Abstract
Phosphorylation of the α-subunit of Na+,K+-ATPase plays an important role in the regulation of this pump. Recent studies suggest that insulin, known to increase solute and fluid reabsorption in mammalian proximal convoluted tubule (PCT), is stimulating Na+,K+-ATPase activity through the tyrosine phosphorylation process. This study was therefore undertaken to evaluate the role of tyrosine phosphorylation of the Na+,K+-ATPase α-subunit in the action of insulin. In rat PCT, insulin and orthovanadate (a tyrosine phosphatase inhibitor) increased tyrosine phosphorylation level of the α-subunit more than twofold. Their effects were not additive, suggesting a common mechanism of action. Insulin-induced tyrosine phosphorylation was prevented by genistein, a tyrosine kinase inhibitor. The site of tyrosine phosphorylation was identified on Tyr-10 by controlled trypsinolysis in rat PCTs and by site-directed mutagenesis in opossum kidney cells transfected with rat α-subunit. The functional relevance of Tyr-10 phosphorylation was assessed by 1) the abolition of insulin-induced stimulation of the ouabain-sensitive 86Rb uptake in opossum kidney cells expressing mutant rat α1-subunits wherein tyrosine was replaced by alanine or glutamine; and 2) the similarity of the time course and dose dependency of the insulin-induced increase in ouabain-sensitive 86Rb uptake and tyrosine phosphorylation. These findings indicate that phosphorylation of the Na+,K+-ATPase α-subunit at Tyr-10 likely participates in the physiological control of sodium reabsorption in PCT.
INTRODUCTION
Approximately 70% of the Na+- and water-filtered load in the kidney are reabsorbed by the proximal convoluted tubule (PCT), making this segment of the nephron a major player in the maintenance of salt and water homeostasis. In PCT, as in other nephron segments, sodium reabsorption is essentially an active process. The generation of a transepithelial Na+ flux by kidney tubule epithelial cells requires the coordinated function of apical Na+ transporters and basolateral Na+,K+-ATPase. Na+,K+-ATPase plays a pivotal role in this process, because it actively extrudes intracellular Na+ to the interstitium and thereby maintains the steep Na gradient, which provides the driving force for apical Na+ entry.
The activity of kidney tubule Na+,K+-ATPase is under tight multihormonal control (Bertorello and Katz, 1993; Ewart and Klip, 1995). Besides long-term regulation by steroid and thyroid hormones (Doucet et al., 1986; Barlet-Bas et al., 1987; Palmer et al., 1993), kidney tubule Na+,K+-ATPase activity can be rapidly modulated through changes in cell surface expression (Beron et al., 1997; Carranza et al., 1998) and/or by modifications of the function of single-pump units (Féraille et al., 1994, 1995). In this regard, it has been demonstrated that the α-subunit of Na+,K+-ATPase can be phosphorylated by PKA and PKC in vitro (Bertorello et al., 1991; Chibalin et al., 1992; Feschenko and Sweadner, 1995) and in intact cells (Middleton et al., 1993; Béguin et al., 1994; Féraille et al., 1995; Fisone et al., 1995; Carranza et al., 1996b). Recent studies in transfected cells indicate that serine–threonine phosphorylation of the catalytic α-subunit by PKA or PKC is a key event in the short-term regulation of Na+,K+-ATPase activity (Fisone et al., 1994; Belusa et al., 1997; Pedemonte et al., 1997; Chibalin et al., 1998). The physiological relevance of this process is supported by the correlation between the phosphorylation level of the α-subunit and the activity of Na+,K+-ATPase in response to PKA (Carranza et al., 1996b) or PKC activation (Carranza et al., 1996a) in isolated rat PCT. On the other hand, PKA and PKC phosphorylations do not fully account for the basal phosphorylation level of the α-subunit observed in various cells (Béguin et al., 1994; Carranza et al., 1996a,b), implying the likely presence of additional phosphorylation sites.
To investigate a potential tyrosine phosphorylation site, the action of insulin on PCT is of interest. Indeed, in vitro microperfusion studies have demonstrated that insulin increases solute and water reabsorption by the mammalian PCT (Baum, 1987) through a stimulation of the active transepithelial Na+ transport (Féraille et al., 1992). Insulin acts, at least in part, through stimulation of Na+,K+-ATPase activity (Féraille et al., 1994). The effect of insulin on Na+,K+-ATPase activity is independent of PKC, prevented by the tyrosine kinase inhibitor genistein and mimicked by the tyrosine phosphatase inhibitor orthovanadate (Féraille et al., 1997). These observations indicate that Na+,K+-ATPase activity is under the control of a tyrosine phosphorylation process in the rat PCT. In the present study, we have investigated whether tyrosine phosphorylation is part of the basal phosphorylation of the Na+,K+-ATPase α-subunit and whether it plays a role in the stimulation of Na+,K+-ATPase activity by insulin in the rat PCT.
MATERIALS AND METHODS
Preparation of Rat Tubules
Studies were performed either on PCT-enriched suspensions or on single microdissected PCT from male Wistar rats (body weight, 150–200 g). Animals were anesthetized with pentobarbital (5 mg/100 g body weight, i.p.), and the kidneys were perfused with ice-cold incubation solution (120 mM NaCl, 5 mM RbCl, 4 mM NaHCO3, 1 mM CaCl2, 1 mM MgSO4, 0.2 mM NaH2PO4, 0.15 mM Na2HPO4, 5 mM glucose, 10 mM lactate, 1 mM pyruvate, 4 mM essential and nonessential amino acids, 0.03 mM vitamins, 20 mM HEPES, 0.1% BSA, pH 7.45), containing 0.18% (wt/vol) collagenase (CLSII, 0.87 U/mg; Serva, Heidelberg, Germany). Single PCTs were isolated by microdissection. A PCT-enriched suspension was obtained by mechanical dissociation of the kidney cortex as described previously (Carranza et al., 1996a).
Site-directed Mutagenesis
The introduction of single point mutations into the cDNA of rat α1-subunit (kindly provided by J. Lingrel) was performed by using the PCR method of Nelson and Long (1989). Rat α1-subunit was subcloned into a pSD5 vector, and the linearized vector was used as template for mutants. Tyrosine 10 was substituted either by alanine (Y10A) or by glutamic acid (Y10E) to abolish or mimic Tyr-10 phosphorylation, respectively. To generate these mutants, DNA was first amplified between the mutated sense oligonucleotide G16–G40 and the antisense oligonucleotide consisting of G310–G324 and a vesiculostomatis virus glycoprotein primer. The mutated DNA was selectively amplified between a sense oligonucleotide encompassing T2653–C2673 of the pSD5 vector and the antisense vesiculostomatis virus glycoprotein oligonucleotide. The mutated DNA fragments were subcloned into the wild-type (WT) pSD5 α1 by using NheI present in the vector and StuI (G184) after partial digestion. WT and mutant rat α1-subunit were then subcloned into the pCI-neo (Promega, Madison, WI) eukaryotic expression vector.
Cell Culture and Transfection
Opossum kidney (OK) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS, l-glutamine (10−6 M), penicillin (10 IU/ml), and streptomycin (100 mg/ml). OK cells were transfected, as previously described (Pedemonte et al., 1997), with the eukaryotic expression vector pCI-neo containing the cDNA for the WT rat α1 subunit or its Y10A and Y10E mutants. The day before transfection, OK cells were seeded on 96-well plates (3500 cells per well), and after 1 d in DMEM, the cells were transfected in 50 μl Opti-MEM I containing 3 μg/ml total DNA and 15 μl/ml liposomes. Five hours after transfection, 200 μl/well of DMEM was added. Two days later, 1 μM ouabain was added to the medium. This concentration completely inhibits the endogenous Na+,K+-ATPase activity, and only cells expressing the transfected rat α1-subunit could survive. After 10 d, single colonies were transferred to a medium containing 10 μM ouabain to select clones expressing the highest level of rat α1-subunit.
The expression of the rat α1-subunit was assessed by the presence of an ouabain-resistant 86Rb uptake (see below). In these transfected cells, the presence of functional ouabain-resistant Na+,K+-ATPase units in the plasma membrane is dependent on the association of the rat α1-subunits with the endogenous β-subunits.
After stable transfection, OK cells were grown in medium supplemented with 5 × 10−6 M ouabain to maintain the selection pressure. Cells were used between passages 10 and 30 and cultured for 6–7 d before the experiments.
Immunoprecipitation
Proximal tubules or OK cells were incubated at 37°C for various times in 1 ml oxygenated (95% O2-5% CO2) incubation solution with or without insulin and/or orthovanadate. Incubation was stopped by centrifugation at 4°C, and the tubules contained in the pellet were lysed for 10 min in 0.5 ml ice-cold immunoprecipitation buffer [20 mM Tris-HCl, 2 mM EGTA, 2 mM EDTA, 30 mM NaF, 30 mM Na4O7P2, 2 mM Na3VO4, 1 mM 4-(2-aminoethyl)-benzenesulfonylfluoride), 10 μg/ml leupeptin, 4 μg/ml aprotinin, 1% Triton X-100, pH 7.45]. After clearing homogenates by centrifugation and determination of protein concentration by the bicinchoninic acid method (BCA assay; Pierce, Rockford, IL), identical or increasing amounts of protein from supernatants were incubated overnight at 4°C with antibodies. Antibodies were either covalently linked to agarose beads or bound to saturating amount of protein G-agarose or protein A-Sepharose beads (Pharmacia, Piscataway, NJ). After two washes with 1 ml ice-cold immunoprecipitation buffer and one wash in 10 mM Tris, pH 7.4, 100 μl of sample buffer (5% SDS, 140 mM Tris, 2.5% β-mercaptoethanol, 6.8% sucrose, 0.003% bromphenol blue) were added, and the samples were warmed up to 65°C for 15 min. Tyrosine-phosphorylated proteins were immunoprecipitated with monoclonal anti-phosphotyrosine antibody PY20 (Transduction Laboratories, Lexington, KY). Na+,K+-ATPase was immunoprecipitated with a previously characterized (Carranza et al., 1996a) rabbit polyclonal anti-Na+,K+-ATPase antibody (anti-NK1) raised against the purified rat kidney holoenzyme. Preliminary experiments have determined that 100 μl agarose-conjugated PY20 antibody or 20 μl anti-NK1 antibody are saturating for up to 200 μg proximal tubule protein. These, antibody:protein ratios were used in all subsequent experiments.
Immunoblotting
Proteins were separated by electrophoresis on 7% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA). For monoclonal antibodies, membranes were blocked in Tris-buffered saline (TBS)-Tween (150 mM NaCl, 50 mM Tris, 0.2% Tween 20, pH 7.5) with 3% BSA for 1 h at room temperature. After three washes in TBS-Tween, the membranes were incubated overnight at 4°C with a first antibody in TBS-Tween containing 1% BSA. The membranes were washed three times and incubated for 1 h at room temperature in TBS-Tween containing 1% BSA and anti-mouse immunoglobulin antibodies coupled to horseradish peroxidase (Amersham, Arlington Heights, IL) at dilution 1:20,000 (vol/vol). After three washes, the immunoreactivity was detected by the chemiluminescence method (Amersham). For polyclonal antibodies, membranes were blocked in TBS with 0.2% (vol/vol) Nonidet-P40 (TBS-NP40) and 5% (wt/vol) nonfat dry milk, washed in TBS-NP40. Antibodies were dissolved in TBS-NP40 supplemented with 5% nonfat dry milk. The secondary antibody was an anti-rabbit immunoglobulin coupled to horseradish peroxidase (Transduction Laboratories) and was used at dilution 1:20,000 (vol/vol). Tyrosine phosphoproteins were detected by the 4G10 antibody (Upstate Biotechnology, Lake Placid, NY) used at dilution of 1:2500 (vol/vol). The Na+,K+-ATPase α-subunit was detected by 1) the mouse monoclonal antibody McK1, which recognizes the KKSKK motif contained in the extreme NH2-terminal end of the rat α1-subunit (Felsenfeld and Sweadner, 1988), and used at dilution of 1:500 (vol/vol); 2) NKP-2, a rabbit polyclonal antibody raised against the purified α-subunit of Bufo Marinus (Girardet et al., 1981), which recognizes the α-subunits from every species tested so far, and used at dilution of 1:20,000 (vol/vol); or 3) a rabbit polyclonal antibody raised against the extreme COOH-terminal ETYY motif (kindly provided by Dr. Jack Kyte, University of California, San Diego, CA), which is conserved in the majority of the cloned α-subunits including rat, and used at dilution of 1:10,000 (vol/vol). The anti-NK1 antibody (see above) was not used for immunoblots, because it recognizes both α- and β-subunits of Na+,K+-ATPase. Results were quantified under conditions of linearity by integration of the density of the total area of each band using a video densitometer and the ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Results are expressed either as arbitrary units of optical density or as percent ± SEM of the control optical density.
32P Labeling and Autoradiography
Proximal tubules were preincubated for 120 min at 30°C in 2.5 ml oxygenated incubation solution containing 1 mCi/ml [32P]orthophosphate (New England Nuclear, Boston, MA) before incubation in the absence or presence of insulin or/and orthovanadate for 30 min at 37°C. Na+,K+-ATPase from 100 μg protein of 32P-labeled proximal tubules was immunoprecipitated (see above), and after separation by 7% SDS-PAGE, proteins were electrotransferred to a polyvinylidene difluoride membrane to reduce the background in autoradiograms. Membranes were dried, and autoradiography was performed at −70°C for 5–7 d with Hyperfilm-MP (Amersham). The results were quantified as described above. Results are expressed as percent ± SEM of the control optical density (absence of insulin or orthovanadate).
Phosphoamino Acid Analysis
Immunoprecipitated Na+,K+-ATPase from 32P-labeled proximal tubules was run on 7% SDS-PAGE, and the dried gels were exposed for autoradiography. The 32P-labeled α-subunits were identified on the gel, cut out, and subjected to two-dimensional phosphoamino acid analysis performed as described by Boyle et al. (1991).
Controlled Trypsinolysis of Na+,K+-ATPase α-Subunit
Proximal tubules were lysed in immunoprecipitation buffer without protease inhibitors. Aliquots of proximal tubule protein (200 μg) were incubated for 5 min at 4°C with trypsin (type XI; Sigma, St. Louis, MO) at a trypsin:protein ratio (wt/wt) from 0.005 to 0.01. Trypsin digestion was stopped by addition of a fivefold excess (wt/wt) of soybean trypsin inhibitor (Sigma). After 10 min at 4°C, samples were subjected to immunoprecipitation with PY20 antibodies or directly to SDS-PAGE.
Measurement of Ouabain-sensitive 86Rb Uptake in OK Cells
The transport activity of Na+,K+-ATPase was estimated in OK cells by measurement of the ouabain-sensitive 86Rb uptake under conditions of initial rates. For this purpose, OK cells were seeded on multiwell plates (22-mm-diameter wells) and grown to 70–80% confluence. After removal of the culture medium, cells were washed twice with 1 ml HEPES-buffered (20 mM, pH 7.4) bicarbonate- and serum-free DMEM. Cells were then preincubated at room temperature for 30 min after addition of 1 ml of the same medium with or without 10−8 M insulin or 10−6 M phorbol 12-myristate 13-acetate (PMA). 86Rb uptake was determined in triplicate samples after addition of 10 μl DMEM containing 86RbCl (Amersham; 100 nCi per sample) and 5.4 mM K+. Incubation was stopped after 15 min by cooling on ice and rapid aspiration of the incubation medium. After three washes with 1 ml ice-cold washing solution containing 150 mM choline-chloride, 1.2 mM MgSO4, 1.2 mM CaCl2, 2 mM BaCl2, 5 mM HEPES, pH 7.4, cells were lysed in 0.5 ml of 1% (wt/vol) sodium deoxycholate, and 0.4 ml of the lysate were transferred into a counting vial. Radioactivity was measured by liquid scintillation counting. The remaining 0.1 ml of the lysate was used to determine the protein content by the bicinchoninic acid assay (BCA; Pierce).
The Rb (K) transport mediated by the Na+,K+-pumps containing the rat α1-subunit was calculated as the difference between the mean values measured in triplicate samples incubated with 5 × 10−6 or 5 × 10−3 M ouabain. Ouabain was introduced at the beginning of the preincubation step. 86Rb uptake was calculated as pmol Rb (K) × μg protein−1 × min−1. Preliminary experiments have shown that 86Rb uptake was linear for at least 20 min (our unpublished results).
Measurement of Ouabain-sensitive 86Rb Uptake in Rat Kidney Tubules
The transport activity of Na+,K+-ATPase was estimated on intact isolated PCTs or proximal tubule suspension by the ouabain-sensitive 86Rb uptake measured under conditions of initial rate in the presence of 5 mM RbCl, as previously described (Féraille et al., 1992). Ouabain-sensitive 86Rb uptake was calculated as the difference between the uptake measured without and with 5 × 10−3 M ouabain, respectively. As previously reported (Féraille et al., 1994), insulin (10−8 M for 30 min at 37°C) stimulated the ouabain-sensitive 86Rb uptake in isolated PCTs (as pmol Rb × mm−1 × min−1 ± SEM; control, 16.0 ± 1.24; insulin, 23.6 ± 2.1; n = 9; p < 0.01). This effect was reproduced in proximal tubule suspension (as pmol Rb × μg proteins−1 × min−1 ± SEM; control, 39.0 ± 6.2; insulin, 71.8 ± 12.9; n = 6; p < 0.05), which is made up to 90% of proximal tubules (Carranza et al., 1996a). Because insulin produced similar effects in both preparations of tubules, 86Rb uptake was measured in isolated PCTs for convenience.
Statistics and Calculations
Statistical analysis were done by Wilcokson rank test or by Friedman test for comparisons between two or more than two groups, respectively. p < 0.05 were considered significant.
Values of the ouabain inhibition of 86Rb uptake were fitted to the following equation describing two independent subpopulations of Na+,K+-ATPase (Féraille et al., 1993): v = V1/(1 + [O]/Ki1) + V2/(1 + [O]/Ki2), where v is the 86Rb uptake measured at a given concentration of ouabain ([O]); V is the 86Rb uptake measured in the absence of ouabain; Ki is the apparent inhibition constant for ouabain of the Na+,K+-ATPase (IC50). Kinetic parameters (V and IC50) were determined by nonlinear regression analysis using Prism 2.0 (GraphPad Software, San Diego, CA).
RESULTS
Insulin and Orthovanadate Increase the Phosphorylation Level of the Na+,K+-ATPase α-Subunit and Its Phosphotyrosine Immunoreactivity in Rat Kidney Proximal Tubules
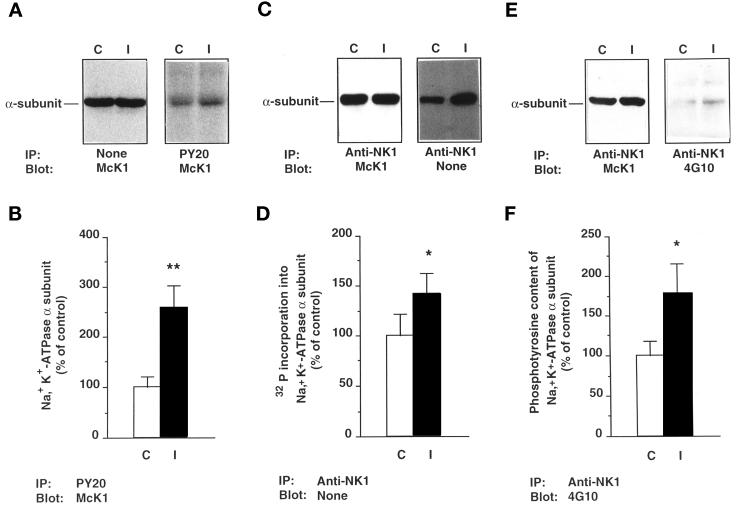
To assess whether insulin increases phosphorylation of Na+,K+-ATPase, α-subunits were immunoprecipitated with anti-NK1 antibody from lysates of 32P-labeled cortical tubules after 30 min incubation at 37°C in the absence or presence of 10−8 M insulin. The immunoprecipitated samples were then subjected either to immunoblotting with McK1 antibodies or directly to SDS PAGE and autoradiography. In the presence of similar amounts of α-subunits (Figure 1C, left panel), insulin increased 32P incorporation by 42 ± 20% (p < 0.05; n = 6; Figure 1C, right panel, and D). The insulin-induced increase in α-subunit phosphorylation is at least in part due to tyrosine phosphorylation, because since insulin increased the amount of α-subunit, detected by immunoblot with the McK1 antibody after immunoprecipitation with the PY20 antibody, by 2.58 ± 0.45-fold (p < 0.01; n = 16; Figure 1A, right panel, and B), at similar expression levels of the α-subunit (Figure 1A, left panel). Similarly, insulin increased the phosphotyrosine immunoreactivity detected by immunoblot with 4G10 antibody by 1.78 ± 0.38-fold (p < 0.05; n = 4; Figure 1, E, right panel, and F) after immunoprecipitation of similar amounts of α-subunit with the anti-NK1 antibody (Figure 1E, left panel). The apparent discrepancy between the magnitude of insulin effects on 32P incorporation and tyrosine phosphorylation level could be accounted for by detection of basal serine and threonine phosphorylation by the former approach. Preliminary experiments have shown that the effect of insulin on phosphorylation of the α-subunit is maximal under the experimental conditions used (also see Figure 10).
Figure 1.
Insulin increases the phosphorylation level of the Na+, K+-ATPase α-subunit and its phosphotyrosine immunoreactivity in rat kidney proximal tubules. Kidney proximal tubules were incubated for 30 min at 37°C in the absence (C) or presence of 10−8 M insulin (I). (A) Immunoblots of tubular extracts with the McK1 antibody before (left panel) and after (right panel) immunoprecipitation with the PY20 antibody. (B) Densitometric quantification of data shown in A (right panel). Results are expressed as percent of the control optical density and are means ± SEM from 12 independent experiments. (C) Lysates from 32P-labeled proximal tubules were prepared, and immunoprecipitation was performed with the anti-NK1 antibody. Samples were subjected to immunoblotting with the McK1 antibody (left panel) or directly to SDS-PAGE and autoradiography (right panel). (D) Densitometric quantification of data shown in C (right panel). Results are expressed as percent of the control optical density and are means ± SEM from six independent experiments. (E) Immunoblots of tubular extracts with McK1 antibody (left panel) and 4G10 antibody (right panel) after immunoprecipitation with the anti-NK1 antibody. (F) Densitometric quantification of data shown in E (right panel). Results are expressed as percent of the control optical density and are means ± SEM from four independent experiments (**, p < 0.01; *, p < 0.05 vs. control).
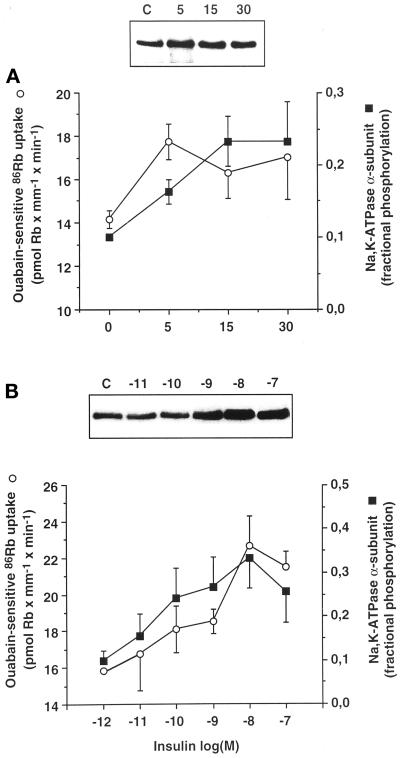
Figure 10.
The time course and the dose dependence of insulin-induced tyrosine phosphorylation of the Na+,K+-ATPase α-subunit and stimulation of the ouabain-sensitive 86Rb uptake are similar in rat PCT. (A) The tyrosine phosphorylation of the α-subunit of Na+,K+-ATPase from kidney proximal tubules (closed squares) and ouabain-sensitive 86Rb uptake by isolated PCTs (open circles) was determined after 30 min preincubation at 37°C. Prewarmed insulin solution (10−8 M final) was added or not for various times (5–30 min). Upper panel, typical immunoblot with McK1 antibody showing the amount of α-subunit immunoprecipitated with the PY20 antibody. Lower panel, averaged results expressed as fractional tyrosine phosphorylation of the α-subunit (assuming that 10% of α-subunits are phosphorylated under baseline) and as pmol Rb × mm−1 × min−1 were plotted on the same graph and are means ± SEM from five or six independent experiments. (B) The tyrosine phosphorylation of the α-subunit of Na+,K+-ATPase from proximal tubules (closed squares) and ouabain-sensitive 86Rb uptake by isolated PCTs (open circles) was determined after preincubation for 30 min in the absence (C) or presence of various concentrations of insulin. Upper panel, typical immunoblot with McK1 antibody showing the amount of α-subunit immunoprecipitated with the PY20 antibody. Lower panel, averaged results expressed as in A were plotted on the same graph and are means ± SEM from five or six independent experiments.
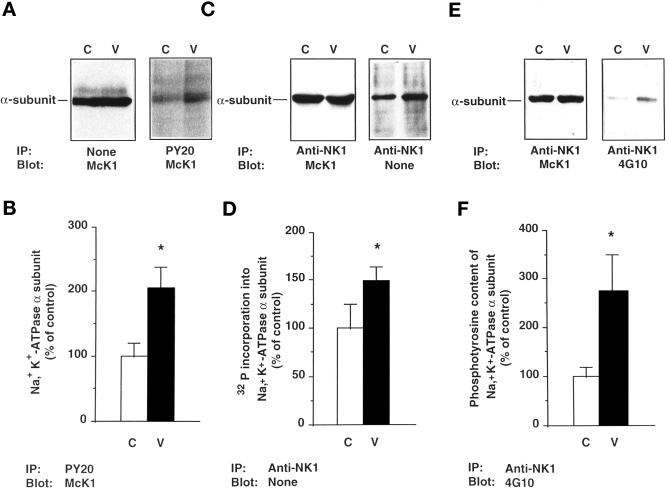
The same results were obtained when kidney cortical tubules were incubated for 30 min at 37°C with 10−3 M orthovanadate, a tyrosine kinase inhibitor (Klarlund, 1985). Orthovanadate increased the total 32P incorporation into the α-subunit by 49 ± 15% (p < 0.05; n = 6; Figure 2, C and D). Furthermore, orthovanadate increased the amount of α-subunit, detected by immunoblot with the McK1 antibody, by 2.05 ± 0.33-fold (p < 0.05; n = 6; Figure 2, A and B), after immunoprecipitation with the PY20 antibody, and the phosphotyrosine immunoreactivity detected by immunoblot with the 4G10 antibody by 2.74 ± 0.75-fold (p < 0.05; n = 4; Figure 2, E and F), after immunoprecipitation of the α-subunit with the anti-NK1 antibody.
Figure 2.
Orthovanadate increases the phosphorylation level of the Na+, K+-ATPase α-subunit and its phosphotyrosine immunoreactivity in rat kidney proximal tubules. Kidney proximal tubules were incubated for 30 min at 37°C in the absence (C) or presence of 10−3 M orthovanadate (V). (A) Immunoblots of tubular extracts with the McK1 antibody before (left panel) and after (right panel) immunoprecipitation with the PY20 antibody. (B) Densitometric quantification of data shown in A (right panel). Results are expressed as percent of the control optical density and are means ± SEM from six independent experiments. (C) Lysates from 32P-labeled proximal tubules were prepared, and immunoprecipitation was performed with the anti-NK1 antibody. Samples were subjected to immunoblotting with the McK1 antibody (left panel) or directly to SDS-PAGE and autoradiography (right panel). (D) Densitometric quantification of data shown in C (right panel). Results are expressed as percent of the control optical density and are means ± SEM from six independent experiments. (E) Immunoblots of tubular extracts with McK1 antibody (left panel) and 4G10 antibody (right panel) after immunoprecipitation with the anti-NK1 antibody. (F) Densitometric quantification of data shown in E (right panel). Results are expressed as percent of the control optical density and are means ± SEM from four independent experiments (**, p < 0.01; *, p < 0.05 vs. control).
Thus, in intact kidney cortical tubules, insulin and orthovanadate increase the phosphorylation level of the Na+,K+-ATPase α-subunit most likely at tyrosine residues.
The Na+,K+-ATPase α-Subunit Is Phosphorylated on Tyrosine Residue(s) in Kidney Proximal Tubules
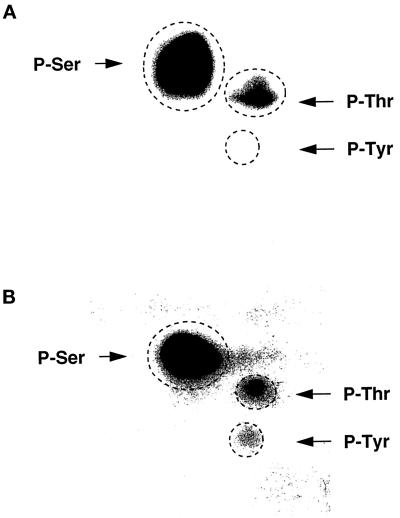
To demonstrate tyrosine phosphorylation of the Na+,K+-ATPase α-subunit, immunoprecipitates from 32P-loaded proximal tubules were subjected to phosphoamino acid analysis. Under control conditions, only serine and threonine phosphorylations of the α-subunit were detected (Figure 3A). After orthovanadate treatment, an additional phosphotyrosine spot was observed without variation of the α-subunit content in phosphoserine and phosphothreonine (Figure 3B). These results indicate that, besides predominant serine and threonine phosphorylation, the rat Na+,K+-ATPase α-subunit can also be phosphorylated on tyrosine residues.
Figure 3.
Phosphoamino acid analysis of the Na+, K+-ATPase α-subunit. Kidney proximal tubules were labeled with 1 mCi/ml [32P]orthophosphoric acid for 2 h at 30°C in the absence (A) or presence of 10−3 M orthovanadate (B). 32P-Labeled Na+, K+-ATPase was immunoprecipitated with anti-NK1 antibody, and the α-subunit was subjected to phosphoamino acid analysis. The dashed circles represent the positions of cold phosphoserine (P-Ser), phosphothreonine (P-Thr), and phosphotyrosine (P-Tyr). This experiment was performed twice with the same results.
The Effect of Insulin Is Prevented by Genistein and Is Not Further Enhanced by Orthovanadate
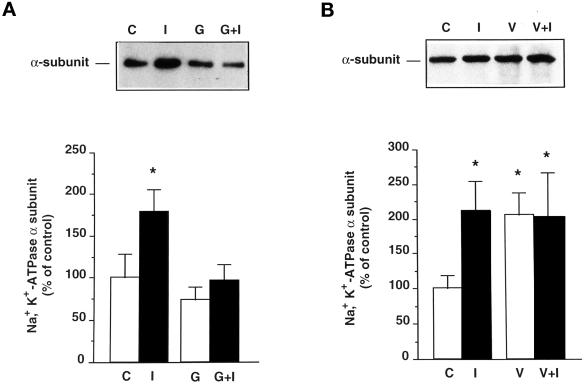
The effect of genistein, a tyrosine kinase inhibitor, on the effect of insulin was examined after 30 min incubation of proximal tubules at 37°C. As shown in Figure 4A, 10−4 M genistein slightly decreased the amount of Na+,K+-ATPase α-subunit immunoprecipitated with the PY20 antibody and prevented most of the effect of insulin (as percent of control ± SEM: insulin, 179 ± 27%; genistein, 73 ± 16%; insulin + genistein, 96 ± 19%). This result indicates that the action of insulin is dependent on tyrosine kinase activity.
Figure 4.
The effect of insulin is prevented by genistein and is not additive to the effect of orthovanadate. (A) Kidney proximal tubules were incubated for 30 min at 37°C in the absence (C) or presence of 10−8 M insulin (I) and/or 10−4 M genistein (G). Upper panel, immunoblot of proximal tubule extracts with McK1 antibody showing the effects of insulin and genistein on the amount of Na+, K+-ATPase α-subunit immunoprecipitated by the PY20 antibody. Lower panel, densitometric quantification of data shown in the upper panel. Results are expressed as percent of control optical density and are means ± SEM from seven independent experiments. (B) Proximal tubules were incubated for 30 min at 37°C in the absence (C) or presence of 10−8 M insulin (I) and/or 10−3 M orthovanadate (V). Upper panel, immunoblot of proximal tubule extracts with McK1 antibody showing the effects of insulin and orthovanadate on the amount of Na+, K+-ATPase α-subunit immunoprecipitated with the PY20 antibody. Lower panel, densitometric quantification of data shown in the upper panel. Results are expressed as percent of control optical density and are means ± SEM from six independent experiments (*, p < 0.05 vs. control).
To examine whether insulin and orthovanadate act through a common pathway, their effect was studied in combination. As depicted in Figure 4B, incubation of proximal tubules in the presence of 10−8 M insulin or 10−3 M orthovanadate increased equally the amount of Na+,K+-ATPase α-subunit immunoprecipitated with PY20 antibody, but these effects were not additive (as percent of control ± SEM: insulin, 211 ± 44%; orthovanadate, 205 ± 33%; insulin + orthovanadate, 202 ± 64%). These results suggest that insulin and orthovanadate share a common signaling pathway.
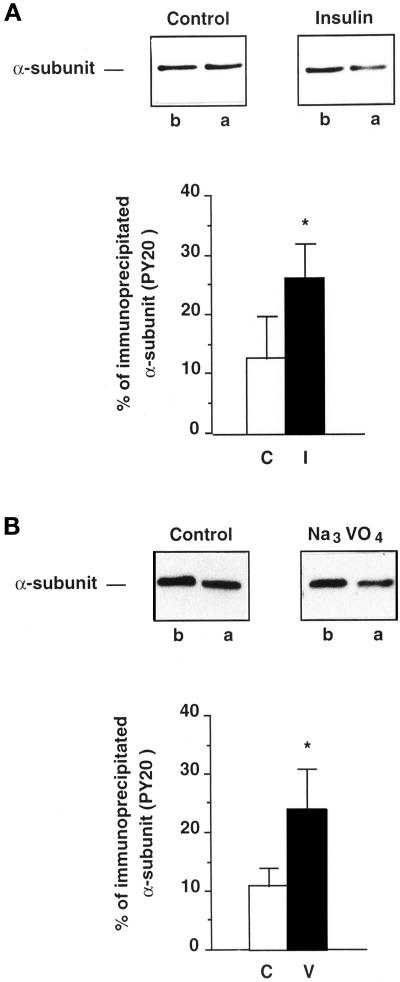
Determination of the Percentage of Na+,K+-ATPase α-Subunits Immunoprecipitated with Anti-Phosphotyrosine Antibodies
The proportion of α-subunits immunoprecipitated by the PY20 antibody was determined as follows. Aliquots of extracts of cortical tubules, previously incubated for 30 min at 37°C in the absence or presence of 10−8 M insulin or 10−3 M orthovanadate, were either subjected directly to immunoblotting with McK1 antibodies or immunoprecipitated first with PY20 antibodies before immunoblotting with McK1 antibodies. The percent decrease in the α-signal detected with the McK1 antibody before and after immunoprecipitation with the PY20 antibody (Figure 5, A and B, upper panels) is a reflection of the proportion of tyrosine-phosphorylated α-subunits in the total α-subunit population. In control conditions, the proportion of α-subunits immunoprecipitated by the PY20 antibody amounts to ∼ 10% of the total α-subunit population (Figure 5, A and B, lower panels). Insulin (Figure 5A, lower panel) or orthovanadate treatment (Figure 5B, lower panel) increased this proportion to 26 ± 6% (p < 0.05; n = 4) or 24 ± 7% (p < 0.05; n = 7), respectively.
Figure 5.
Estimation of the percentage of Na+, K+-ATPase α-subunits immunoprecipitated with antiphosphotyrosine antibodies under control or stimulated conditions. Proximal tubules were preincubated for 30 min at 37°C without or with stimulators. Extracts were prepared and aliquots were subjected to immunoblotting with the McK1 antibody before (b) or after (a) immunoprecipitation with the PY20 antibody. (A) Tyrosine phosphorylation of the α-subunit from control or insulin-treated (10−8 M) tubules. Upper panels, immunoblots. Lower panel, quantification of data shown in upper panels and expressed as percent of the total α-subunit population immunoprecipitated with the PY20 antibody. Results are means ± SEM from four independent experiments. (B) Tyrosine phosphorylation of the α-subunit from control or orthovanadate-treated (10−3 M) tubules. Upper panels, immunoblots. Lower panel, quantification of data shown in upper panels and expressed as percent of α-subunits of the total α-subunit population immunoprecipitated with the PY20 antibody (*, p < 0.05 vs. control).
Localization of the Tyrosine Phosphorylation site of the Na+,K+-ATPase α-Subunit
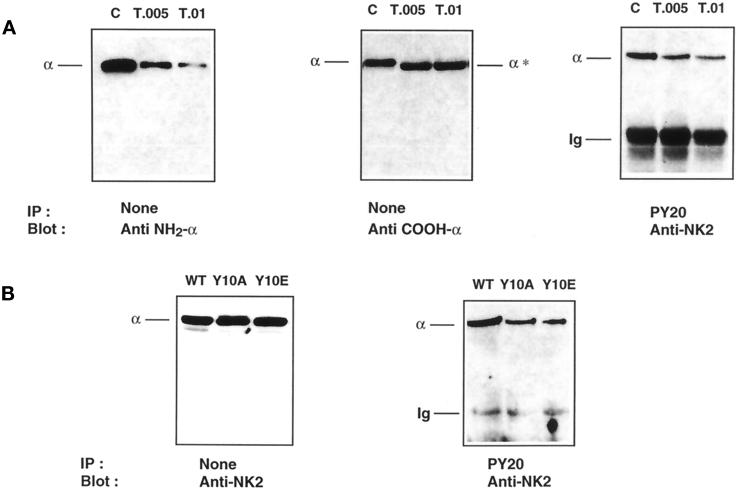
To identify the structural domain of tyrosine phosphorylation, controlled trypsinolysis of the Na+,K+-ATPase α-subunit was performed in Triton X-100 extracts of insulin- and orthovanadate-treated proximal tubules. Under the experimental conditions used, tryptic cleavage of the α-subunit should occur at the T2 site and only remove the first 30 amino acids of the NH2 terminus (Jorgensen and Collins, 1986). Indeed, limited tryptic digestion of the α-subunit cleaved the extreme NH2 terminus of the α-subunit (Figure 6A, left panel), as shown by the decrease in α-signal with McK1 antibody (Anti NH2-α; see MATERIALS AND METHODS) and by the moderate shift in apparent molecular weight of the α-subunit (Figure 6A, middle panel) detected by an antibody raised against its extreme COOH terminus (Anti COOH-α; see MATERIALS AND METHODS). The extreme COOH terminus of the α-subunit was not cleaved by trypsin, as indicated by the constant level of the α-signal detected with anti COOH-α antibody (Figure 6, middle panel). Limited trypsinolysis did not produce tryptic fragments of lower molecular mass consistent with the sole removal of the most NH2-terminal amino acids as described previously using similar proteolysis conditions (Béguin et al., 1994). After limited trypsinolysis, the amount of Na+,K+-ATPase α-subunit immunoprecipitated by the PY20 antibody (anti-phosphotyrosine) was dramatically decreased, as shown by the reduction in α-signal detected with the anti-NK2 antibody that recognizes both intact and digested α-subunits (Figure 6, right panel). The α-signal detected after immunoprecipitation by the PY20 antibody is proportional to α-signal detected with the McK1 antibody (Anti NH2-α) of nonprecipitated samples. This result indicates that a tyrosine phosphorylation site is located in the proximal portion of the NH2 terminus of the α-subunit.
Figure 6.
The Na+, K+-ATPase α-subunit is phosphorylated at tyrosine 10. (A) Proximal tubules were preincubated for 30 min at 37°C with 10−8 M insulin, and 200 μg protein were incubated for 5 min at 4°C without (C) or with trypsin (T) at a trypsin:protein ratio (wt/wt) of 0.005–0.01. Immunoblots of total protein with McK1 antibody (Anti NH2-α; left panel) and with an antibody raised against the extreme COOH-terminal ETYY motif (Anti COOH-α; middle panel) show that tryptic cleavage of the α-subunit has occurred exclusively at the extreme NH2 terminus. This limited trypsinolysis removed the tyrosine phosphorylation site recognized by PY20 antibody (right panel). Results are representative of four independent experiments. (B) OK cells were stably transfected with the WT rat α1-subunit (WT) or its Y10A or Y10E mutants. Immunoblot of total protein (left panel) shows that transfected cell lines express similar amounts of α-subunit, but substitution of Tyr-10 by Ala or Glu greatly reduces the amount of α-subunit immunoprecipitated by PY20 antibody (right panel).
Tyrosine-10 of the Na+,K+-ATPase α-subunit is the best candidate for phosphorylation, because it is conserved in all cloned Na+,K+-ATPase α1-subunits (i.e., the renal isoform), and the related gastric H+,K+-ATPase α-subunit is phosphorylated at this position (Togawa et al., 1995). Therefore, we substituted Tyr-10 of the rat α1-subunit by Ala or Glu and studied the phosphorylation and the functional properties of this mutant after its expression in OK cells, a model of proximal tubule cells. OK cells stably transfected with WT rat α1-subunit cDNA or the Y10A and Y10E α-mutant cDNAs expressed similar levels of Na+,K+-ATPase α-subunit (Figure 6B, left panel). However, the amount of α-subunit immunoprecipitated by the PY20 antibody was significantly reduced in OK cells expressing either the Y10A or the Y10E mutants compared with those expressing the WT rat α1-subunit (Figure 6B, right panel). Similar results were obtained with two different OK cell clones. The residual α-signal detected in OK cells expressing the Y10A and Y10E α1 mutants after immunoprecipitation with anti-phosphotyrosine antibodies most likely represents endogenous oppossum α-subunits revealed by the polyclonal anti-NK2 antibody (our unpublished results).
Altogether, these results strongly support that Tyr-10 is phosphorylated under basal conditions and that phosphorylation increases in response to insulin or orthovanadate in proximal tubule cells.
Phosphorylation of the α-Subunit at Tyr-10 Is Required for the Insulin-induced Stimulation of Na+,K+-ATPase Activity in Kidney Proximal Tubule Cells
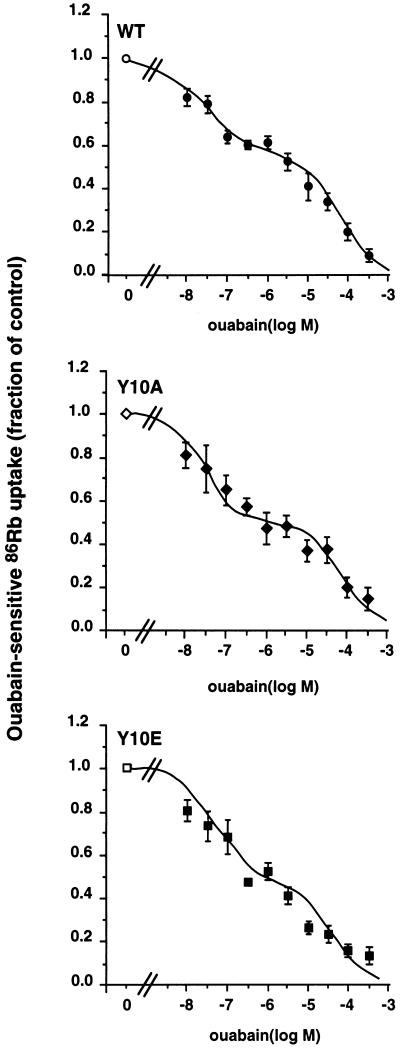
The functional role of Tyr-10 phosphorylation was studied in OK cells stably expressing the WT rat α1-subunit or its Y10A and Y10E mutants. The expression of functional ouabain-resistant Na+,K+-ATPase in transfected OK cells was revealed by the dose–inhibition curve of 86Rb (K) uptake by ouabain (from 10−8 to 5 × 10−3 M). Figure 7 shows that OK cells expressing the WT rat α1 subunit or its tyrosine phosphorylation site mutants (Y10A and Y10E) exhibited a biphasic inhibition pattern. The calculated IC50 value of the Na+,K+-ATPase population with high ouabain sensitivity (2.2–3.8 × 10−8 M) and that with low ouabain sensitivity (3.0 to 8.7 × 10−5 M) is in agreement with the IC50 values of endogenous OK cells (Pedemonte et al., 1997) and rat Na+,K+-ATPase (Jewell and Lingrel, 1991). These results show that the endogenous β subunits of OK cells associate with WT or mutant rat α1 subunits to form functional ouabain-resistant α–β complexes which account for the 46–57% residual 86Rb (K) uptake measured in the presence of 5 × 10−6 M ouabain. All subsequent experiments were done in the presence of 5 × 10−6 M ouabain to measure the activity of the exogenous Na+,K+-ATPase independently of the endogenous pumps.
Figure 7.
Functional expression of WT or mutant rat α1-subunits in stably transfected OK cells. 86Rb (K) uptake was measured under initial rate in OK cells preincubated in the absence or presence of increasing concentrations of ouabain. The Na+,K+-ATPase-mediated 86Rb uptake was obtained by subtraction of ouabain-insensitive 86Rb (K) uptake measured in the presence of 5 × 10−3 M ouabain. Results from three or four independent experiments are expressed as percent of control (absence of ouabain) ± SEM. OK cells expressing the WT (WT) rat α1-subunit or its tyrosine phosphorylation site mutants (Y10A and Y10E) exhibited a bimodal ouabain inhibition pattern. The IC50 values of Na+,K+-ATPase with high ouabain sensitivity were WT, 2.2 ± 0.9 × 10−8 M; Y10A, 3.8 ± 1.5 × 10−8 M; and Y10E, 3.8 ± 2.5 × 10−8 M. The IC50 values of Na+,K+-ATPase with low ouabain sensitivity were WT, 4.3 ± 0.9 × 10−5 M; Y10A, 8.7 ± 2.7 × 10−5 M; and Y10E, 3.0 ± 1.6 × 10−5 M. The percentages of 86Rb (K) uptake mediated by the ouabain-resistant Na+,K+-ATPase were WT, 57.1 ± 2.6%; Y10A, 47.6 ± 3.0%; and Y10E, 45.8 ± 5.7%.
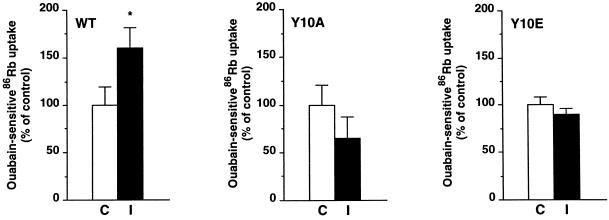
In OK cells stably expressing the WT rat α1-subunit, insulin stimulated the exogenous Na+,K+-ATPase-mediated 86Rb (K) uptake from 0.91 ± 0.18 to 1.47 ± 0.22 pmol Rb (K) × μg protein−1 [tiems] min−1 (p < 0.05; n = 7; Figure 8), similar to data obtained in isolated rat PCT (Féraille et al., 1994). The stimulatory effect of insulin was abolished in cells expressing the Y10A mutant, which prevents tyrosine phosphorylation. Similarly, OK cells expressing the Y10E mutant, which contains a negative charge that may mimic the effect of phosphorylation, did not exhibit insulin-stimulated 86Rb (K) uptake. Pumps containing the Y10E mutation might be already maximally stimulated under control conditions. This is supported by the observation that the basal exogenous Na+,K+-ATPase-mediated 86Rb (K) uptake is 50% higher in cells expressing the Y10E α1 mutant than in cells expressing the WT rat α1-subunit (as pmol × ug protein−1 [tiems] min−1 ± SEM: WT, 0.91 ± 0.20; Y10E, 1.40 ± 0.13; p < 0.05; n = 7). Finally, the effect of insulin on 86Rb uptake was preserved in OK cells expressing a PKC phosphorylation site mutant (S16A) of rat α1-subunit (our unpublished results). Results are means from pooled data obtained in two independent stably transfected clones for OK cells expressing the WT rat α1-subunit or its Y10A or Y10E mutants.
Figure 8.
Substitution of Tyr-10 by either Ala (Y10A) or Glu (Y10E) abolishes the stimulation of Na+,K+-ATPase by insulin in transfected OK cells. 86Rb (K) uptake was measured in the presence of 5 × 10−6 M ouabain under initial rates of influx in OK cells expressing the WT rat α1-subunit, its tyrosine phosphorylation site mutants (Y10A and Y10E). Cells were preincubated for 30 min in the absence (C) or presence of 10−8 M insulin (I). Results are expressed as a percentage of control and are means ± SEM from seven independent experiments (*, p < 0.05 vs. control). In each cell line studied, the ouabain-insensitive 86Rb (K) uptake was not altered by insulin.
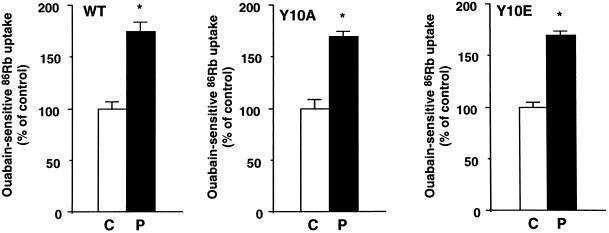
The specificity of the effect of Tyr-10 mutants on the insulin-induced stimulation of ouabain-sensitive 86Rb (K) uptake was assessed by measuring the effect of PKC activation by phorbol esters. As shown in Figure 9, 10−6 M PMA for 30 min stimulated the exogenous Na+,K+-ATPase-mediated 86Rb (K) uptake by ∼80% in OK cells expressing either the WT rat α1-subunit or its Y10A and Y10E mutants.
Figure 9.
Substitution of Tyr-10 by either Ala (Y10A) or Glu (Y10E) does not alter the stimulation of Na+,K+-ATPase by PMA in transfected OK cells. 86Rb (K) uptake was measured in the presence of 5 × 10−6 M ouabain under initial rates of influx in OK cells expressing the WT rat α1-subunit and its tyrosine phosphorylation site mutants (Y10A and Y10E). Cells were preincubated for 30 min in the absence (C) or presence of 10−6 M PMA (P). Results are expressed as a percentage of control and are means ± SEM from six independent experiments (* p < 0.05 vs. control). In each cell line studied, the ouabain-insensitive 86Rb (K) uptake was not altered by PMA.
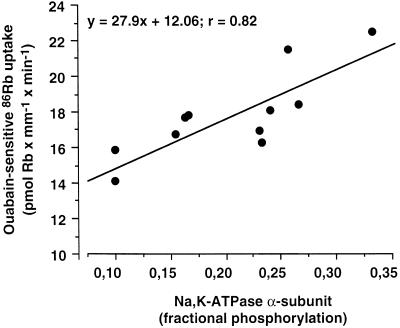
The physiological relevance of tyrosine phosphorylation of the Na+,K+-ATPase α-subunit was further evaluated by comparing the time course and the concentration dependence of the insulin-induced increase in tyrosine phosphorylation to the activity of Na+,K+-ATPase in kidney proximal tubules (Figure 10). The α-subunit signal revealed by immunoblot after immunoprecipitation with the PY20 antibody was expressed as fractional tyrosine phosphorylation of the α-subunit, assuming that 10% of α-subunits are phosphorylated under basal condition (see Figure 5). The increase in fractional tyrosine phosphorylation of the α-subunit and the stimulation of the ouabain-sensitive 86Rb uptake in response to insulin were submaximal after 5 min incubation at 37°C (Figure 10A). The insulin-dependent increase in fractional tyrosine phosphorylation of the α-subunit and stimulation of 86Rb uptake exhibited a similar dose dependence (Figure 10B) with an apparent threshold level close to 10−10 M and a saturation at 10−8 M insulin. To further assess whether there is a possible quantitative relationship between the effects of insulin on the activity of Na+,K+-ATPase and its tyrosine phosphorylation, the changes in ouabain-sensitive 86Rb uptake were plotted as a function of the changes in phosphorylation level of the α-subunit. Figure 11, drawn from the data presented in Figure 10, shows that the level of insulin-dependent tyrosine phosphorylation of Na+,K+-ATPase was linearly correlated (r2 = 0.87) with the stimulatory action of insulin on the pump activity.
Figure 11.
Stimulation of Na+,K+-ATPase activity by insulin is linearly correlated and cosaturates with the tyrosine phosphorylation level of its α-subunit. The ouabain-sensitive 86Rb uptake was plotted as a function of the fractional tyrosine phosphorylation of the α-subunit of Na+,K+-ATPase. Values were from the experiments depicted in Figure 10.
Altogether, these results strongly suggest that phosphorylation of the α-subunit at Tyr-10 participates in the stimulation of Na+,K+-ATPase activity by insulin in kidney proximal tubule cells.
DISCUSSION
The present work shows that phosphorylation of the Na+,K+-ATPase α1-subunit at Tyr-10 is required for the insulin-induced stimulation of its activity in kidney proximal tubule cells.
Several studies using purified Na+,K+-ATPase preparations (Bertorello et al., 1991; Chibalin et al., 1992), transfected cells (Béguin et al., 1994; Fisone et al., 1994), and native nephron segments (Carranza et al., 1996a,b) have shown that the α-subunit of Na+,K+-ATPase is phosphorylated on serine–threonine residues by PKA and PKC. However, the presence of additional phosphorylation sites was suspected because neither removal of the identified phosphorylation sites (Béguin et al., 1994) nor specific PKA or PKC inhibitors (Béguin et al., 1994; Carranza et al., 1996a,b) suppressed completely the basal phosphorylation of the α-subunit in intact cells. The present work demonstrates that, in addition to previously characterized serine–threonine phosphorylation (Bertorello et al., 1991; Chibalin et al., 1992), the Na+,K+-ATPase α-subunit is also phosphorylated on tyrosine residues (Figures 1–3). The tyrosine phosphorylation is located in the cytoplasmic NH2-terminal tail of the α1-subunit at Tyr-10 (Figure 6). This finding is in agreement with the following observations: 1) Tyr-10 is surrounded by acidic residues at positions −2 and +1, positions characterizing a consensus phosphorylation site for nonreceptor tyrosine kinases of the Src family (Zhou et al., 1995); 2) Tyr-10 is conserved in all cloned Na+,K+-ATPase α1-subunits (i.e., the renal isoform); and 3) the closely related gastric H+,K+-ATPase α-subunit is also phosphorylated at Tyr-10 (Togawa et al., 1995).
Assuming that α-subunits immunoprecipitated by anti-phosphotyrosine antibodies are phosphorylated on Tyr-10, the proportion of tyrosine-phosphorylated α-subunits was 10 and 25% of the total α-subunit population in unstimulated and stimulated proximal tubule cells, respectively (Figure 5). The low level of phosphotyrosine detected by phosphoamino acid analysis under stimulated conditions may indicate that 1) α-subunits were also immunoprecipitated through interaction with another tyrosine-phosphorylated protein; or 2) phosphotyrosine has a very slow turnover; therefore incubation with 32P was too short to allow detection of phosphotyrosine under basal conditions. Nevertheless, the large decrease in the amount of α-subunis after immunoprecipitation with anti-phosphotyrosine antibodies together with the abolition of the insulin-induced stimulation of Na+,K+-ATPase after point mutation of Tyr-10 (Figures 6 and 8) strongly suggests that Tyr-10 is indeed phosphorylated.
The stimulation of Na+,K+-ATPase activity by insulin in proximal tubule cells is most likely mediated by α-subunit tyrosine phosphorylation, because 1) substitution of Tyr-10 with Ala (Y10A) abolished the stimulation of ouabain-sensitive 86Rb uptake by insulin in OK cells; 2) the basal exogenous Na+,K+-ATPase-mediated 86Rb uptake is higher in OK cells expressing the Y10E mutant α1-subunit mimicking the effect of the negative charge introduced by phosphorylation; 3) stimulation of ouabain-sensitive 86Rb uptake and phosphorylation of Na+,K+-ATPase occurred with the same time course and within the same range of insulin concentrations (Figure 10) and cosaturated (Figure 11) in rat PCTs; and 4) the proportion of tyrosine-phosphorylated α-subunit under stimulated conditions is large enough (∼25%) to elicit a physiological effect in rat PCTs (Figure 6). Whether tyrosine phosphorylation directly alters the function of Na+,K+-ATPase or acts through phosphotyrosine binding of regulatory protein(s) containing Src homology region 2 or phosphotyrosine-binding (PTB) domains (Koch et al., 1991; Kavanaugh and Williams, 1994) remains to be determined.
The insulin-induced increase in phosphorylation level of the Na+,K+-ATPase α-subunit in proximal tubule cells contrasts with the previously reported α-subunit dephosphorylation in response to insulin in skeletal muscle cells (Ragolia et al., 1997). The different results most likely reflect α-subunit isoform- and cell-specific regulation. In proximal tubule cells, insulin increases the Na+ affinity (Féraille et al., 1994) of the predominantly expressed α1-isoform of Na+,K+-ATPase (McDonough et al., 1994; Lücking et al., 1996), whereas in skeletal muscle cells, insulin increases the cell surface expression (Hundal et al., 1992; Marette et al., 1993) of the predominantly expressed α2-isoform (Hsu and Guidotti, 1991).
An obvious candidate for tyrosine phosphorylation of the Na+,K+-ATPase α-subunit is the insulin receptor itself. Stimulation of the protein-tyrosine kinase activity of the insulin receptor leads to detectable phosphorylation of substrate proteins within 1 min (White et al., 1985; Kadowaki et al., 1987). The time course of insulin-induced phosphorylation of the Na+,K+-ATPase α-subunit (Figure 10) remains compatible with this possibility. Phosphorylation of the α-subunit induced by insulin and the tyrosine-phosphatase inhibitor orthovanadate (Klarlund, 1985) were not additive (Figure 4), suggesting a convergent signaling pathway. Therefore, insulin- and orthovanadate-induced increase in tyrosine phosphorylation of the α-subunit could be secondary to the stimulation of a nonreceptor tyrosine kinase (Elberg et al., 1994; Evans et al., 1994; Sun et al., 1996). Alternatively, insulin may inhibit a tyrosine-phosphatase and prevent dephosphorylation of the Na+,K+-ATPase α-subunit.
In summary, besides serine and threonine phosphorylation, the α-subunit of Na+,K+-ATPase is phosphorylated on a tyrosine residue. The α-subunit tyrosine phosphorylation site is located at Tyr-10, and its integrity is required for the stimulation of Na+,K+-ATPase activity by insulin in proximal tubule cells. Therefore, phosphorylation of the α-subunit at Tyr-10 is likely to play a major role in the control of renal Na+ and fluid reabsorption.
ACKNOWLEDGMENTS
We are greatly indebted to Dr. K.J. Sweadner and Dr. J. Kite for the kind gift McK1 antibody and anti-α-subunit COOH-terminal antibody, respectively. We especially thank Dr. B. Thorens for help in performing phosphoamino acid analysis. This work was supported in part by Swiss National Science Foundation grants 31-40386.94 and 31-50643.97 to H.F. and E.F. and 31-42954.95 to K.G., by National Institutes of Health grant RO1DK53460 to C.P., and by a grant from the Foundation Carlos and Elsie de Reuter to H.F. and E.F.
Abbreviations used:
- DMEM
Dulbecco’s modified Eagle’s medium
- OK
Opossum kidney
- PCT
proximal convoluted tubule
- PMA
phorbol 12-myristate 13-acetate
- TBS
Tris-buffered saline
- WT
wild-type
REFERENCES
- Barlet-Bas C, Khadouri C, Marsy S, Doucet A. Sodium-independent in vitro induction of Na+,K+-ATPase by aldosterone in renal target cells: permissive effect of triiodothyronine. Proc Natl Acad Sci USA. 1987;85:1707–1711. doi: 10.1073/pnas.85.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest. 1987;79:1104–1109. doi: 10.1172/JCI112925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P, Beggah AT, Chibalin AV, Burgener-Kairuz P, Jaisser F, Mathews PM, Rossier BC, Cotecchia S, Geering K. Phosphorylation of the Na+,K+-ATPase α-subunit by protein kinase A and C in vitro and in intact cells. Identification of a novel motif for PKC-mediated phosphorylation. J Biol Chem. 1994;269:24437–24445. [PubMed] [Google Scholar]
- Belusa R, Wang Z-M, Matsubara T, Sahlgen B, Dulubova I, Nairn AC, Ruoslahti E, Greengard P, Aperia A. Mutation of the protein kinase C phosphorylation site on rat α1 Na+,K+-ATPase alters regulation of intracellular Na+ and pH and influences cell shape and adhesiveness. J Biol Chem. 1997;272:20179–20184. doi: 10.1074/jbc.272.32.20179. [DOI] [PubMed] [Google Scholar]
- Beron J, Forster I, Béguin P, Geering K, Verrey F. Phorbol 12-myristate 13-acetate down-regulates Na,K-ATPase independent of its protein kinase C site: decrease in basolateral cell surface area. Mol Biol Cell. 1997;8:387–398. doi: 10.1091/mbc.8.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello AM, Aperia A, Walaas SI, Nairn AC, Greengard P. Phosphorylation of the catalytic subunit of Na+,K+-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci USA. 1991;88:11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello AM, Katz AI. Short-term regulation of renal Na-K-ATPase activity: physiological relevance and cellular mechanisms. Am J Physiol. 1993;265:F743–F755. doi: 10.1152/ajprenal.1993.265.6.F743. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Van Der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimentional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Carranza ML, Féraille E, Favre H. Protein kinase C-dependent phosphorylation of the Na+,K+-ATPase α-subunit in rat kidney cortical tubules. Am J Physiol. 1996a;271:C136–C143. doi: 10.1152/ajpcell.1996.271.1.C136. [DOI] [PubMed] [Google Scholar]
- Carranza ML, Féraille E, Kiroytcheva M, Rousselot M, Favre H. Stimulation of ouabain-sensitive 86Rb+ uptake and Na+,K+-ATPase α-subunit phosphorylation by a cAMP-dependent signaling pathway in intact cells from rat kidney cortex. FEBS Lett. 1996b;396:309–314. doi: 10.1016/0014-5793(96)01121-0. [DOI] [PubMed] [Google Scholar]
- Carranza ML, Rousselot M, Chibalin AV, Bertorello AM, Favre H, Féraille E. Protein kinase A induces recruitment of active Na+,K+-ATPase units to the plasma membrane of rat proximal convoluted tubule. J Physiol. 1998;511:235–243. doi: 10.1111/j.1469-7793.1998.235bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalin AV, Pedemonte CH, Katz AI, Féraille E, Berggren P-O, Bertorello AM. Phosphorylation of the catalytic α-subunit constitutes a triggering signal for Na+,K+-ATPase endocytosis. J Biol Chem. 1998;273:8814–8819. doi: 10.1074/jbc.273.15.8814. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Vasilets LA, Hennekes H, Pralong D, Geering K. Phosphorylation of Na,K-ATPase α-subunits in microsomes and in homogenates of Xenopus oocytes resulting from the stimulation of protein kinase A and protein kinase C. J Biol Chem. 1992;267:22378–22384. [PubMed] [Google Scholar]
- Doucet A, Hus-Citharel A, Morel F. In vitro stimulation of Na-K-ATPase in rat thick ascending limb by dexamethasone. Am J Physiol. 1986;251:F851–F857. doi: 10.1152/ajprenal.1986.251.5.F851. [DOI] [PubMed] [Google Scholar]
- Elberg G, Li J, Shechter Y. Vanadium activates or inhibits receptor and nonreceptor protein tyrosine kinase in cell-free experiments, depending on its oxidation state. J Biol Chem. 1994;269:9521–9527. [PubMed] [Google Scholar]
- Evans GA, Garcia GG, Erwin R, Howard OMZ, Farrar WL. Pervanadate simulates the effects of interleukin-2 (IL-2) in human T cells and provides evidence for the activation of two distinct tyrosine kinase pathways by IL-2. J Biol Chem. 1994;269:23407–23412. [PubMed] [Google Scholar]
- Ewart HS, Klip A. Hormonal regulation of the Na+-K+-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am J Physiol. 1995;269:C295–C311. doi: 10.1152/ajpcell.1995.269.2.C295. [DOI] [PubMed] [Google Scholar]
- Felsenfeld DP, Sweadner KJ. Fine specificity mapping and topography of an isozyme-specific epitope of the Na,K-ATPase catalytic subunit. J Biol Chem. 1988;263:10932–10942. [PubMed] [Google Scholar]
- Féraille E, Carranza ML, Buffin-Meyer B, Rousselot M, Doucet A, Favre H. Protein kinase C-dependent stimulation of Na+-K+-ATPase in rat proximal convoluted tubules. Am J Physiol. 1995;268:C1277–C1283. doi: 10.1152/ajpcell.1995.268.5.C1277. [DOI] [PubMed] [Google Scholar]
- Féraille E, Carranza ML, Rousselot M, Favre H. Insulin enhances sodium sensivity of Na-K-ATPase in isolated rat proximal convoluted tubule. Am J Physiol. 1994;267:F55–F62. doi: 10.1152/ajprenal.1994.267.1.F55. [DOI] [PubMed] [Google Scholar]
- Féraille E, Carranza ML, Rousselot M, Favre H. Modulation of Na+,K+-ATPase activity by tyrosine phosphorylation process in rat proximal convoluted tubule. J Physiol. 1997;498:99–108. doi: 10.1113/jphysiol.1997.sp021844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féraille E, Marsy S, Cheval L, Barlet-Bas C, Khadouri C, Favre H, Doucet A. Sites of antinatriuretic action of insulin along rat nephron. Am J Physiol. 1992;263:F175–F179. doi: 10.1152/ajprenal.1992.263.1.F175. [DOI] [PubMed] [Google Scholar]
- Féraille E, Vogt B, Rousselot M, Barlet-Bas C, Cheval L, Doucet A, Favre H. Mechanism of enhanced Na-K-ATPase activity in cortical collecting duct from rats with nephrotic syndrome. J Clin Invest. 1993;91:1295–1300. doi: 10.1172/JCI116328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feschenko MS, Sweadner KJ. Structural basis for species-specific differences in the phosphorylation of Na,K-ATPase by protein kinase C. J Biol Chem. 1995;270:14072–14077. doi: 10.1074/jbc.270.23.14072. [DOI] [PubMed] [Google Scholar]
- Fisone G, et al. Identification of the phosphorylation site for cAMP-dependent protein kinase on Na+,K+-ATPase and effects of site-directed mutagenesis. J Biol Chem. 1994;269:9368–9373. [PubMed] [Google Scholar]
- Fisone G, Snyder GL, Fryckstedt J, Caplan MJ, Aperia A, Greengard P. Na+,K+-ATPase in the choroid plexus. Regulation by serotonin/protein kinase C pathway. J Biol Chem. 1995;270:2427–2430. doi: 10.1074/jbc.270.6.2427. [DOI] [PubMed] [Google Scholar]
- Girardet M, Geering K, Frantes JM, Geser D, Rossier BC, Kraehenbuhl J-P, Bron C. Immunochemical evidence for a transmembrane orientation of both the Na,K-ATPase subunits. Biochemistry. 1981;20:6684–6691. doi: 10.1021/bi00526a025. [DOI] [PubMed] [Google Scholar]
- Hsu Y-M, Guidotti G. Effects of hypokalemia on the properties and expression of the (Na,K)-ATPase of rat skeletal muscle. J Biol Chem. 1991;266:427–433. [PubMed] [Google Scholar]
- Hundal HS, Marette A, Mitsumoto Y, Ramlal T, Blostein R, Klip A. Insulin induces translocation of the α2 and β1 subunits of the Na+/K +-ATPase from intracellular compartments to the plasma membrane in mammalian skeletal muscle. J Biol Chem. 1992;267:5040–5043. [PubMed] [Google Scholar]
- Jewell EA, Lingrel JB. Comparison of the substrate dependence properties of the rat Na,K-ATPase α1, α2, and α3 isoforms expressed in HeLa cells. J Biol Chem. 1991;266:16925–16930. [PubMed] [Google Scholar]
- Jorgensen PL, Collins JH. Tryptic and chemotryptic cleavage sites in sequence of α-subunit of Na+,K+-ATPase from outer medulla of mammalian kidney. Biochim Biophys Acta. 1986;860:570–576. doi: 10.1016/0005-2736(86)90555-9. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Koyasu S, Nishida E, Tobe K, Izumi T, Takaku F, Sakai H, Yahara I, Kasuga M. Tyrosine phosphorylation of common and specific sets of cellular proteins rapidly induced by insulin, insulin-like growth factor I, and epidermal growth factor in an intact cell. J Biol Chem. 1987;262:7342–7350. [PubMed] [Google Scholar]
- Kavanaugh WM, Williams LT. An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science. 1994;266:1862–1865. doi: 10.1126/science.7527937. [DOI] [PubMed] [Google Scholar]
- Klarlund JK. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985;41:707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Koch CA, Anderson D, Moran M, Ellis C, Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Lücking K, Nielsen JM, Pedersen PA, Jorgensen PL. Na-K-ATPase isoform (α3, α2, α1) abundance in rat kidney estimated by competitive RT-PCR and ouabain binding. Am J Physiol. 1996;271:F253–F260. doi: 10.1152/ajprenal.1996.271.2.F253. [DOI] [PubMed] [Google Scholar]
- Marette A, Krischer J, Lavoie L, Ackerlay C, Carpentier J-L, Klip A. Insulin increases the Na+-K+-ATPase α2-subunit in the surface of rat skeletal muscle: morphological evidence. Am J Physiol. 1993;265:C1716–C1722. doi: 10.1152/ajpcell.1993.265.6.C1716. [DOI] [PubMed] [Google Scholar]
- McDonough AA, Magyar CE, Komatsu Y. Expression of Na+-K+-ATPase α- and β-subunits along rat nephron: isoform specificity and response to hypokalemia. Am J Physiol. 1994;267:C901–C908. doi: 10.1152/ajpcell.1994.267.4.C901. [DOI] [PubMed] [Google Scholar]
- Middleton JP, Khan WA, Collinsworth G, Hannun YA, Medford RM. Heterogeneity of protein kinase C-mediated rapid regulation of Na/K-ATPase in kidney epithelial cells. J Biol Chem. 1993;268:15958–15964. [PubMed] [Google Scholar]
- Nelson RM, Long GL. A general method of site-specific mutagenesis using a modification of the Thermus aquaticus PCR. Anal Biochem. 1989;180:147–151. doi: 10.1016/0003-2697(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Palmer LG, Antonian L, Frindt G. Regulation of the Na-K pump of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993;102:43–57. doi: 10.1085/jgp.102.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte CH, Pressley TA, Lokhandwala MF, Cinelli AR. Regulation of Na,K-ATPase transport activity by protein kinase C. J Membr Biol. 1997;155:219–227. doi: 10.1007/s002329900174. [DOI] [PubMed] [Google Scholar]
- Ragolia L, Cherpalis B, Srinivasan M, Begum N. Role of serine/threonine protein phosphatases in insulin regulation of Na+,K+-ATPase activity in cultured rat skeletal muscle cells. J Biol Chem. 1997;272:23653–23658. doi: 10.1074/jbc.272.38.23653. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Pons S, Asano T, Myers MG, Jr, Glasheen E, White MF. The Fyn tyrosine kinase binds IRS-1 and forms a distinct signaling complex during insulin stimulation. J Biol Chem. 1996;271:10583–10587. doi: 10.1074/jbc.271.18.10583. [DOI] [PubMed] [Google Scholar]
- Togawa K, Ishiguro T, Kaya S, Shimada A, Imagawa T, Tanigushi K. Reversible phosphorylation of both Tyr7 and Tyr10 in the α-chain of pig stomach H,K-ATPase by a membrane bound kinase and a phosphatase. J Biol Chem. 1995;270:15475–15478. doi: 10.1074/jbc.270.26.15475. [DOI] [PubMed] [Google Scholar]
- White MF, Maron R, Kahn CR. Insulin rapidly stimulates tyrosine phosphorylation of a M-185,000 protein in intact cells. Nature. 1985;318:183–187. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Zhou S, et al. Catalytic specificity of protein tyrosine kinases is critical for selective signaling. Nature. 1995;373:536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]