Abstract
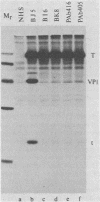
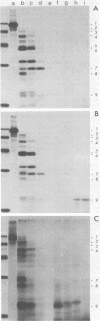
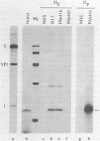
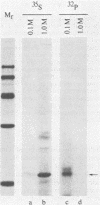
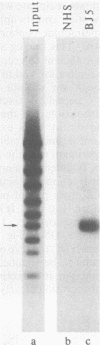
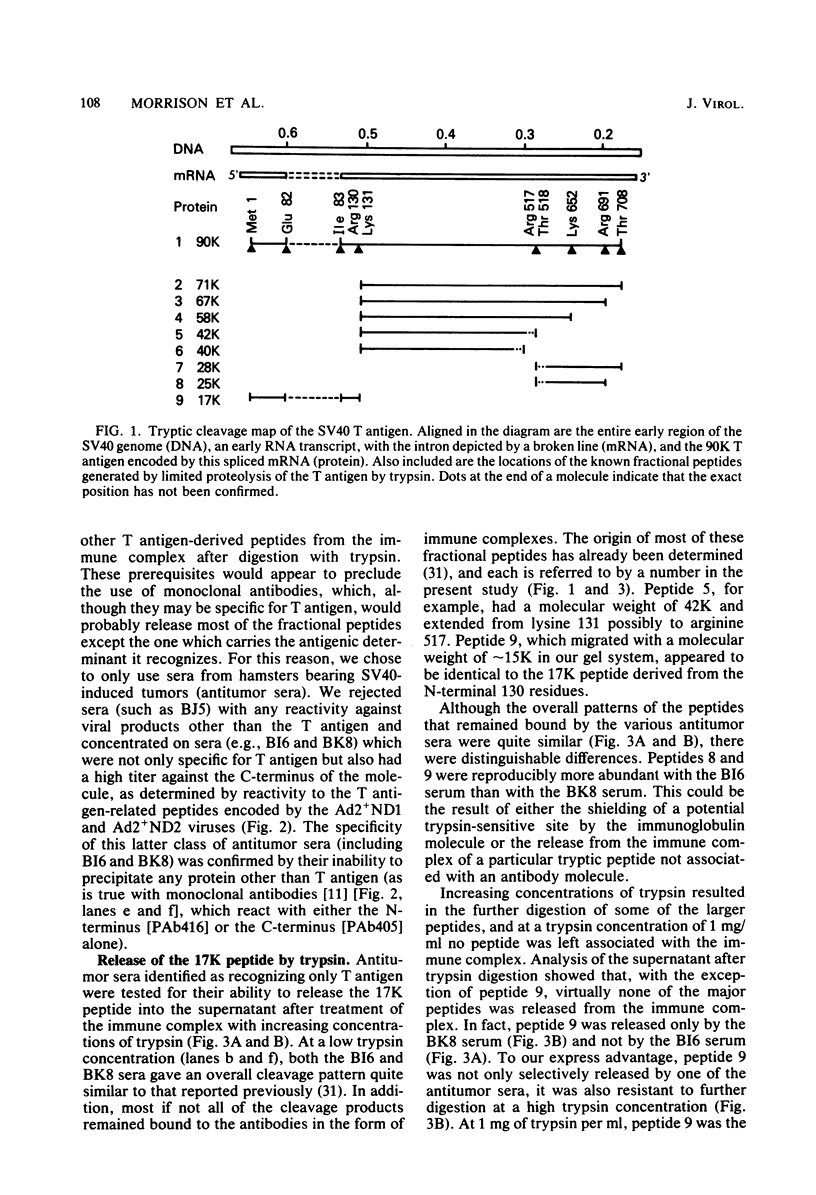
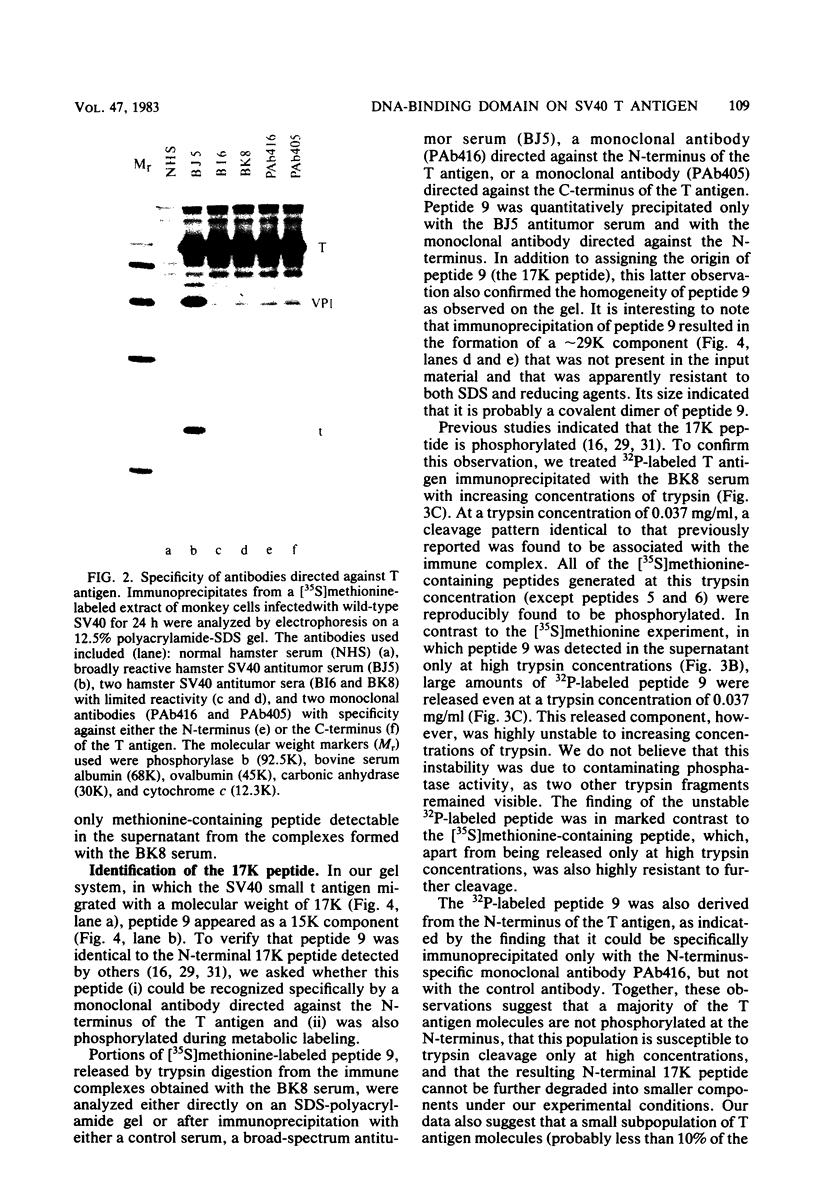
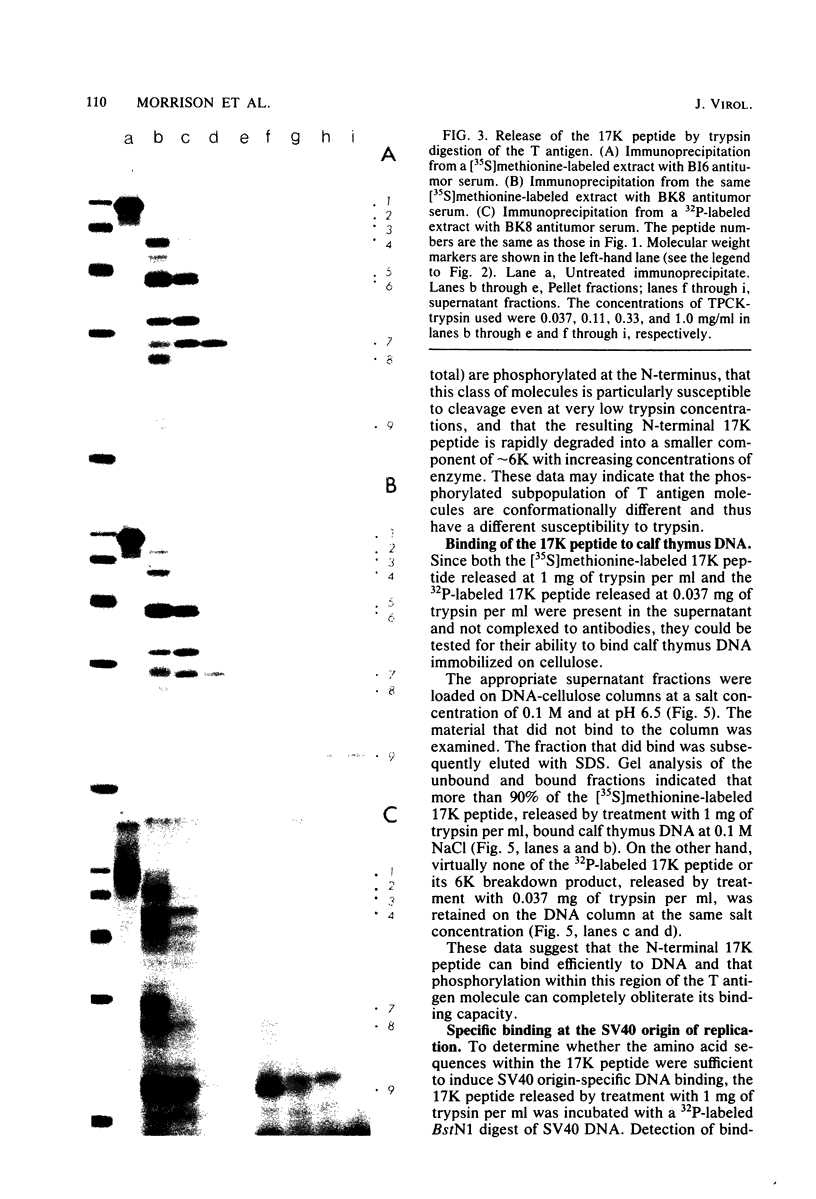
To localize the origin-specific DNA-binding domain on the simian virus 40 tumor (T) antigen molecule, we used limited proteolysis with trypsin to generate fractional peptides for analysis. A 17,000-Mr peptide was found to be capable of binding not only to calf thymus DNA, but also specifically to the simian virus 40 origin of DNA replication. This approximately 130-amino-acid peptide was derived from the extreme N-terminus of the T antigen and represented less than one-fifth of the entire molecule. The coding sequence for this tryptic peptide was located approximately between 0.51 and 0.67 map units (excluding the intron, which maps between 0.54 and 0.59). Since the first 82 amino acids are shared between large T and small t antigens, and since the latter does not bind DNA, it can be concluded that the sequence between isoleucine 83 and approximately arginine 130 is necessary for origin-specific binding by the T antigen. We also observed that in vivo phosphorylation of the T antigen within this region completely abolished the ability of the 17,000-Mr peptide to bind DNA. This observation is consistent with the idea that DNA binding by the T antigen is regulated by posttranslational modifications.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Chang C., Martin R. G., Livingston D. M., Luborsky S. W., Hu C. P., Mora P. T. Relationship between T-antigen and tumor-specific transplantation antigen in simian virus 40-transformed cells. J Virol. 1979 Jan;29(1):69–75. doi: 10.1128/jvi.29.1.69-75.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry F., Harvey R., Smith A. E. Structure and biochemical functions of four simian virus 40 truncated large-T antigens. J Virol. 1982 Oct;44(1):54–66. doi: 10.1128/jvi.44.1.54-66.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Crawford L. V., Berg P. Simian virus 40 mutants with deletions at the 3' end of the early region are defective in adenovirus helper function. J Virol. 1979 Jun;30(3):683–691. doi: 10.1128/jvi.30.3.683-691.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S. E., Botchan M. R. Isolation and characterization of human DNA fragments with nucleotide sequence homologies with the simian virus 40 regulatory region. Mol Cell Biol. 1982 Aug;2(8):949–965. doi: 10.1128/mcb.2.8.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Burger C., Gurney E. G. Comparison of T antigen-associated host phosphoproteins from SV40-infected and -transformed cells of different species. J Gen Virol. 1981 Aug;55(Pt 2):367–378. doi: 10.1099/0022-1317-55-2-367. [DOI] [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A poly(dT)-stimulated ATPase activity associated with simian virus 40 large T antigen. J Biol Chem. 1979 Sep 10;254(17):8113–8116. [PubMed] [Google Scholar]
- Gidoni D., Scheller A., Barnet B., Hantzopoulos P., Oren M., Prives C. Different forms of simian virus 40 large tumor antigen varying in their affinities for DNA. J Virol. 1982 May;42(2):456–466. doi: 10.1128/jvi.42.2.456-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Ahrens B. SV40 early mutants that are defective for viral DNA synthesis but competent for transformation of cultured rat and simian cells. Virology. 1982 Nov;123(1):78–92. doi: 10.1016/0042-6822(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Complex of simian virus 40 large tumor antigen and 48,000-dalton host tumor antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):105–109. doi: 10.1073/pnas.78.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Jay F. T., Chang C., Friedman R. M., Levine A. S. Tumor-specific transplantation antigen: use of the Ad2+ND1 hybrid virus to identify the protein responsible for simian virus 40 tumor rejection and its genetic origin. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3055–3059. doi: 10.1073/pnas.75.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Khoury G., DeLeo A. B., Dippold W. G., Old L. J. p53 transformation-related protein: detection of an associated phosphotransferase activity. Proc Natl Acad Sci U S A. 1981 May;78(5):2932–2936. doi: 10.1073/pnas.78.5.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Jay G., Shiu R. P., Jay F. T., Levine A. S., Pastan I. Identification of a transformation-specific protein induced by a Rous sarcoma virus. Cell. 1978 Mar;13(3):527–534. doi: 10.1016/0092-8674(78)90326-4. [DOI] [PubMed] [Google Scholar]
- Kress M., Resche-Rigon M., Feunteun J. Phosphorylation pattern of large T antigens in mouse cells infected by simian virus 40 wild type or deletion mutants. J Virol. 1982 Sep;43(3):761–771. doi: 10.1128/jvi.43.3.761-771.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- May E., May P., Weil R. Analysis of the events leading to SV40-induced chromosome replication and mitosis in primary mouse kidney cell cultures. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1208–1211. doi: 10.1073/pnas.68.6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Singer M. F. DNA sequences similar to those around the simian virus 40 origin of replication are present in the monkey genome. Proc Natl Acad Sci U S A. 1981 Jan;78(1):95–99. doi: 10.1073/pnas.78.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. D. Binding of a simian virus 40 T antigen-related protein to DNA. J Mol Biol. 1981 Jan 25;145(3):471–488. doi: 10.1016/0022-2836(81)90540-4. [DOI] [PubMed] [Google Scholar]
- Montenarh M., Henning R. Simian virus 40 T-antigen phosphorylation is variable. FEBS Lett. 1980 May 19;114(1):107–110. doi: 10.1016/0014-5793(80)80870-2. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Williams R. C., Tjian R. Oligomeric structure of a simian virus 40 T antigen in free form and bound to DNA. J Mol Biol. 1981 Jun 5;148(4):347–353. doi: 10.1016/0022-2836(81)90180-7. [DOI] [PubMed] [Google Scholar]
- Prives C., Barnet B., Scheller A., Khoury G., Jay G. Discrete regions of simian virus 40 large T antigen are required for nonspecific and viral origin-specific DNA binding. J Virol. 1982 Jul;43(1):73–82. doi: 10.1128/jvi.43.1.73-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Beck Y., Shure H. DNA binding properties of simian virus 40 T-antigens synthesized in vivo and in vitro. J Virol. 1980 Feb;33(2):689–696. doi: 10.1128/jvi.33.2.689-696.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller A., Covey L., Barnet B., Prives C. A small subclass of SV40 T antigen binds to the viral origin of replication. Cell. 1982 Jun;29(2):375–383. doi: 10.1016/0092-8674(82)90154-4. [DOI] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Shortle D. R., Margolskee R. F., Nathans D. Mutational analysis of the simian virus 40 replicon: pseudorevertants of mutants with a defective replication origin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6128–6131. doi: 10.1073/pnas.76.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Robbins A. Enzymatic activities associated with a purified simian virus 40 T antigen-related protein. Proc Natl Acad Sci U S A. 1979 Feb;76(2):610–614. doi: 10.1073/pnas.76.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. T antigen binding and the control of SV40 gene expression. Cell. 1981 Oct;26(1 Pt 1):1–2. doi: 10.1016/0092-8674(81)90026-x. [DOI] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]