Abstract
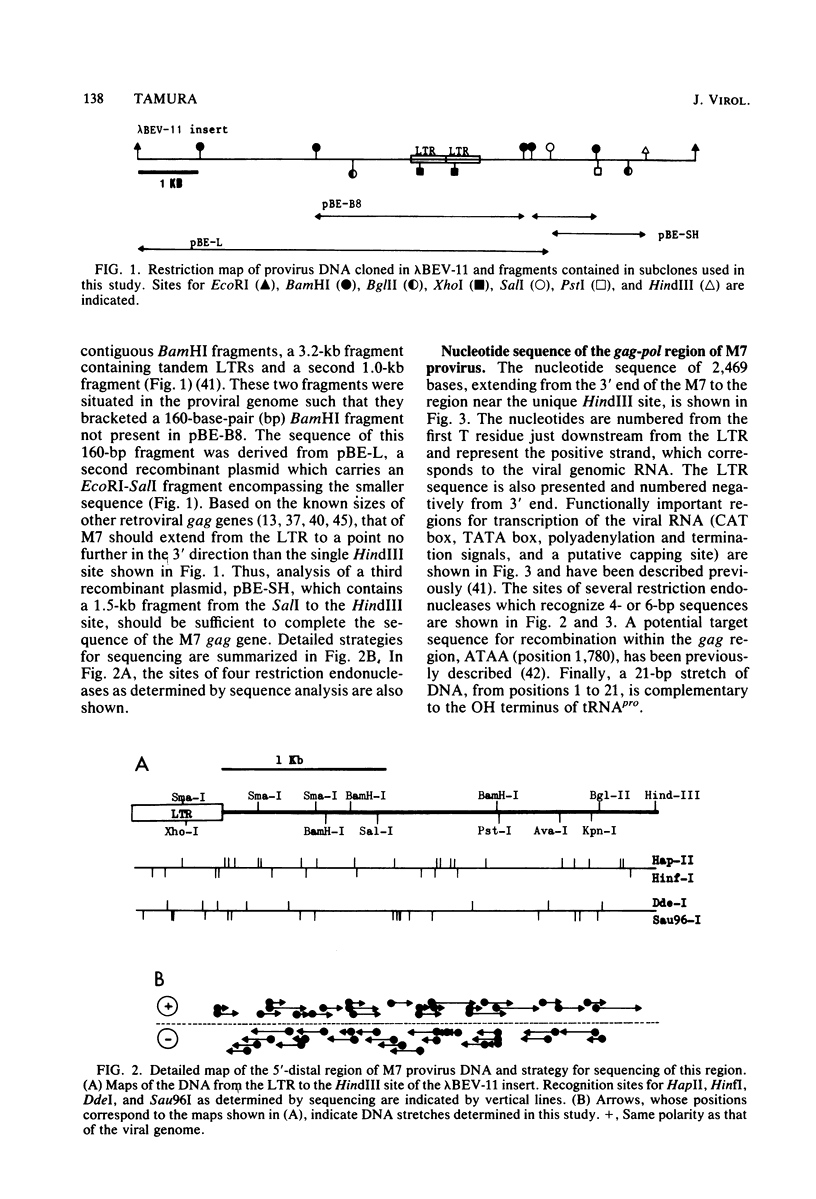
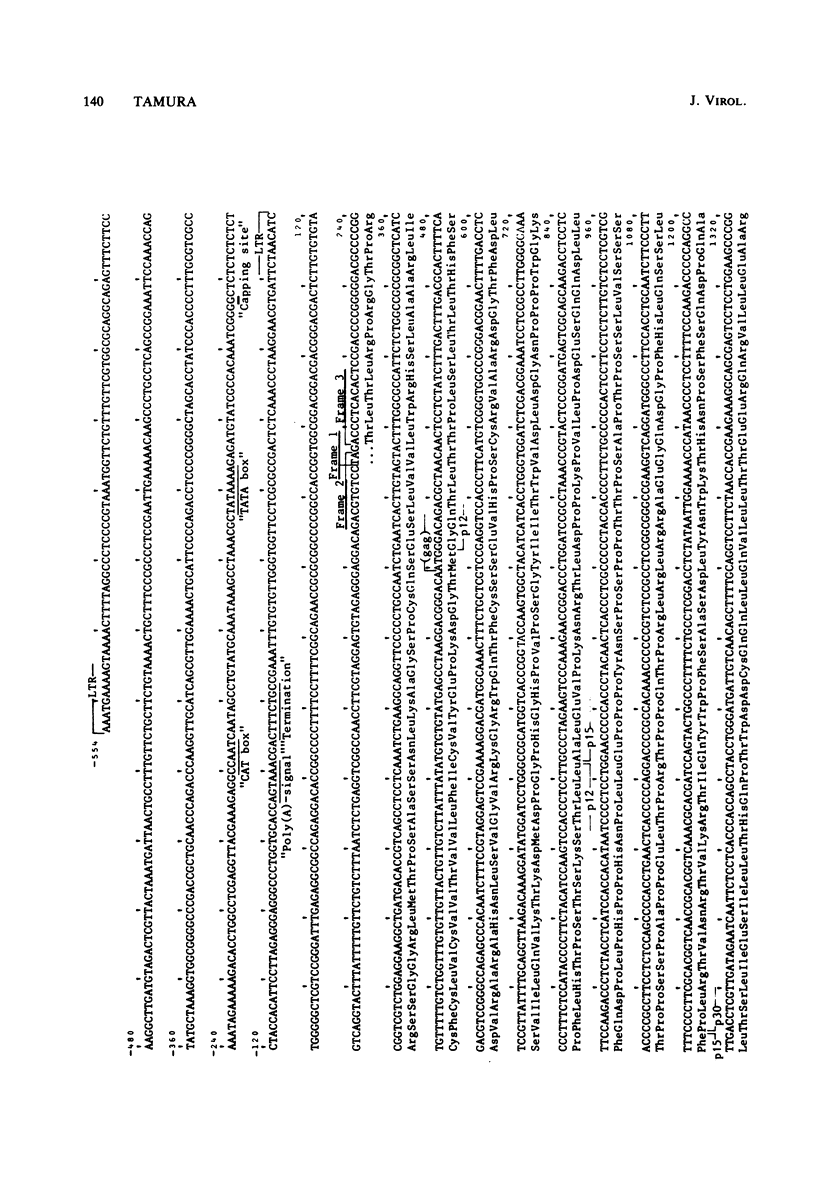
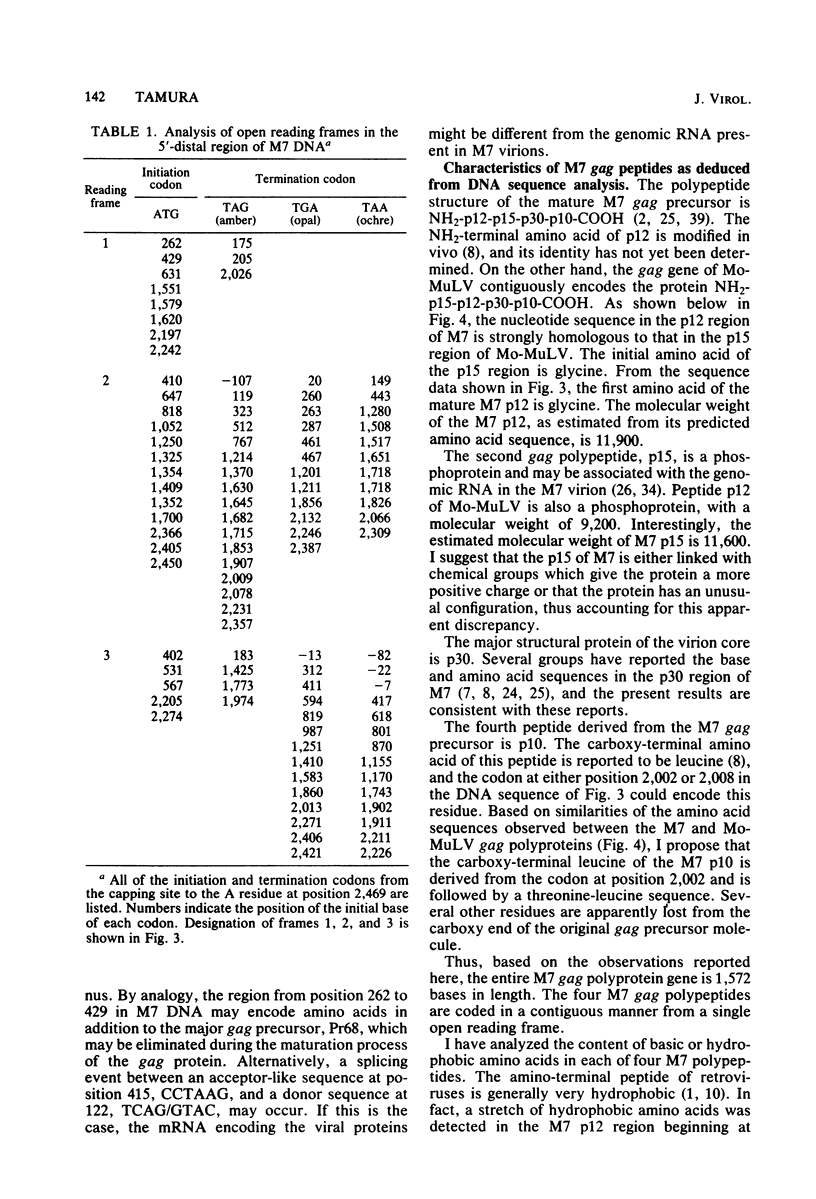
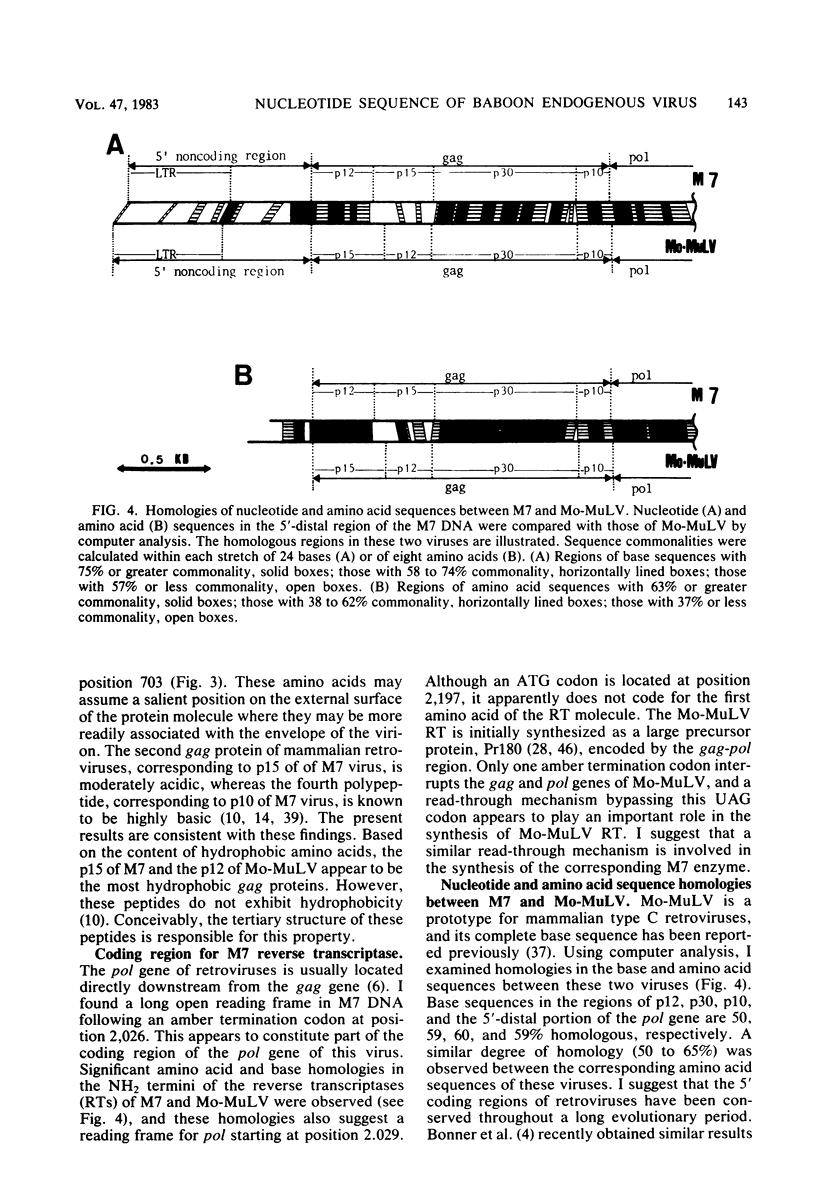
A 3,023-base nucleotide sequence of the M7 baboon endogenous virus genome, spanning the 5' noncoding region as well as the entire gag gene and part of the pol gene, is reported. Within the 562-base 5' noncoding region, a 21-base sequence complementary to the OH terminus of tRNApro is located immediately downstream from the long terminal repeat. Amino acid sequences were deduced from the 1,596 nucleotides comprising the gag gene, and the four structural gag polypeptides, p12, p15, p30, and p10, appeared to be coded contiguously. Only one termination codon interrupted the M7 gag and pol genes. The data suggest that 55 additional amino acids may be attached to the NH2 terminus of the gag precursor protein. However, such a sequence was not detected in virions or in virus-infected cells. With the exception of the p15 region, nucleotide and amino acid sequences of the gag and pol regions of M7 virus exhibited strong homologies to those of Moloney leukemia virus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Aaronson S. A. Membrane properties of the gag gene-coded p15 protein of mouse type-C RNA tumor viruses. J Biol Chem. 1978 Mar 10;253(5):1408–1414. [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. Evolutionary relationships between gag gene-coded proteins of murine and primate endogenous type C RNA viruses. Cell. 1977 Apr;10(4):641–648. doi: 10.1016/0092-8674(77)90097-6. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., O'Connell C., Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M., Rein A., Stephens R. M., O'Connell C., Gilden R. V., Shure M., Nicolson M. O., McAllister R. M., Davidson N. Baboon endogenous virus genome: molecular cloning and structural characterization of nondefective viral genomes from DNA of a baboon cell strain. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5207–5211. doi: 10.1073/pnas.78.8.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland T. D., Henderson L. E., Vanlaningham-Miller E. S., Stephenson J. R., Smythers G. W., Oroszlan S. Amino- and carboxyl-terminal sequences of proteins coded by gag gene of endogenous baboon and cat type C viruses. Virology. 1981 Feb;109(1):13–24. doi: 10.1016/0042-6822(81)90467-0. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Zuker M., Spahr P. F. Structure-function relationship of Rous sarcoma virus leader RNA. Nucleic Acids Res. 1982 Sep 11;10(17):5183–5196. doi: 10.1093/nar/10.17.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. gag-Related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J Virol. 1979 May;30(2):551–563. doi: 10.1128/jvi.30.2.551-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Fan H., Verma I. M. Size analysis and relationship of murine leukemia virus-specific mRNA's: evidence for transposition of sequences during synthesis and processing of subgenomic mRNA. J Virol. 1978 May;26(2):468–478. doi: 10.1128/jvi.26.2.468-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner E., Tress E. Isolation of a ribonucleoprotein structure from oncornaviruses. J Virol. 1973 Dec;12(6):1612–1615. doi: 10.1128/jvi.12.6.1612-1615.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Swanstrom R., Varmus H. E., Bishop J. M. The leader sequence of the subgenomic mRNA's of Rous sarcoma virus is approximately 390 nucleotides. J Virol. 1982 Feb;41(2):527–534. doi: 10.1128/jvi.41.2.527-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Campos D., 3rd, Arlinghaus R. B. Cell-free synthesis of Rauscher murine leukemia virus "gag" and "env" gene products from separate cellular mRNA species. Virology. 1979 Mar;93(2):293–302. doi: 10.1016/0042-6822(79)90234-4. [DOI] [PubMed] [Google Scholar]
- Noda M., Wagatsuma M., Tamura T., Takano T., Matsubara K. Structure of the baboon endogenous virus genome: cloning of circular virus DNA in bacteriophage lambda. Nucleic Acids Res. 1981 May 11;9(9):2173–2185. doi: 10.1093/nar/9.9.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T., Summers M. R., Smythers G., Gilden R. V. Amino acid sequence homology of mammalian type C RNA virus major internal proteins. J Biol Chem. 1975 Aug 25;250(16):6232–6239. [PubMed] [Google Scholar]
- Oroszlan S., Summers M., Gilden R. V. Amino-terminal sequence of baboon type C virus p30. Virology. 1975 Apr;64(2):581–583. doi: 10.1016/0042-6822(75)90138-5. [DOI] [PubMed] [Google Scholar]
- Pal B. K., Roy-Burman P. Phosphoproteins: structural components of oncornaviruses. J Virol. 1975 Mar;15(3):540–549. doi: 10.1128/jvi.15.3.540-549.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Harada F., Dahlberg J. E., Panet A., Haseltine W. A., Baltimore D. Low-molecular-weight RNAs of Moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977 Mar;21(3):1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Aaronson S. A. Complete nucleotide sequence and organization of the Moloney murine sarcoma virus genome. Science. 1981 Oct 23;214(4519):445–450. doi: 10.1126/science.6170110. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. Murine leukemia virus gag polyproteins: the peptide chain unique to Pr80 is located at the amino terminus. Virology. 1978 Dec;91(2):481–486. doi: 10.1016/0042-6822(78)90395-1. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Endogenous feline (RD-114) and baboon type C viruses have related specific RNA-binding proteins and genome binding sites. Virology. 1978 Jan;84(1):99–107. doi: 10.1016/0042-6822(78)90221-0. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Devare S. G., Reynolds F. H. Biochemical and immunological properties of gag genecoded structural proteins of endogenous tyep C RNA tumor viruses of diverse mammalian species. J Biol Chem. 1977 Nov 10;252(21):7818–7825. [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. Nucleotide sequence of the 5' noncoding region and part of the gag gene of Rous sarcoma virus. J Virol. 1982 Feb;41(2):535–541. doi: 10.1128/jvi.41.2.535-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Noda M., Takano T. Structure of the baboon endogenous virus genome: nucleotide sequences of the long terminal repeat. Nucleic Acids Res. 1981 Dec 11;9(23):6615–6626. doi: 10.1093/nar/9.23.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M. An analysis of the role of tRNA species as primers for the transcription into DNA of RNA tumor virus genomes. Biochim Biophys Acta. 1977 Mar 21;473(1):57–71. doi: 10.1016/0304-419x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Goddard J. G., Berns A., Verma I. M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5' long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]


