Abstract
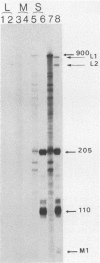
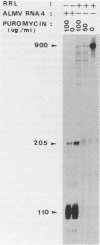
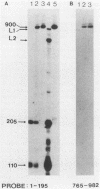
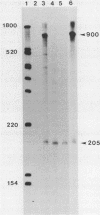
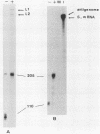
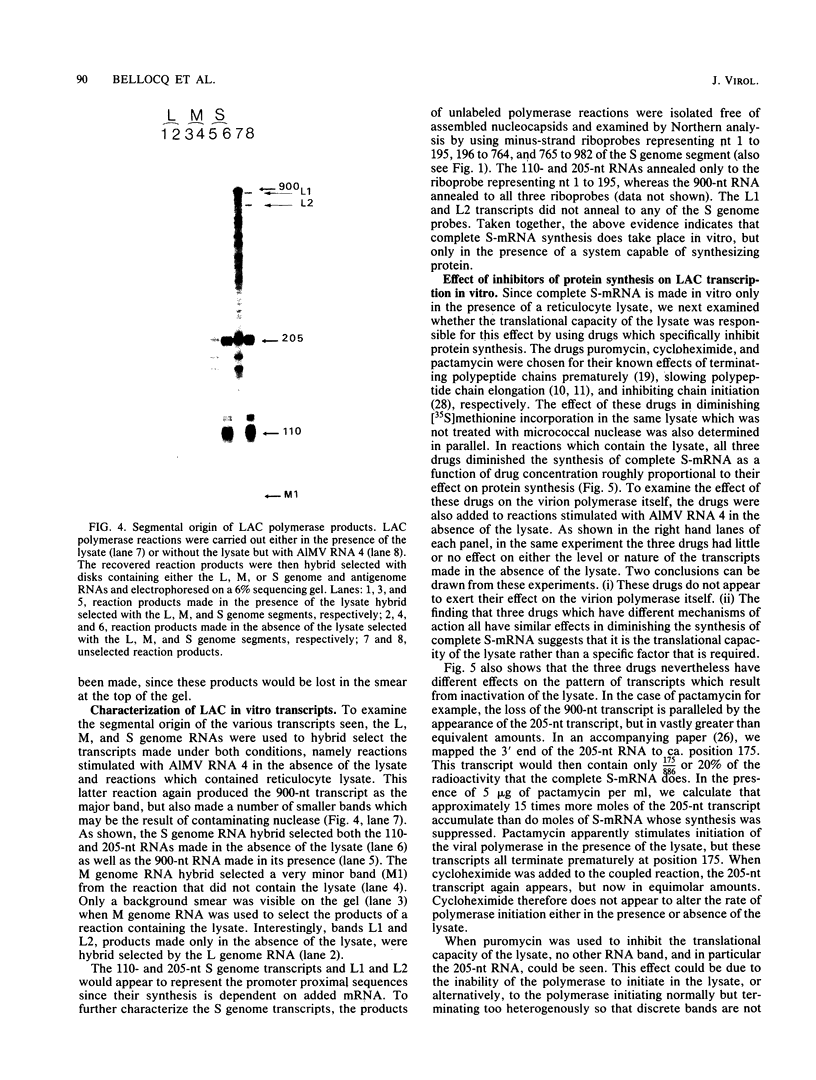
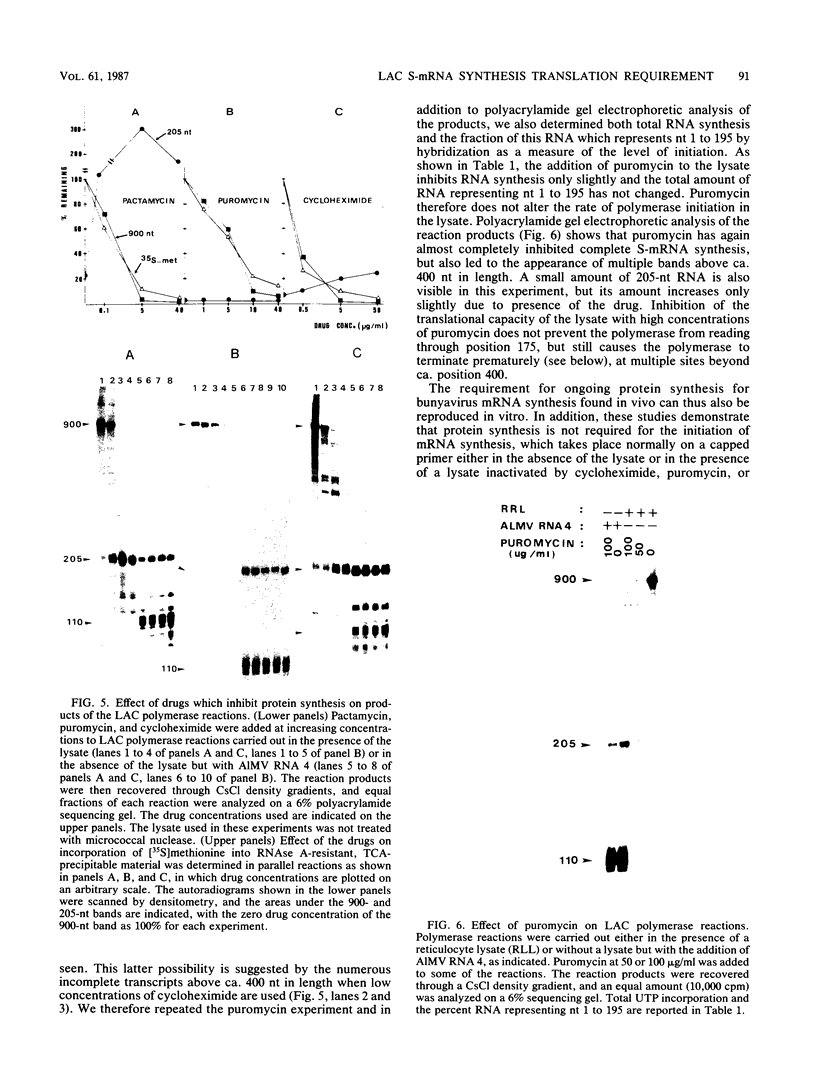
The exceptional requirement of La Crosse virus mRNA synthesis for ongoing protein synthesis in vivo was examined in vitro by using purified virions and a reticulocyte lysate. Transcription from the S genome produced two incomplete transcripts (110 and 205 nucleotides [nt]) in the absence of the lysate, whereas S-mRNA (900 nt) was predominantly made when the lysate was present. The addition of drugs which inhibit protein synthesis also inhibited the synthesis of S-mRNA, and in some cases led to the reappearance of the 205-nt RNA. Reconstruction experiments demonstrated that the incomplete transcripts were not the result of rapid and selective degradation of S-mRNA but were due to premature termination of the polymerase at defined sites. The requirement for ongoing protein synthesis for productive transcription in vitro is not at the level of chain initiation but for elongation of the nascent RNA beyond these sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Pattnaik A. K. Early RNA synthesis in Bunyamwera virus-infected cells. J Gen Virol. 1983 Jun;64(Pt 6):1277–1290. doi: 10.1099/0022-1317-64-6-1277. [DOI] [PubMed] [Google Scholar]
- Akashi H., Bishop D. H. Comparison of the sequences and coding of La Crosse and snowshoe hare bunyavirus S RNA species. J Virol. 1983 Mar;45(3):1155–1158. doi: 10.1128/jvi.45.3.1155-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheiter H., Davis N. L., Wertz G., Schubert M., Lazzarini R. A. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell. 1985 May;41(1):259–267. doi: 10.1016/0092-8674(85)90079-0. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Gay M. E., Matsuoko Y. Nonviral heterogeneous sequences are present at the 5' ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983 Sep 24;11(18):6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Giorgi C., Roux L., Raju R., Dowling P., Chollet A., Kolakofsky D. Sequence determination of the Sendai virus HN gene and its comparison to the influenza virus glycoproteins. Cell. 1985 May;41(1):269–278. doi: 10.1016/0092-8674(85)90080-7. [DOI] [PubMed] [Google Scholar]
- Curran J. A., Quinn J. P., Hoey E. M., Martin S. J., Rima B. K. cDNA cloning of the nucleocapsid and nucleocapsid-associated protein genes of mumps virus. J Gen Virol. 1985 May;66(Pt 5):977–985. doi: 10.1099/0022-1317-66-5-977. [DOI] [PubMed] [Google Scholar]
- Gehrke L., Auron P. E., Quigley G. J., Rich A., Sonenberg N. 5'-Conformation of capped alfalfa mosaic virus ribonucleic acid 4 may reflect its independence of the cap structure or of cap-binding protein for efficient translation. Biochemistry. 1983 Oct 25;22(22):5157–5164. doi: 10.1021/bi00291a015. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Adamson S. D., Herbert E. Effects of cycloheximide on polyribosome function in reticulocytes. J Mol Biol. 1967 Jul 14;27(1):57–72. doi: 10.1016/0022-2836(67)90351-8. [DOI] [PubMed] [Google Scholar]
- Jaye M. C., Godchaux W., 3rd, Lucas-Lenard J. Further studies on the inhibition of cellular protein synthesis by vesicular stomatitis virus. Virology. 1982 Jan 15;116(1):148–162. doi: 10.1016/0042-6822(82)90410-x. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–149. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Palmer E. L., Murphy F. A. Segmented genome and nucleocapsid of La Crosse virus. J Virol. 1976 Dec;20(3):664–675. doi: 10.1128/jvi.20.3.664-675.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Murphy F. A. Bunyaviridae: recent biochemical developments. J Gen Virol. 1977 Oct;37(1):1–14. doi: 10.1099/0022-1317-37-1-1. [DOI] [PubMed] [Google Scholar]
- Patterson J. L., Holloway B., Kolakofsky D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J Virol. 1984 Oct;52(1):215–222. doi: 10.1128/jvi.52.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Kolakofsky D. Characterization of La Crosse virus small-genome transcripts. J Virol. 1984 Mar;49(3):680–685. doi: 10.1128/jvi.49.3.680-685.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Abraham G. Identification of four complementary RNA species in Akabane virus-infected cells. J Virol. 1983 Sep;47(3):452–462. doi: 10.1128/jvi.47.3.452-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pestka S. Peptidyl-puromycin synthesis on polyribosomes from Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):624–628. doi: 10.1073/pnas.69.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Klessig D. F. Normal translation of human adenovirus mRNA in cell-free lysates prepared from abortively as well as productively infected monkey cells. J Virol. 1982 Oct;44(1):426–430. doi: 10.1128/jvi.44.1.426-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Inhibitors of protein synthesis inhibit both La Crosse virus S-mRNA and S genome syntheses in vivo. Virus Res. 1986 Jul;5(1):1–9. doi: 10.1016/0168-1702(86)90061-4. [DOI] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Translational requirement of La Crosse virus S-mRNA synthesis: in vivo studies. J Virol. 1987 Jan;61(1):96–103. doi: 10.1128/jvi.61.1.96-103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier C., Patterson J., Kolakofsky D. La Crosse virus small genome mRNA is made in the cytoplasm. J Virol. 1986 May;58(2):647–650. doi: 10.1128/jvi.58.2.647-650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Determination of the gene sequence of poliovirus with pactamycin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2852–2856. doi: 10.1073/pnas.68.11.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]