Abstract
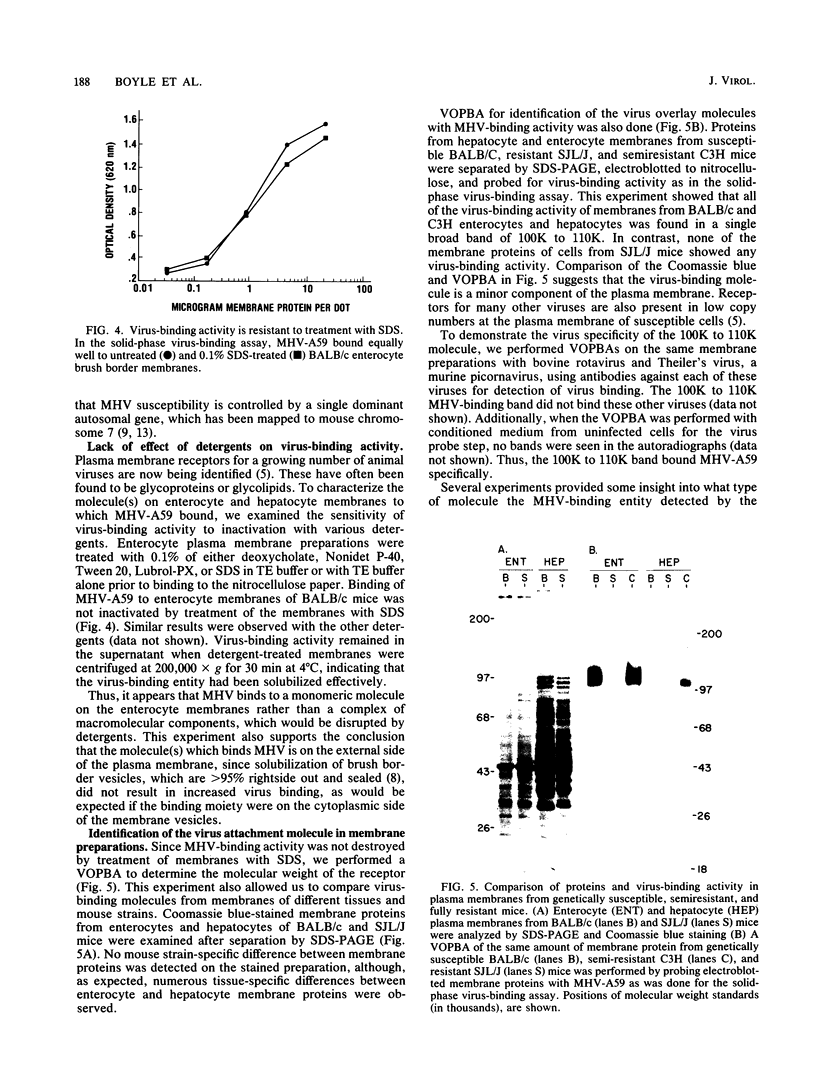
The molecular mechanism of genetic resistance of inbred mouse strains to mouse hepatitis virus, a murine coronavirus, was studied by comparing virus binding to plasma membranes of intestinal epithelium or liver from susceptible BALB/c and resistant SJL/J mice with a new solid-phase assay for virus-binding activity. Virus bound to isolated membranes from susceptible mice, but not to membranes from resistant mice. F1 progeny of SJL/J X BALB/c mice had an intermediate level of virus-binding activity on their enterocyte and hepatocyte membranes. This correlated well with previous studies showing that susceptibility to mouse hepatitis virus strain A59 is controlled by a single autosomal dominant gene (M. S. Smith, R. E. Click, and P. G. W. Plagemann, J. Immunol. 133:428-432). Because virus binding was not prevented by treating membranes with sodium dodecyl sulfate, the virus-binding molecule could be identified by a virus overlay protein blot assay. Virus bound to a single broad band of Mr 100,000 to 110,000 in membranes from hepatocytes or enterocytes of susceptible BALB/c and semisusceptible C3H mice, but no virus-binding band was detected in comparable preparations of resistant SJL/J mouse membranes. Therefore, SJL/J mice may be resistant to mouse hepatitis virus A59 infection because they lack a specific virus receptor which is present on the plasma membranes of target cells from genetically susceptible BALB/c and semisusceptible C3H mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Baechi T., Haller O. Adult mouse hepatocytes in primary monolayer culture express genetic resistance to mouse hepatitis virus type 3. J Immunol. 1982 Sep;129(3):1275–1281. [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Smith A. L. Mouse hepatitis virus strain--related patterns of tissue tropism in suckling mice. Arch Virol. 1984;81(1-2):103–112. doi: 10.1007/BF01309300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Hirano N., Murakami T., Taguchi F., Fujiwara K., Matumoto M. Comparison of mouse hepatitis virus strains for pathogenicity in weanling mice infected by various routes. Arch Virol. 1981;70(1):69–73. doi: 10.1007/BF01320795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Welsh R. M., Haspel M. V. Natural cytotoxicity against mouse hepatitis virus-infected target cells. I. Correlation of cytotoxicity with virus binding to leukocytes. J Immunol. 1986 Feb 15;136(4):1446–1453. [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Haspel M. V., Oldstone M. B. Mouse hepatitis virus type 4 (JHM strains). induced fatal central nervous system disease. I. genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981 Apr 1;153(4):832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzystyniak K., Dupuy J. M. Early interaction between mouse hepatitis virus 3 and cells. J Gen Virol. 1981 Nov;57(Pt 1):53–61. doi: 10.1099/0022-1317-57-1-53. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Smith M. S., Click R. E., Plagemann P. G. Control of mouse hepatitis virus replication in macrophages by a recessive gene on chromosome 7. J Immunol. 1984 Jul;133(1):428–432. [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Takemoto K. K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972 Oct;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Kawamura S., Fujiwara K. Replication of mouse hepatitis viruses with high and low virulence in cultured hepatocytes. Infect Immun. 1983 Feb;39(2):955–959. doi: 10.1128/iai.39.2.955-959.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Woyciechowska J. L., Trapp B. D., Patrick D. H., Shekarchi I. C., Leinikki P. O., Sever J. L., Holmes K. V. Acute and subacute demyelination induced by mouse hepatitis virus strain A59 in C3H mice. J Exp Pathol. 1984 Fall;1(4):295–306. [PubMed] [Google Scholar]