Abstract
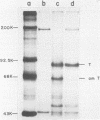
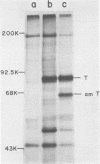
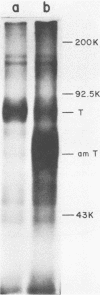
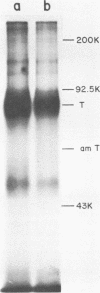
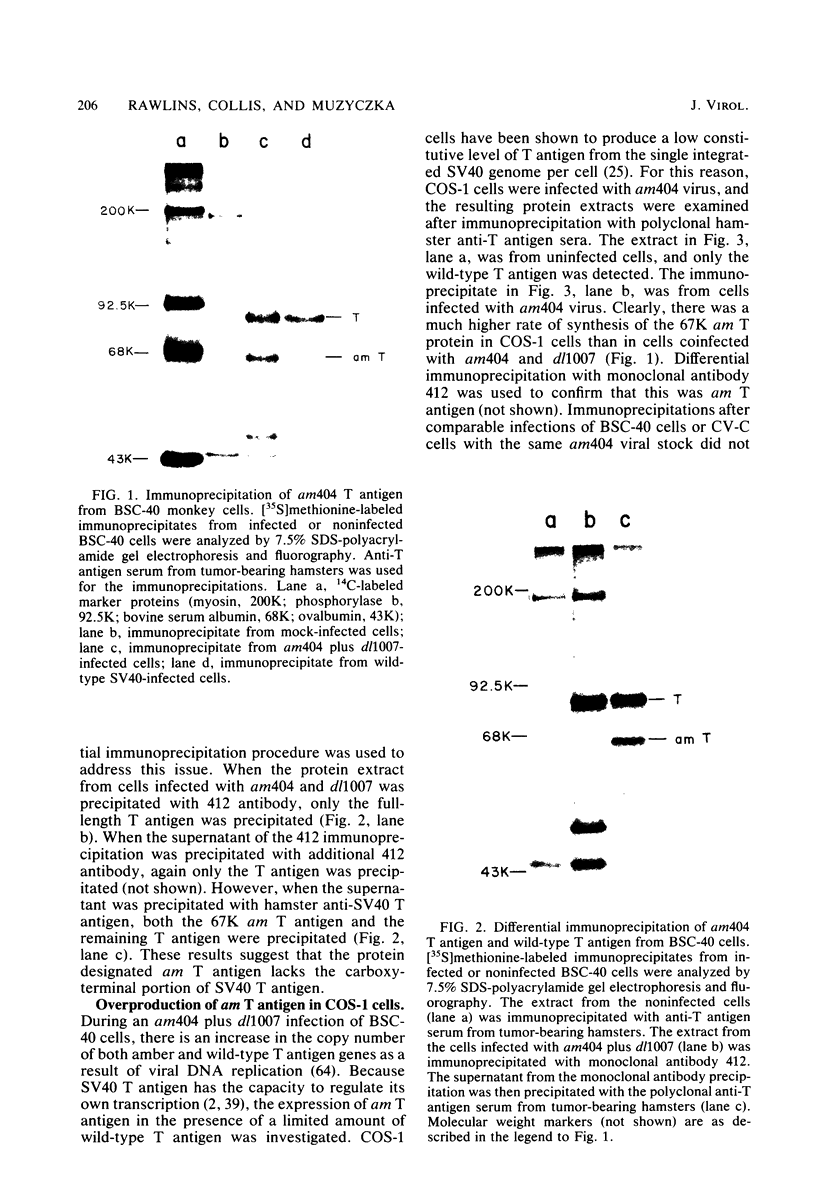
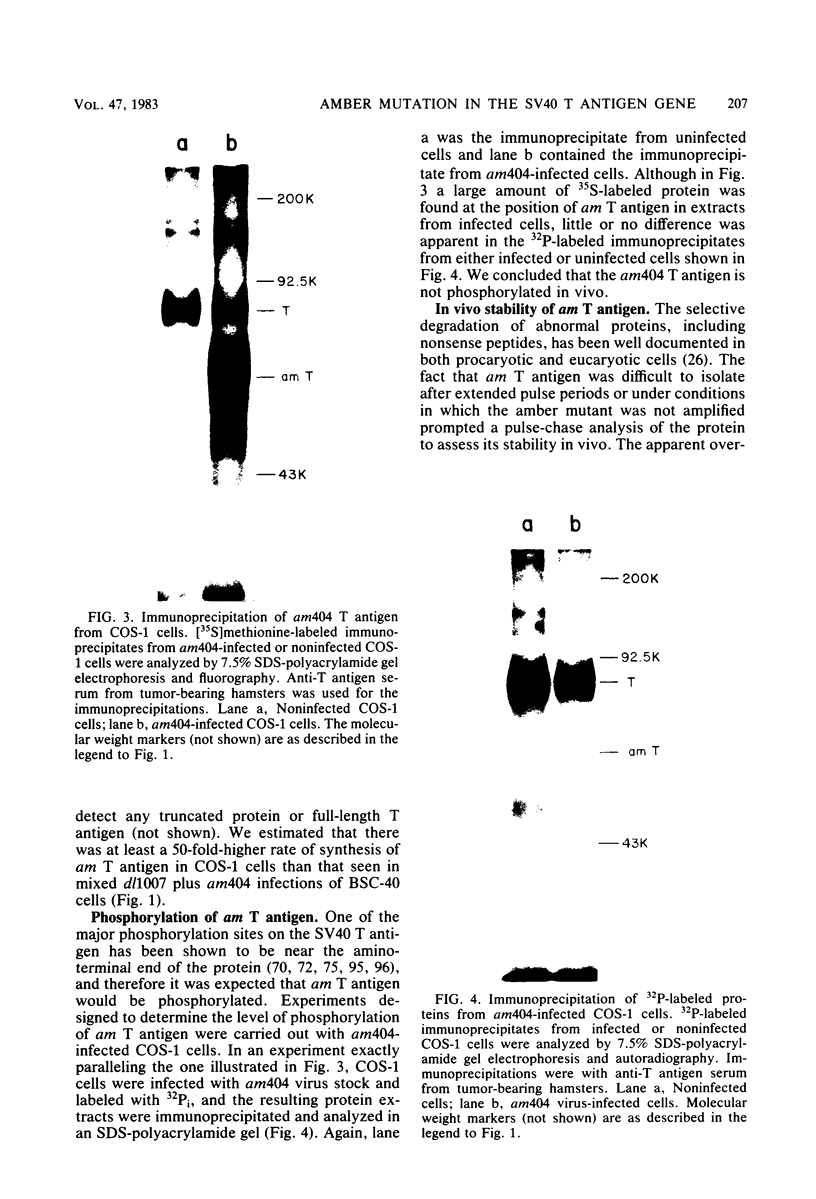
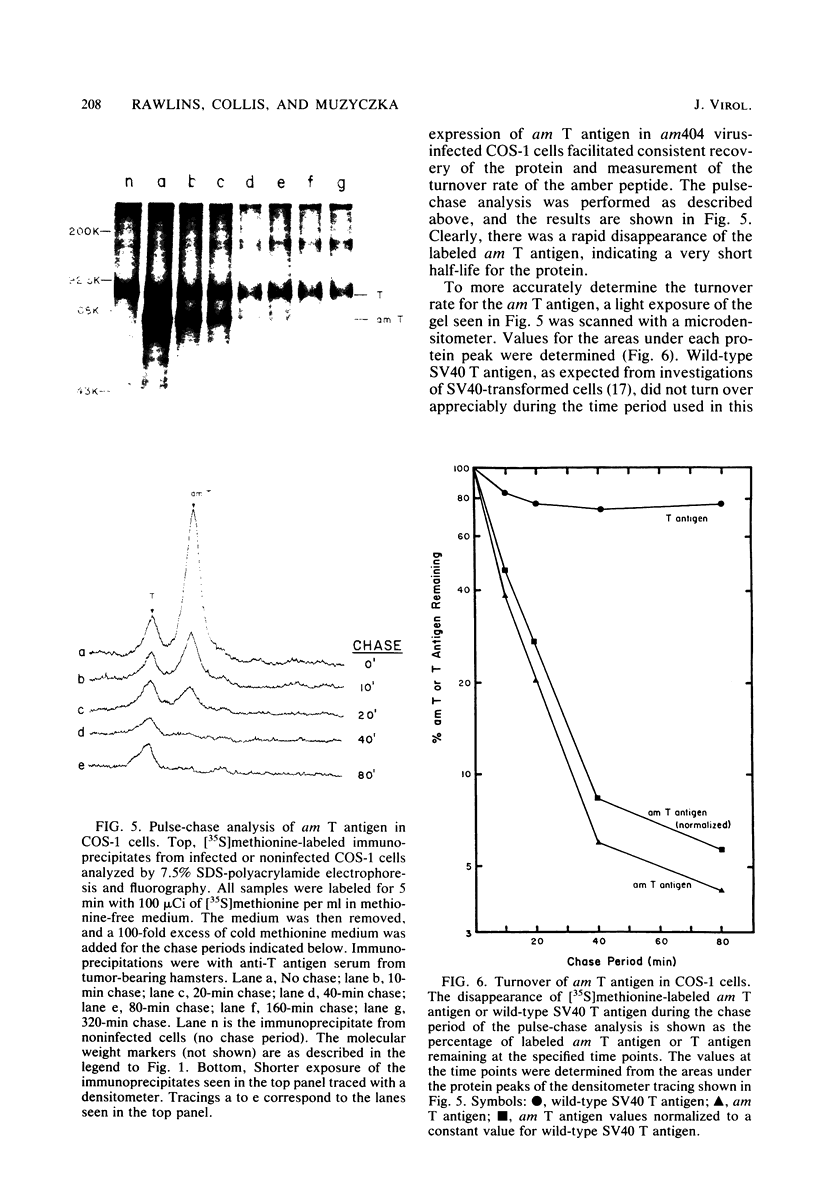
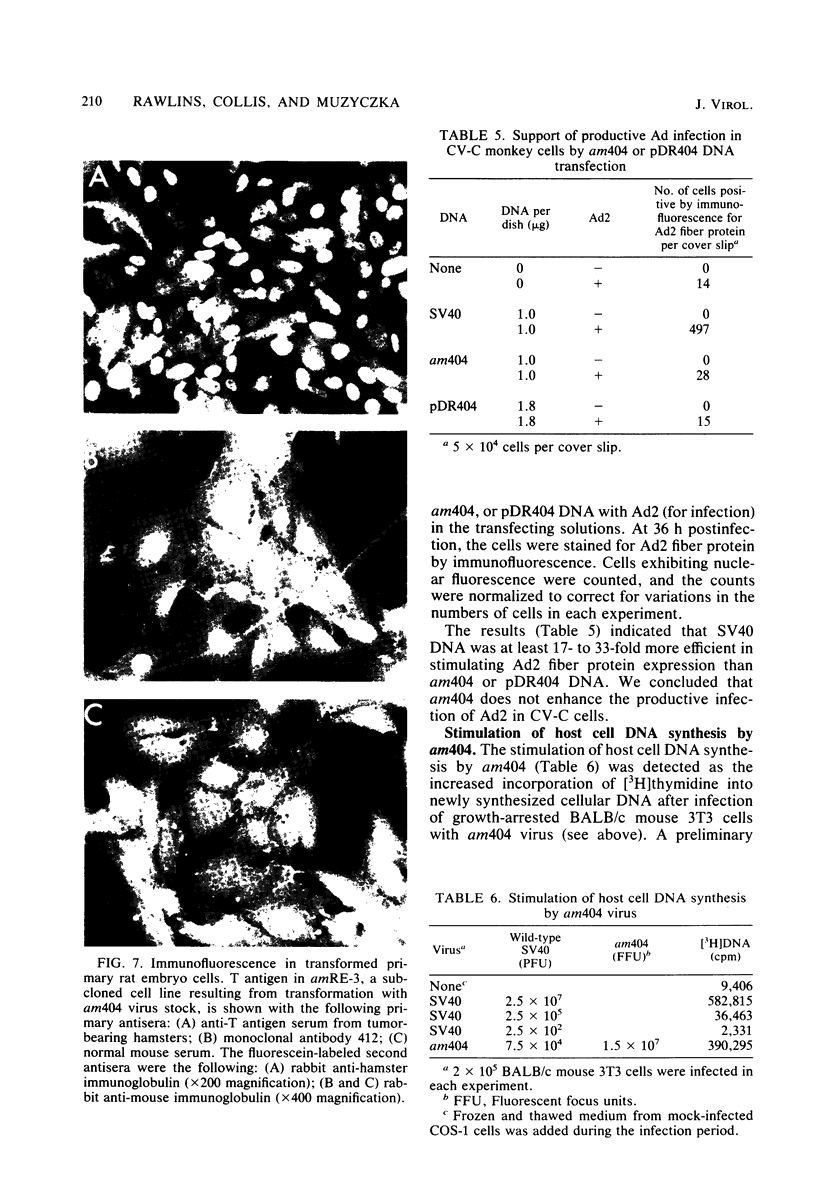
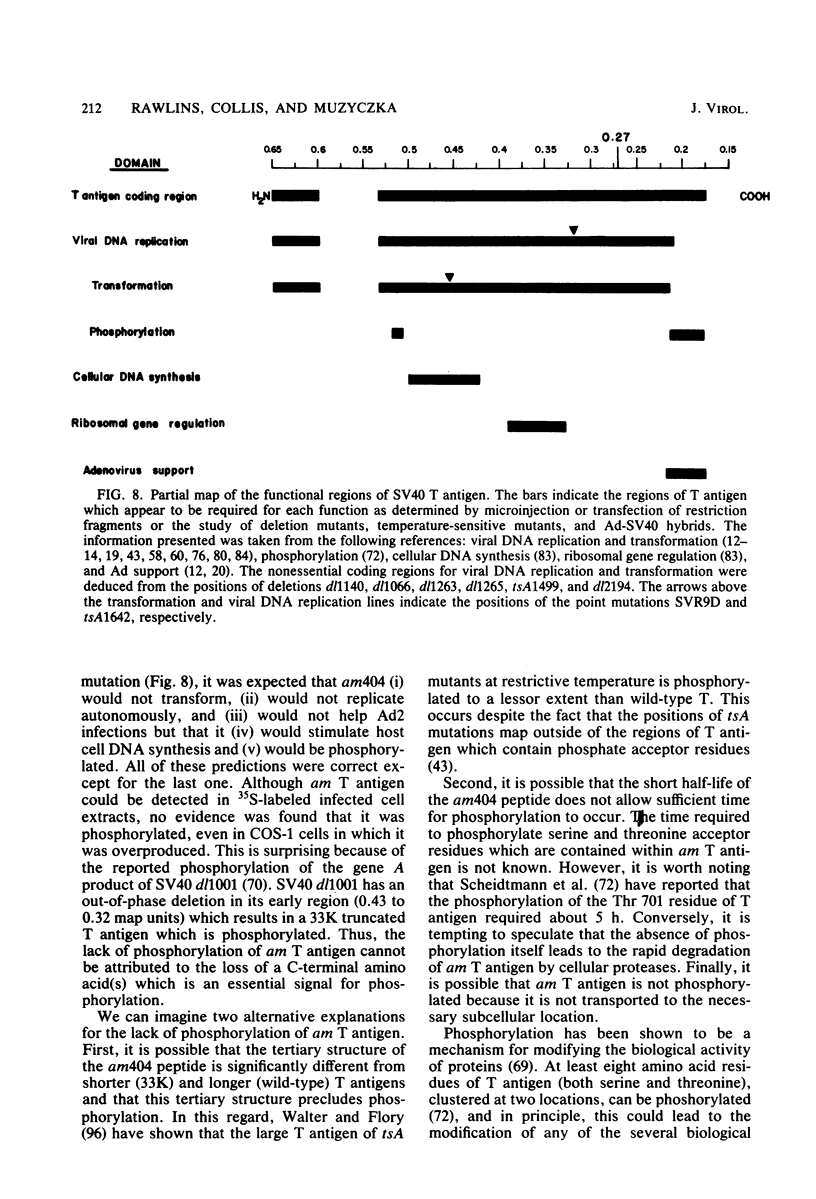
We analyzed the biological activity of an amber mutation, am404, at map position 0.27 in the T antigen gene of simian virus 40. Immunoprecipitation of extracts from am404-infected cells demonstrated the presence of an amber protein fragment (am T antigen) of the expected molecular weight (67,000). Differential immunoprecipitation with monoclonal antibody demonstrated that am T antigen was missing the carboxy-terminal antigenic determinants. The amber mutant was shown to be defective for most of the functions associated with wild-type T antigen. The mutant did not replicate autonomously, but this defect could be complemented by a helper virus (D. R. Rawlins and N. Muzyczka, J. Virol. 36:611-616, 1980). The mutant failed to transform nonpermissive rodent cells and did not relieve the host range restriction of adenovirus 2 in monkey cells. However, stimulation of host cell DNA, whose functional region domain has been mapped within that portion of the protein synthesized by the mutant, could be demonstrated in am404-infected cells. A number of unexpected observations were made. First, the am T antigen was produced in unusually large amounts in a simian virus 40-transformed monkey cell line (COS-1), but overproduction was not seen in nontransformed monkey cells regardless of whether or not a helper virus was present. This feature of the mutant was presumably the result of the inability of am T antigen to autoregulate, the level of wild-type T antigen in COS-1 cells, and the unusually short half-life of am T antigen in vivo. Pulse-chase experiments indicated that am T antigen had an intracellular half-life of approximately 10 min. In addition, although the am T antigen retained the major phosphorylation site found in simian virus 40 T antigen, it was not phosphorylated. Thus, phosphorylation of simian virus 40 T antigen is not required for the stimulation of host cell DNA synthesis. Finally, fusion of am404-infected monkey cells with Escherichia coli protoplasts containing appropriate procaryotic suppressor tRNAs showed that am404 is a suppressible nonsense mutation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Brockman W. W., Nathans D. The isolation of simian virus 40 variants with specifically altered genomes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):942–946. doi: 10.1073/pnas.71.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Haar R. A., Capecchi N. E., Sveda M. M. The isolation of a suppressible nonsense mutant in mammalian cells. Cell. 1977 Oct;12(2):371–381. doi: 10.1016/0092-8674(77)90113-1. [DOI] [PubMed] [Google Scholar]
- Carbon J., Shenk T. E., Berg P. Biochemical procedure for production of small deletions in simian virus 40 DNA. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1392–1396. doi: 10.1073/pnas.72.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Temple G. F., Trecartin R. F., Kan Y. W. Suppression of the nonsense mutation in homozygous beta 0 thalassaemia. Nature. 1979 Oct 18;281(5732):602–603. doi: 10.1038/281602a0. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Crawford L. V., Berg P. Simian virus 40 mutants with deletions at the 3' end of the early region are defective in adenovirus helper function. J Virol. 1979 Jun;30(3):683–691. doi: 10.1128/jvi.30.3.683-691.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D. J., Tevethia M. J. Characterization of a temperature-sensitive, DNA-positive, nontransforming mutant of simian virus 40. Virology. 1981 Jul 30;112(2):605–624. doi: 10.1016/0042-6822(81)90306-8. [DOI] [PubMed] [Google Scholar]
- Cremer K. J., Bodemer M., Summers W. P., Summers W. C., Gesteland R. F. In vitro suppression of UAG and UGA mutants in the thymidine kinase gene of herpes simplex virus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):430–434. doi: 10.1073/pnas.76.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Gurney E. G., Harrison R. O. Monoclonal antibodies against simian virus 40 tumor antigens: analysis of antigenic binding sites, using adenovirus type 2-simian virus 40 hybrid viruses. J Virol. 1981 Jan;37(1):478–482. doi: 10.1128/jvi.37.1.478-482.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A., Khoury G., Martin R. G. Phosphorylation of T-antigen and control T-antigen expression in cells transformed by wild-type and tsA mutants of simian virus 40. J Virol. 1979 Feb;29(2):753–762. doi: 10.1128/jvi.29.2.753-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Carmichael G., Nicolas J. C., Kress M. Mutant carrying deletions in the two simian virus 40 early genes. J Virol. 1981 Dec;40(3):625–634. doi: 10.1128/jvi.40.3.625-634.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey G., Lewis J. B., Grodzicker T., Bothwell A. Characterization of a fused protein specified by the adenovirus type 2-simian virus 40 hybrid Ad2+ND1 dp2. J Virol. 1979 Apr;30(1):201–217. doi: 10.1128/jvi.30.1.201-217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. P., Lyons M. J., Ginsberg H. S. Biochemical consequences of type 2 adenovirus and Simian virus 40 double infections of African green monkey kidney cells. J Virol. 1970 May;5(5):586–597. doi: 10.1128/jvi.5.5.586-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Galanti N., Jonak G. J., Soprano K. J., Floros J., Kaczmarek L., Weissman S., Reddy V. B., Tilghman S. M., Baserga R. Characterization and biological activity of cloned simian virus 40 DNA fragments. J Biol Chem. 1981 Jun 25;256(12):6469–6474. [PubMed] [Google Scholar]
- Gesteland R. F., Wills N., Lewis J. B., Grodzicker T. Identification of amber and ochre mutants of the human virus Ad2+ND1. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4567–4571. doi: 10.1073/pnas.74.10.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldman N., Brown M., Khoury G. Modification of SV40 T antigen by poly ADP-ribosylation. Cell. 1981 May;24(2):567–572. doi: 10.1016/0092-8674(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Simian virus 40 large T antigen isoelectric focuses as multiple species with varying phosphate content. Virology. 1979 Dec;99(2):413–416. doi: 10.1016/0042-6822(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spangler G., Livingston D. M. Protein kinase activity associated with simian virus 40 T antigen. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2610–2614. doi: 10.1073/pnas.76.6.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Lewis J. B., Anderson C. W. Conditional lethal mutants of adenovirus type 2-simian virus 40 hybrids. II. Ad2+ND1 host-range mutants that synthesize fragments of the Ad2+ND1 30K protein. J Virol. 1976 Aug;19(2):559–571. doi: 10.1128/jvi.19.2.559-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. I. In permissive cells. J Virol. 1979 May;30(2):590–599. doi: 10.1128/jvi.30.2.590-599.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. II. In nonpermissive cells. J Virol. 1981 Feb;37(2):802–812. doi: 10.1128/jvi.37.2.802-812.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak R. M., Laski F. A., RajBhandary U. L., Sharp P. A., Capecchi M. R. Establishment of mammalian cell lines containing multiple nonsense mutations and functional suppressor tRNA genes. Cell. 1982 Nov;31(1):137–146. doi: 10.1016/0092-8674(82)90413-5. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J Virol. 1973 Sep;12(3):643–652. doi: 10.1128/jvi.12.3.643-652.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M., Resche-Rigon M., Feunteun J. Phosphorylation pattern of large T antigens in mouse cells infected by simian virus 40 wild type or deletion mutants. J Virol. 1982 Sep;43(3):761–771. doi: 10.1128/jvi.43.3.761-771.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Belagaje R., RajBhandary U. L., Sharp P. A. An amber suppressor tRNA gene derived by site-specific mutagenesis: cloning and function in mammalian cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5813–5817. doi: 10.1073/pnas.79.19.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Mann K., Hunter T. Phosphorylation of SV40 large T antigen in SV40 nucleoprotein complexes. Virology. 1980 Dec;107(2):526–532. doi: 10.1016/0042-6822(80)90320-7. [DOI] [PubMed] [Google Scholar]
- May P., May E., Bordé J. Stimulation of cellular RNA synthesis in mouse-kidney cell cultures infected with SV40 virus. Exp Cell Res. 1976 Jul;100(2):433–436. doi: 10.1016/0014-4827(76)90175-0. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- McKay R., DiMaio D. Binding of an SV40 T antigen-related protein to the DNA of SV40 regulatory mutants. Nature. 1981 Feb 26;289(5800):810–813. doi: 10.1038/289810a0. [DOI] [PubMed] [Google Scholar]
- Mellor A., Smith A. E. Characterization of the amino-terminal tryptic peptide of simian virus 40 small-t and large-T antigens. J Virol. 1978 Dec;28(3):992–996. doi: 10.1128/jvi.28.3.992-996.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Henning R. Simian virus 40 T-antigen phosphorylation is variable. FEBS Lett. 1980 May 19;114(1):107–110. doi: 10.1016/0014-5793(80)80870-2. [DOI] [PubMed] [Google Scholar]
- Mueller C., Graessmann A., Graessmann M. Mapping of early SV40-specific functions by microinjection of different early viral DNA fragments. Cell. 1978 Oct;15(2):579–585. doi: 10.1016/0092-8674(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Muzyczka N. Construction of an SV40-derived cloning vector. Gene. 1980 Oct;11(1-2):63–77. doi: 10.1016/0378-1119(80)90087-6. [DOI] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Induction of cellular mRNA synthesis in BSC-1 cells infected by SV40. Virology. 1968 Jul;35(3):439–444. doi: 10.1016/0042-6822(68)90222-5. [DOI] [PubMed] [Google Scholar]
- Oren M., Winocour E., Prives C. Differential affinities of simian virus 40 large tumor antigen for DNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):220–224. doi: 10.1073/pnas.77.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W., Pipas J. M., Pearson-White S., Nathans D. Isolation of mutants of an animal virus in bacteria. Science. 1980 Sep 19;209(4463):1392–1396. doi: 10.1126/science.6251547. [DOI] [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G., Thimmappaya B., Swerdlow B., Shenk T. SV40 mutant tsA1499 is heat-sensitive for lytic growth but generates cold-sensitive rat-cell transformants. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):305–309. doi: 10.1101/sqb.1980.044.01.035. [DOI] [PubMed] [Google Scholar]
- Pipas J. M., Adler S. P., Peden K. W., Nathans D. Deletion mutants of SV40 that affect the structure of viral tumor antigens. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):285–291. doi: 10.1101/sqb.1980.044.01.032. [DOI] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Rawlins D. R., Muzyczka N. Construction of a specific amber codon in the simian virus 40 T-antigen gene by site-directed mutagenesis. J Virol. 1980 Nov;36(2):611–616. doi: 10.1128/jvi.36.2.611-616.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Tevethia S. S., Tevethia M. J., Weissman S. M. Nonselective expression of simian virus 40 large tumor antigen fragments in mouse cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2064–2067. doi: 10.1073/pnas.79.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Ferguson J., Davis R. W., Stark G. R. T antigen binds to simian virus 40 DNA at the origin of replication. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1605–1609. doi: 10.1073/pnas.72.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. 3. Characterization of a temperature-sensitive mutant blocked at an early stage of productive infection in monkey cells. J Virol. 1972 Jun;9(6):956–968. doi: 10.1128/jvi.9.6.956-968.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Kaiser A., Carbone A., Walter G. Phosphorylation of threonine in the proline-rich carboxy-terminal region of simian virus 40 large T antigen. J Virol. 1981 Apr;38(1):59–69. doi: 10.1128/jvi.38.1.59-69.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutzbank T., Robinson R., Oren M., Levine A. J. SV40 large tumor antigen can regulate some cellular transcripts in a positive fashion. Cell. 1982 Sep;30(2):481–490. doi: 10.1016/0092-8674(82)90245-8. [DOI] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Scott W. A., Brockman W. W., Nathans D. Biological activities of deletion mutants of simian virus 40. Virology. 1976 Dec;75(2):319–334. doi: 10.1016/0042-6822(76)90031-3. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Shaw S. B., Tegtmeyer P. Binding of dephosphorylated A protein to SV40 DNA. Virology. 1981 Nov;115(1):88–96. doi: 10.1016/0042-6822(81)90091-x. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano K. J., Dev V. G., Croce C. M., Baserga R. Reactivation of silent rRNA genes by simian virus 40 in human-mouse hybrid cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3885–3889. doi: 10.1073/pnas.76.8.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano K. J., Galanti N., Jonak G. J., McKercher S., Pipas J. M., Peden K. W., Baserga R. Mutational analysis of simian virus 40 T antigen: stimulation of cellular DNA synthesis and activation of rRNA genes by mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):214–219. doi: 10.1128/mcb.3.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R. Mutant of simian virus 40 large T-antigen that is defective for viral DNA synthesis, but competent for transformation of cultured rat cells. J Virol. 1982 Jun;42(3):854–864. doi: 10.1128/jvi.42.3.854-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Dozy A. M., Roy K. L., Kan Y. W. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for beta-thalassaemia. Nature. 1982 Apr 8;296(5857):537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Robbins A. Enzymatic activities associated with a purified simian virus 40 T antigen-related protein. Proc Natl Acad Sci U S A. 1979 Feb;76(2):610–614. doi: 10.1073/pnas.76.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Takemoto K. K. "Rescued" SV40: increased transforming efficiency in mouse and human cells. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1031–1037. doi: 10.1073/pnas.62.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Phosphorylation patterns of tumour antigens in cells lytically infected or transformed by simian virus 40. J Virol. 1981 Oct;40(1):28–44. doi: 10.1128/jvi.40.1.28-44.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Flory P. J., Jr Phosphorylation of SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):165–169. doi: 10.1101/sqb.1980.044.01.019. [DOI] [PubMed] [Google Scholar]
- Weiss R. L. Methods for protoplast formation in Escherichia coli. Methods Cell Biol. 1978;20:141–147. doi: 10.1016/s0091-679x(08)62016-6. [DOI] [PubMed] [Google Scholar]
- Zorn G. A., Anderson C. W. Adenovirus type 2 expresses fiber in monkey-human hybrids and reconstructed cells. J Virol. 1981 Feb;37(2):759–769. doi: 10.1128/jvi.37.2.759-769.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]