996; Pullan et al., 1996). To be certain that cell death was not enhanced significantly in the cell populations treated with β1- and α6-blocking antibodies, we assayed the relative viability of each treated population using a vital dye. Cell viability was assayed in cultures of prerounded cells under conditions identical to those used for β-casein assays. Cells were exposed to laminin, hormones, and each of the function-blocking antibodies for 4 d, 1 d beyond the usual end point of the β-casein assay, to capture any cell death that might have been initiated when the β-casein was assayed. A slight reduction of cell viability was observed in the population treated with β1-blocking antibodies, but this was no greater than the effects observed for α5- and αv-treated cells, which showed no inhibition of β-casein expression (Figure 3C). Therefore, the inhibition of β-casein expression by β1- and α6-blocking antibodies was not caused by enhanced cell death.
Both β1 Integrin and α6β4 Integrin Functions Are Required to Signal β-Casein Expression
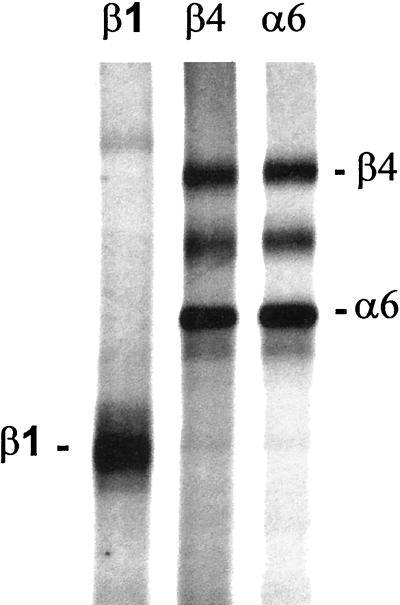
The α6 subunit is a component of two laminin receptors, the α6β1 and α6β4 integrins, both of which are reported to bind the laminin E8 fragment (Hall et al., 1990; Lee et al., 1992). Therefore, the α6-blocking antibody, GoH3, could target either the α6β1 or the α6β4 heterodimer, or both. The β1-blocking antibody, HA2/5, would target all β1 integrin heterodimers (Mendrick and Kelly, 1993). Because both of these antibodies inhibited β-casein expression, one could conclude that the α6β1 integrin is the receptor responsible for the signaled β-casein expression. Alternatively, the two antibodies could perturb β-casein expression by distinct mechanisms, one through blocking the α6β4 integrin and the other through blocking one or more β1 integrins. The second possibility was found to be the case by immunoprecipitation of the α6 integrins from the SCp2 cell line. Immunoprecipitations of the α6 integrins using the GoH3 antibody revealed that the β4 subunit was the exclusive partner of the α6 subunit in the SCp2 cells (Figure 4). The quantity of α6 subunit immunoprecipitated was the same whether the α6 or the β4 antibody was used, and the β1 subunit was undetectable in the α6 subunit precipitations. The absence of the α6β1 heterodimer in cells expressing both the β1 and β4 subunits has been reported by several other laboratories and demonstrates a dominant preference of the α6 subunit for dimerization with the β4 subunit (Lee et al., 1992; Delcommenne and Streuli, 1995; Spinardi et al., 1995; DiPersio et al., 1997; Hodivala-Dilke et al., 1998). In addition, the 9EG7 antibody, reported to alternately block or stimulate the function of a subset of β1 integrins, including the α6β1 integrin (Lenter et al., 1993; Driessens et al., 1995), did not inhibit or stimulate β-casein expression in our assays (our unpublished results). Therefore, the α6β1 integrin is not involved in signaling β-casein expression, but the α6β4 integrin is essential for transmitting signals for β-casein expression in mammary epithelial cells. The inhibition of β-casein expression by the β1-blocking antibody occurs by a different mechanism, either through blocking yet another laminin receptor (e.g., the α3β1 integrin) or through events unrelated to signaling from laminin (e.g., disruption of other β1 integrin functions not related to binding laminin).
Figure 4.
Immunoprecipitation of α6 integrins from the SCp2 cells. The β1, β4, and α6 integrins were immunoprecipitated from [35S]methionine-labeled cell extracts and separated on a 7% SDS-polyacrylamide gel. Bands for both the β4 and α6 subunits are evident in the immunoprecipitations using the β4 and α6 subunit antibodies, demonstrating the presence of the α6β4 heterodimer. The β1 subunit was not detectable in precipitations of the α6 integrins, demonstrating the absence of the α6β1 heterodimer in the SCp2 cells. The exclusive dimerization of α6 with the β4 subunit is further supported by the fact that the yield of the α6 and β4 subunits is equal between the α6 and β4 immunoprecipitations, indicating that most or all of the α6 subunit is dimerized with the β4 subunit in these cells.
A Receptor for the E3 Domain of Laminin Mediates the Cell Shape Changes, Independent of β1 and β4 Integrins
The results described above demonstrated that both the α6β4 integrin and β1 integrins are required for induction of β-casein expression, but neither are required to mediate the cell shape changes induced by laminin. Therefore, the receptor mediating the prerequisite cell shape change appeared not to be among the known integrin laminin receptors. Other receptors that might perform this function include those that bind the laminin E3 domain. Previous studies in our laboratory, with primary cell cultures and CID-9 cells, had identified a role for the E3 domain of laminin in signaling β-casein expression; purified E3 laminin fragment inhibited β-casein expression (Streuli et al., 1995). Because neither the α6β4 integrin nor β1 integrins are thought to bind the laminin E3 domain (with the possible exception of α3β1 [Gehlsen et al., 1992[), the mechanism by which the E3 fragment inhibited β-casein was not clear.
We hypothesized that the E3 laminin fragment may inhibit β-casein expression through inhibition of the receptor(s) mediating changes in cell shape. The E3 and E8 laminin fragments alone, or in combination, did not signal either the cell shape change or β-casein expression in SCp2 cells. However, the ability of cells to round and cluster when exposed to laminin was strongly inhibited by the E3 fragment but not by the E8 fragment or the BSA control (Figure 5A, a–f). Titration of the E3 fragment showed strong inhibition of cell rounding at 100 μg/ml, with diminishing effects at lower concentrations. The concentration of E3 fragment necessary to affect cell shape paralleled the concentrations needed to block β-casein expression in primary cell cultures (Streuli et al., 1995). This indicated that the E3 laminin fragment perturbs a laminin receptor that mediates the cell shape change.
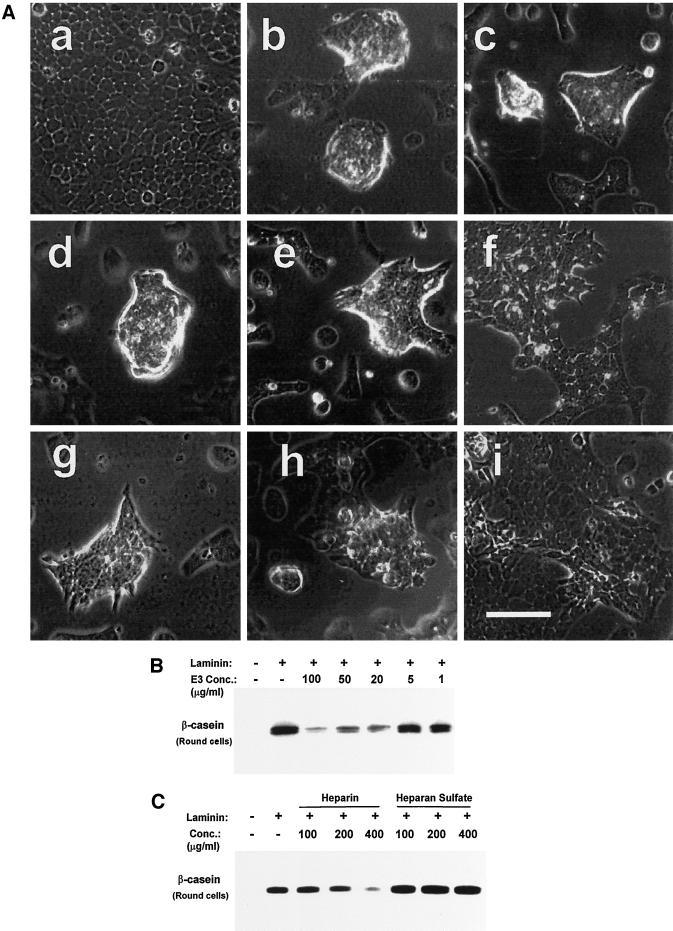
Figure 5.
Morphogenic changes and β-casein expression are blocked by the laminin E3 fragment and by heparin. (A) Assays for the cell shape changes induced by laminin in SCp2 mammary epithelial cells were performed on cells initially spread on plastic and subsequently exposed to medium containing laminin plus or minus the elastase-generated laminin fragments E3 and E8. In the absence of added laminin (a), cells remain attached and spread on the plastic and continue to grow to confluence. Cells exposed to laminin (b) undergo cell rounding, and cells sharing cell-cell contacts cluster into multicellular aggregates. Cells continue to undergo the cell shape changes when exposed to laminin in the presence of BSA at 100 μg/ml (c), the laminin E8 fragment at 100 μg/ml (d), the laminin E3 fragment at 50 μg/ml (e), heparansulfate at 400 μg/ml (g), or heparin at 100 μg/ml (h). However, cells are strongly inhibited from undergoing the cell rounding and clustering when exposed to laminin in the presence of the laminin E3 fragment at 100 μg/ml (f) or heparin at 400 μg/ml (i). (B) The E3 laminin fragment was tested as a competitive inhibitor in assays of β-casein expression in prerounded cells. The E3 fragment continues to inhibit β-casein expression even in assays of prerounded cells, with partial inhibition at concentrations as low as 20 μg/ml. (C) Heparin and heparan sulfate were tested as competitive inhibitors in assays of β-casein expression in prerounded cells. Heparin inhibits β-casein expression in assays of prerounded cells at 400 μg/ml, whereas heparan sulfate has no effect at the same concentration.
The E3 laminin fragment could inhibit β-casein expression solely through effects on cell shape, or it could perturb additional signaling functions required for β-casein expression. To distinguish between these two possibilities, the laminin fragments were tested in assays of β-casein expression in both flat and prerounded cells. Immunoblots for the resulting β-casein expression showed that the E3 fragment inhibited β-casein expression in both flat and rounded SCp2 cells (Figure 5B and data not shown). Therefore, the inhibition of β-casein expression by the E3 fragment occurs through both effects on cell shape and inhibition of other functions that are yet to be determined. The laminin E8 fragment and the BSA control did not inhibit β-casein expression at concentrations up to 100 μg/ml (data not shown).
Receptors reported to bind the laminin E3 domain include syndecan-1 and dystroglycan, which are believed to bind, in part, through carbohydrate interactions with the heparin-binding region of laminin (Ervasti and Campbell, 1993; Salmivirta et al., 1994). Consequently, their interactions with laminin are inhibited by heparin. We tested whether heparin also inhibited signals for the cell shape change and β-casein expression. Heparin strongly inhibited the cell shape change at a concentration of 400 μg/ml (Figure 5Ai). Heparan sulfate and chondroitin sulfates A, B, and C were not effective inhibitors of cell rounding at the same concentration (Figure 5Ag, and data not shown). In assays of β-casein expression, heparin mimicked the activity of the laminin E3 fragment, whereas heparan sulfate did not. Heparin inhibited the induction of β-casein expression in assays of both flat and prerounded cells at the same concentrations that inhibit cell rounding (Figure 5C and data not shown). This indicates that the heparin-binding region within the laminin E3 domain participates in the interaction of laminin with the E3 laminin receptor. Heparan sulfate did not inhibit β-casein expression at concentrations up to 400 μg/ml.
The Requirement of α6β4 Integrin to Signal β-Casein Expression Is Obscured in Primary Cell Cultures because of Paracrine Signaling Leading to Formation of Endogenous Basement Membrane
The results described above, using the clonal mammary epithelial cell line SCp2, differed in part from our previously published results with primary mammary epithelial cell cultures and the CID-9 mammary epithelial cell line. In the previous studies, the GoH3 antibody was found not to inhibit the induction of β-casein expression (Streuli et al., 1991). Therefore, either the SCp2 cell line had acquired a new signaling requirement for β-casein expression or some common aspect of the primary cultures and CID-9 cell line obscured or circumvented the requirement for the α6β4 integrin.
We hypothesized that endogenous basement membrane formation, occurring in primary cultures and the heterogeneous CID-9 cell line, may interfere with the detection of signaling by the α6β4 integrin. It has been established previously that paracrine signaling between the mesenchymal and epithelial compartments results in the deposition of an endogenous basement membrane (Reichmann et al., 1989; Cunha and Hom, 1996). The principal differences between the primary cultures, CID-9, and the SCp2 cell lines are that the latter is clonal and unable to form a functional basement membrane (Desprez et al., 1993; Roskelley et al., 1994). In mixed cultures, preformed α6β4-laminin complexes might resist disruption by the GoH3 antibody.
This hypothesis was tested by two independent means. First, if primary cultures were able to form an endogenous basement membrane, then it would follow that β-casein expression could be induced in primary cultures by simply forcing a rounded cell conformation in the presence of lactogenic hormones but without the addition of exogenous laminin. The induction of β-casein expression was assayed in parallel cultures of primary murine mammary epithelial cells plated either onto tissue culture plastic, where they attached and spread, or in wells coated with polyHEMA, where they remained in suspension and maintained a clustered and rounded conformation. After 2 d, both cultures were treated with medium containing lactogenic hormones without the addition of laminin, and after 3 d of exposure to hormones, the cells were extracted and assayed for β-casein expression. As predicted, primary cells cultured on plastic (flat cells) did not produce significant β-casein; however, the same cells cultured on polyHEMA showed an induction of β-casein expression despite the absence of exogenously added laminin (Figure 6A). In contrast, the clonal SCp2 cell line expressed little or no β-casein, regardless of cell shape, if exogenous laminin was not present (Figures 1B, 3B, and 6A). Second, we tested whether the previous results obtained with primary cultures could be duplicated with the SCp2 cells if we added a mesenchymal component. Mesenchymal cells such as NIH3T3 fibroblasts do not express milk proteins. SCp2 cells were cocultured with NIH3T3 fibroblasts at a 10:1 ratio (epithelial cells:fibroblasts) and tested for β-casein expression in the absence of exogenous laminin. Coculture of the SCp2 cells and NIH3T3 fibroblasts resulted in expression of β-casein when cultured on polyHEMA but not when cultured on plastic, in which case they remained flat (Figure 6A). The resulting cell behavior of the cocultured epithelial cells and fibroblasts was identical to that of primary cell cultures, in which β-casein expression was induced by cell rounding without the addition of exogenous laminin.
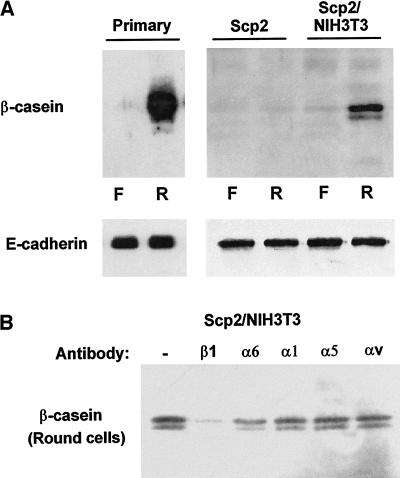
Figure 6.
Assays of β-casein expression, in the absence of added laminin, in primary cell cultures, clonal epithelial cells, and cocultures of clonal epithelial cells and fibroblasts. (A) Primary murine mammary epithelial cell cultures, SCp2 clonal epithelial cells, and cocultures of SCp2 cells and NIH3T3 fibroblasts (10:1) were assayed for β-casein expression in both flat cells (F) and rounded cells (R) (suspension culture) exposed to prolactin in the absence of added laminin. Cell rounding in suspension cultures permitted the induction of β-casein in primary cultures but not in the clonal SCp2 cell line. Coculture of SCp2 cells with a mesenchymal component (NIH3T3 fibroblasts) permitted the induction of β-casein. The same immunoblot filters were also probed for E-cadherin to demonstrate normalization for equal cell number. (B) SCp2 cells and NIH3T3 fibroblasts were cocultured in suspension (prerounded) and exposed to prolactin in the presence of function-blocking antibodies against the β1, α6, α1, α5, and αv integrin subunits, without the addition of laminin. β-Casein expression induced by endogenous basement membrane formation was inhibited by the β1 integrin-blocking antibody but was not inhibited effectively by the α6 integrin-blocking antibody GoH3.
Finally, the inhibition of the β-casein signal by the integrin-blocking antibodies was tested in cocultured SCp2 and NIH3T3 cells. The cocultured cells were exposed to the function-blocking antibodies in suspension without the addition of exogenous laminin. Under these conditions, the signaled β-casein in the coculture experiments was still inhibited by the β1-blocking antibody but was less efficiently inhibited by the GoH3 antibody (Figure 6B), consistent with results previously described for primary cell cultures (Streuli et al., 1991). Therefore, the ability or inability to produce a functional basement membrane appears to be responsible for the different results obtained with the SCp2 cells as opposed to primary mammary epithelial and CID-9 cultures.
DISCUSSION
Division of Labor
The division of labor among laminin receptors has been presumed on the basis of the unique structural properties of the different receptors and on the basis of a limited number of functional studies in vivo and in cell culture. Structurally, the cytoplasmic domains of different laminin receptors are distinct from each other, yet highly conserved, reflecting the selective conservation of unique functions within each different receptor (Sastry and Horwitz, 1993). Receptor knockout experiments, in which the function of a number of the known laminin receptors has been eliminated, lead to different phenotypes, most being lethal at various stages of development (Hynes, 1996; Williamson et al., 1997). Among laminin receptors expressed in epithelial cells, the integrin α3 and α6 subunit knockouts displayed distinct alterations in the cell-basement membrane junctions (DiPersio et al., 1997). In culture, cell binding to either the E3 or E8 domains of laminin had different effects in assays of kidney and salivary gland morphogenesis (Klein et al., 1988; Sorokin et al., 1992; Durbeej et al., 1995; Kadoya et al., 1995). In addition, ligation of different laminin receptors resulted in distinct downstream signaling events, including differences in protein phosphorylation and Shc activation (Kornberg et al., 1991; Jewell et al., 1995; Mainiero et al., 1995; Wary et al., 1996; Xia et al., 1996). Finally, laminin receptors were found to localize to different membrane-cytoskeleton junctions in both muscle and epithelial cells (Bao et al., 1993; Burgeson and Christiano, 1997). Therefore, the different functions of laminin receptors can be tied to their roles as mediators of unique membrane-cytoskeleton interactions in addition to their different signaling properties.
Despite the extensive characterization of different laminin receptors, little is known about their downstream influence on cell function beyond cell adhesion. We demonstrate here the distinct roles of at least two laminin receptors in signals leading to transcription of the milk protein β-casein in mammary epithelial cells. In addition to resolving the different signaling functions of laminin receptors, this work assigns clear downstream consequences of cell behavior to ligation of specific laminin receptors: morphogenic changes are induced by an E3 laminin receptor; and β-casein expression requires signaling by the α6β4 integrin, β1 integrins, and an E3 laminin receptor.
The key reagents used in this study, the α6- and β1-blocking antibodies and the laminin E3 fragment, not only resolved the distinct functions of laminin receptors but also revealed their partial independence. The integrin-blocking antibodies did not perturb the cell shape changes mediated by the E3 laminin receptor. The independence of these receptors suggests that they do not associate at the cell surface to enact their functions but more likely segregate to distinct membrane-cytoskeleton junctions. Indeed, the α6β4 is a hemidesmosome component known to interact with the intermediate filament cytoskeleton, whereas all receptors so far reported to bind the E3 domain of laminin are thought to interact with the actin cytoskeleton (Gehlsen et al., 1992; Henry and Campbell, 1996; Carey, 1997). Although these receptors function independently for the cell shape change, the integrins and E3 laminin receptor are codependent for signaling β-casein expression. Because the α6β4 integrin has been reported to bind the laminin E8 domain, it was surprising that the E8 fragment failed to inhibit β-casein expression. One possible explanation is that the purified E8 fragment does not compete efficiently with intact laminin, but other interpretations may have to be considered. It should be noted also that the E8 fragment has a molecular mass four times greater than that of the E3 fragment. Therefore, much higher E8 protein concentrations may be required for inhibition to be observed in these assays.
Cell Shape
The mechanism by which cell shape participates in the β-casein signaling pathway is unknown. Although shape dependence of signaling pathways has been demonstrated for many functions, the underlying molecular mechanisms are just beginning to be revealed (e.g., see Kheradmand et al., 1998). The four known signaling pathways for β-casein expression emanate from the α6β4 integrin, β1 integrins, an E3 laminin receptor, and the prolactin receptor. Whether only one of these pathways is cell shape dependent, or whether all require a particular cell structure before they can signal, remains to be determined. Shape dependence implies a requirement for a particular cytoskeletal organization. Both the α6β4 integrin and β1 integrins are associated with the cytoskeleton, and this may be true also for the E3 laminin receptor. Therefore, signaling through one or all of these receptors may be altered by the organization of the cytoskeleton.
α6β4 Integrin Function
What is the role of α6β4 in signaling β-casein expression? Although biochemical signals have been shown to emanate from the α6β4 integrin (Giancotti, 1996; Mainiero et al., 1997), this receptor is also a mediator of epithelial architecture. Ligation of the α6β4 integrin to laminin is considered to be the nucleating event in hemidesmosome formation (Giancotti, 1996), which in turn organizes components of the intermediate filament cytoskeleton. Therefore, the induction of β-casein expression by α6β4 ligation may operate, at least in part, through effects on cell architecture that in turn permit other pathways to function (e.g., those responding to lactogenic hormones). Previous results from our laboratory have shown that a program of normal epithelial morphogenesis in cultured human breast cells can be perturbed by blocking α6β4 integrin function, leading to disorganized and uncontrolled cell growth (Weaver et al., 1997). The question of whether cell polarity per se is a requirement for β-casein expression has been addressed previously, and it was determined not to be essential because single cells (by definition apolar) embedded in a laminin-rich ECM produced β-casein (Streuli et al., 1991). However, a much higher proportion of cells expressed β-casein when allowed to form multicellular aggregates. Furthermore, the time course for detection of signals for β-casein expression is uncharacteristically slow for simple biochemical signaling, requiring a minimum of 8 h for detection, even in prerounded cells (Roskelley et al., 1994). Therefore, we propose that structural reorganization of the cell is one essential component of β-casein signaling, whether it is mediated by the α6β4 integrin, β1 integrins, the E3 laminin receptor, or all three.
E3 Laminin Receptor Function
In addition to assigning a function to signaling from the α6β4 integrin, we now have revealed a clear consequence of cell interaction with the laminin E3 domain on cell morphology and function. The mechanism by which cell binding to the E3 domain induces cell rounding is unknown. As described for α6β4, the E3 laminin receptor could mediate its function through biochemical signaling or through direct effects on cytoskeletal organization, or both. This rounding function is insensitive to the tyrosine kinase inhibitor genistein (Roskelley et al., 1994), so tyrosine phosphorylation events may not be required; however, the activity is inhibited by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate which affects the cytoskeleton. The fact that the E3 domain alone could not induce the rounding response, but was instead inhibitory, indicates that simple ligand binding is insufficient for this signaling event to occur and that a higher molecular organization of laminin is required.
The E3 laminin receptor responsible for inducing the cell shape change remains to be identified. However, dystroglycan is a strong candidate (Henry and Campbell, 1996). Dystrolgycan is expressed in the SCp2 cells as well as in mammary epithelial cells in vivo (Durbeej et al., 1998; our unpublished results). Dystroglycan is reported to bind the laminin E3 domain, and this binding is inhibited by heparin, but less effectively by heparan sulfate, and not at all by chondroitin sulfates (Pall et al., 1996). Moreover, the high concentration of heparin required to inhibit cell rounding in our assays (200–400 μg/ml) corresponds to the concentration of heparin required to inhibit laminin binding to muscle α-dystroglycan, which inhibits at a 50% inhibitory concentration of 250 μg/ml (Pall et al., 1996). Dystroglycan was shown recently to mediate the assembly of laminin at the cell surface (Henry and Campbell, 1998). Based on these results, it has been suggested that dystroglycan might act as a coreceptor for laminin and may thereby influence the function of other laminin receptors at the cell surface. Interpreting our results through this model, one can propose that basement membrane assembly by dystroglycan is required for correct signaling through the α6β4 or β1 integrins. This model offers an attractive explanation for why at least two laminin receptors are required to signal β-casein expression and why the laminin E3 domain function is required continuously. On the other hand, it is still possible that these receptors each contribute essential but entirely independent functions.
Other candidate E3 receptors include syndecan-1, whose binding to the G domain of laminin has been implicated in acinar formation in epithelial cells of the salivary gland (Hoffman et al., 1998). Syndecan-1 is expressed in SCp2 cells (our unpublished results), but it is unknown whether the laminin-binding isoform (Salmivirta et al., 1994) is present. Unlike dystroglycan, syndecan-1 binding to laminin-1 is not differentially inhibited by heparin, heparan sulfate, and chondroitin sulfate, although these interactions were not assayed in mammary epithelial cells (Salmivirta et al., 1994; Hoffman et al., 1998). In addition, the AG73 peptide, reported to compete with laminin for syndecan-1 binding (Hoffman et al., 1998), did not induce or perturb significantly the cell shape change in our assays (our unpublished results). Aside from dystroglycan and syndecan-1, many cell surface proteoglycans have the potential to bind laminin through heparin-binding domains such as the E3 domain of laminin. It is possible that multiple cell surface molecules can perform this function; however, all redundancy must exist among E3 laminin receptors because the E3 fragment alone was able to inhibit cell rounding. Other cell surface molecules may also be required to effect the cell shape change, in cooperation with the E3 receptor, but so far we have found that only an E3 laminin receptor is essential.
β1 Integrin Function
The mechanism of inhibition of β-casein expression by the β1-blocking antibody remains to be deciphered. Inhibition might occur through the blocking of yet another required laminin receptor. The α3β1 integrin is a logical candidate because it functions in epithelial interactions with laminin and is expressed in the SCp2 cells in culture (our unpublished results). Function-perturbing antibodies for the integrin α3 subunit, however, are still not available in the mouse system, but once available they will allow a resolution of this question. Alternatively, it is possible that the inhibition of β-casein expression could result from the blocking of other β1 integrins, independent of effects on any laminin receptor. The β1-blocking antibody might induce some form of trans-dominant inhibition of the α6β4 integrin, E3 laminin receptor, or other molecules, as has been described previously for some integrins (Diaz-Gonzalez et al., 1996; Hodivala-Dilke et al., 1998). So far, we know that blocking of α1, α5, and αv integrins had no observable effect on β-casein expression.
Finally, an absolute requirement for β1 integrin, α6β4 integrin, or E3 laminin receptor signaling in lactation remains to be demonstrated in vivo. Knockouts of the α6, β4, and β1 subunits have proven lethal at the neonatal and early embryonic stages (Fassler and Meyer, 1995; Stephens et al., 1995; Dowling et al., 1996; Georges-Labouesse et al., 1996; van der Neut et al., 1996), long before lactation could be assessed. One recent study, however, demonstrated that perturbation of β1 integrin function, in transgenic mice expressing a chimeric β1 integrin/CD4 molecule, led to decreased expression of milk proteins, including β-casein (Faraldo et al., 1998).
The Interference of Endogenous Basement Membrane Deposition
The current study was made possible by the use of a clonal epithelial cell line instead of mixed cultures containing both epithelial and mesenchymal cell types. Earlier studies from our laboratory had concluded that the E3 domain of laminin alone may be the only domain of laminin required for β-casein expression and that the GoH3 antibody was not inhibitory (Streuli et al., 1991, 1995). However, these studies used either primary cell cultures or the CID-9 cell line, both of which contain some mesenchymal components. Paracrine signaling between mesenchymal and mammary epithelial cells results in the deposition of an endogenous basement membrane, which, in turn, can induce β-casein expression in the presence of lactogenic hormones (Reichmann et al., 1989). In the present study, we concluded that the presence of an endogenous basement membrane in primary and CID-9 cultures had obscured the two-step signaling requirement; mechanical cell rounding was sufficient to induce β-casein expression in both primary and “mixed” (SCp2/NIH3T3) cultures without the addition of exogenous laminin. We propose that the GoH3 antibody was less effective at inhibiting β-casein expression in experiments in which mixed cell types were present because it does not efficiently disrupt the preformed complexes of α6β4 integrins bound to endogenous laminin deposits. These results demonstrate the usefulness of homogeneous, but functional, epithelial cell lines for studies of extracellular matrix signaling from laminin. They also underscore the importance of defining the contribution of endogenously deposited ECM molecules when cultured cells are used for functional studies.
ACKNOWLEDGMENTS
The authors thank Dinah Levy for technical assistance and Marina Simian for assistance with primary cell cultures. We also thank Todd Mathis and Holly Colognato for assistance with laminin fragment preparation. We are grateful to Drs. Valerie Weaver, Michael Henry, and Zena Werb for helpful discussion. This work was sponsored by National Institutes of Health grant NIH-CA57621 and Department of Energy grant DE-AC03-76-SF00098. J.M. was supported by a National Institutes of Health Postdoctoral Fellowship and by a Department of Defense Breast Cancer Research Fellowship.
REFERENCES
- Bao ZZ, Lakonishok M, Kaufman S, Horwitz AF. Alpha 7 beta 1 integrin is a component of the myotendinous junction on skeletal muscle. J Cell Sci. 1993;106:579–589. doi: 10.1242/jcs.106.2.579. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–658. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- Carey DJ. Syndecans: multifunctional cell-surface coreceptors. Biochem J. 1997;327:1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997a;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen L, Shick V, Matter ML, Laurie SM, Ogle RC, Laurie GW. Laminin E8 alveolarization site: heparin sensitivity, cell surface receptors, and role in cell spreading. Am J Physiol. 1997b;272:494–503. doi: 10.1152/ajplung.1997.272.3.L494. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hom YK. Role of mesenchymal-epithelial interactions in mammary gland development. J Mammary Gland Biol Neoplasia. 1996;1:21–35. doi: 10.1007/BF02096300. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Streuli CH. Control of integrin expression by extracellular matrix. J Biol Chem. 1995;270:26794–26801. doi: 10.1074/jbc.270.45.26794. [DOI] [PubMed] [Google Scholar]
- Desprez P-Y, Roskelley C, Campisi J, Bissell M. Isolation of functional cell lines from a mouse mammary epithelial cell strain: the importance of basement and cell-cell interaction. Mol Cell Diff. 1993;1:99–110. [Google Scholar]
- Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg MH. Trans-dominant inhibition of integrin function. Mol Biol Cell. 1996;7:1939–1951. doi: 10.1091/mbc.7.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala DK, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessens MH, Van Rijthoven EA, Kemperman H, Roos E. Adhesion of lymphoma cells to fibronectin: differential use of alpha 4 beta 1 and alpha 5 beta 1 integrins and stimulation by the 9EG7 mAb against the murine beta 1 integrin subunit. Cell Adhes Commun. 1995;3:327–336. doi: 10.3109/15419069509081017. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46:449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Larsson E, Ibraghimov BO, Roberds SL, Campbell KP, Ekblom P. Nonmuscle alpha-dystroglycan is involved in epithelial development. J Cell Biol. 1995;130:79–91. doi: 10.1083/jcb.130.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of beta1-integrin function alters the development of murine mammary gland. EMBO J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Gehlsen KR, Sriramarao P, Furcht LT, Skubitz AP. A synthetic peptide derived from the carboxy terminus of the laminin A chain represents a binding site for the alpha 3 beta 1 integrin. J Cell Biol. 1992;117:449–459. doi: 10.1083/jcb.117.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Signal transduction by the alpha 6 beta 4 integrin: charting the path between laminin binding and nuclear events. J Cell Sci. 1996;109:1165–1172. doi: 10.1242/jcs.109.6.1165. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol. 1996;8:625–631. doi: 10.1016/s0955-0674(96)80103-7. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- Hinek A. Biological roles of the nonintegrin elastin/laminin receptor. Biol Chem. 1996;377:471–480. [PubMed] [Google Scholar]
- Hodivala-Dilke KM, Michael DiPersio C, Kreidberg JA, Hynes RO. Novel roles for alpha3beta1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol. 1998;142:1357–1369. doi: 10.1083/jcb.142.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MP, Nomizu M, Roque E, Lee S, Jung DW, Yamada Y, Kleinman HK. Laminin-1 and laminin-2 G-domain synthetic peptides bind syndecan-1 and are involved in acinar formation of a human submandibular gland cell line. J Biol Chem. 1998;273:28633–28641. doi: 10.1074/jbc.273.44.28633. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Targeted mutations in cell adhesion genes: what have we learned from them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- Jewell K, Kapron-Bras C, Jeevaratnam P, Dedhar S. Stimulation of tyrosine phosphorylation of distinct proteins in response to antibody-mediated ligation and clustering of alpha 3 and alpha 6 integrins. J Cell Sci. 1995;108:1165–1174. doi: 10.1242/jcs.108.3.1165. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995;129:521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel CS, Ray DB. Immunochemical characterization with monoclonal antibodies of three major caseins and alpha-lactalbumin from rat milk. J Dairy Sci. 1984;67:64–75. doi: 10.3168/jds.S0022-0302(84)81267-9. [DOI] [PubMed] [Google Scholar]
- Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci USA. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci USA. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CQ, Dempsey PJ, Coffey RJ, Bissell MJ. Extracellular matrix regulates whey acidic protein gene expression by suppression of TGF-alpha in mouse mammary epithelial cells: studies in culture and in transgenic mice. J Cell Biol. 1995;129:1115–1126. doi: 10.1083/jcb.129.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Murgia C, Wary KK, Curatola AM, Pepe A, Blumemberg M, Westwick JK, Der CJ, Giancotti FG. The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the alpha 6 beta 4 integrin: distinct beta 4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrick DL, Kelly DM. Temporal expression of VLA-2 and modulation of its ligand specificity by rat glomerular epithelial cells in vitro. Lab Invest. 1993;69:690–702. [PubMed] [Google Scholar]
- Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- Pall EA, Bolton KM, Ervasti JM. Differential heparin inhibition of skeletal muscle alpha-dystroglycan binding to laminins. J Biol Chem. 1996;271:3817–3821. doi: 10.1074/jbc.271.7.3817. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov JL, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996;109:631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- Reichmann E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1989;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Bissell MJ. Dynamic reciprocity revisited: a continuous, bidirectional flow of information between cells and the extracellular matrix regulates mammary epithelial cell function. Biochem Cell Biol. 1995;73:391–397. doi: 10.1139/o95-046. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signaling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmivirta M, Mali M, Heino J, Hermonen J, Jalkanen M. A novel laminin-binding form of syndecan-1 (cell surface proteoglycan) produced by syndecan-1 cDNA-transfected NIH-3T3 cells. Exp Cell Res. 1994;215:180–188. doi: 10.1006/excr.1994.1330. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol. 1993;5:819–831. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- Schittny JC, Yurchenco PD. Terminal short arm domains of basement membrane laminin are critical for its self-assembly. J Cell Biol. 1990;110:825–832. doi: 10.1083/jcb.110.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Extracellular matrix and hormones transcriptionally regulate bovine beta-casein 5′ sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci USA. 1990;87:9118–9122. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin LM, Conzelmann S, Ekblom P, Battaglia C, Aumailley M, Timpl R. Monoclonal antibodies against laminin A chain fragment E3 and their effects on binding to cells and proteoglycan and on kidney development. Exp Cell Res. 1992;201:137–144. doi: 10.1016/0014-4827(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. A recombinant tail-less integrin beta 4 subunit disrupts hemidesmosomes, but does not suppress alpha 6 beta 4-mediated cell adhesion to laminins. J Cell Biol. 1995;129:473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung U, O’Rear JJ, Yurchenco PD. Cell and heparin binding in the distal long arm of laminin: identification of active and cryptic sites with recombinant and hybrid glycoprotein. J Cell Biol. 1993;123:1255–1268. doi: 10.1083/jcb.123.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov BO, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- Xia Y, Gil SG, Carter WG. Anchorage mediated by integrin alpha6beta4 to laminin 5 (epiligrin) regulates tyrosine phosphorylation of a membrane-associated 80-kDa protein. J Cell Biol. 1996;132:727–740. doi: 10.1083/jcb.132.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]