Abstract
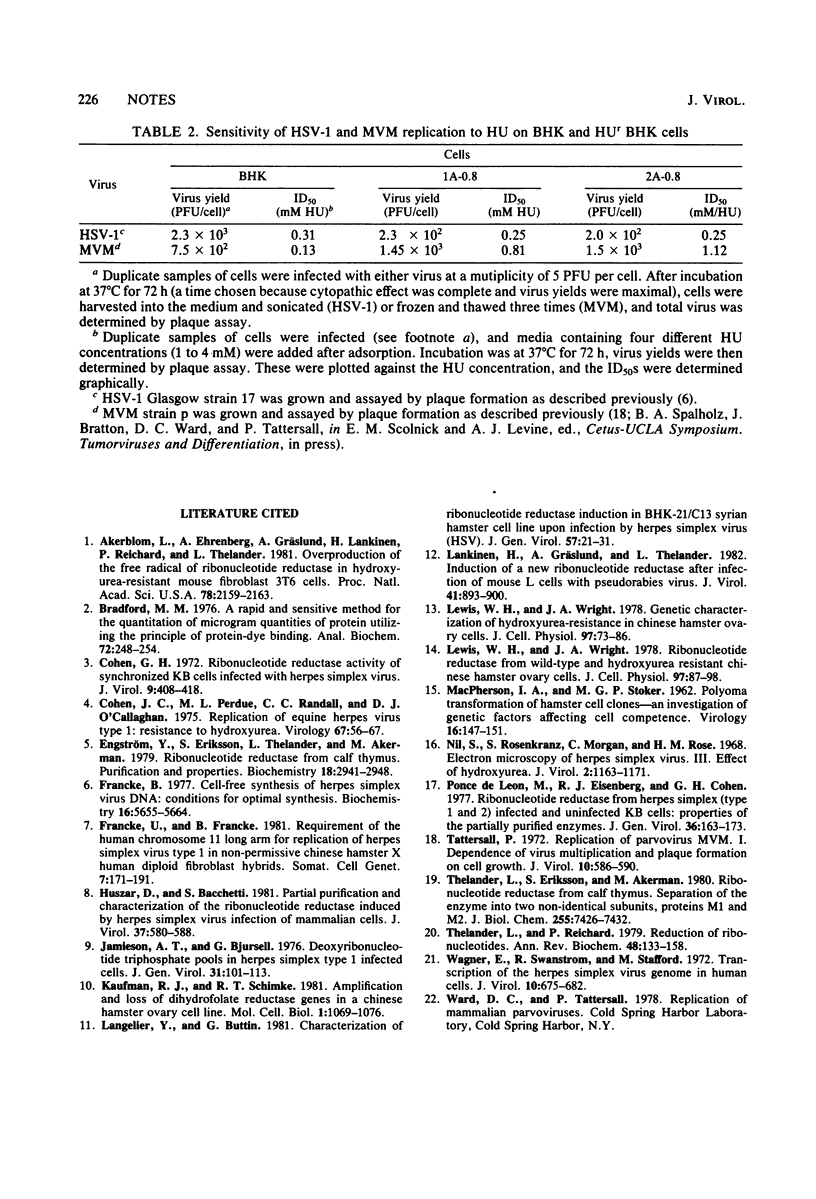
Hydroxyurea-resistant (HUr) baby hamster kidney cells were isolated, subcloned, and characterized. One clonal line, which contained elevated levels of ribonucleotide reductase, lost its HU resistance during passage in the absence of the inhibitor, whereas another clonal line was stably resistant. The replication of herpes simplex virus type 1 on these cells was compared with that of the parvovirus minute virus of mice. Herpes simplex virus type 1 was found to be as sensitive to HU on both lines of HUr baby hamster kidney cells as it was on parental (HU-sensitive) cells, whereas parvovirus replication was about eight times more resistant on HUr baby hamster kidney cells compared with the parental cells. The results suggest that herpes simplex virus type 1 cannot use the cellular reductase and may code for its own.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom L., Ehrenberg A., Gräslund A., Lankinen H., Reichard P., Thelander L. Overproduction of the free radical of ribonucleotide reductase in hydroxyurea-resistant mouse fibroblast 3T6 cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2159–2163. doi: 10.1073/pnas.78.4.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Perdue M. L., Randall C. C., O'Callaghan D. J. Replication of equine herpesvirus type I: resistance to hydroxyurea. Virology. 1975 Sep;67(1):56–67. doi: 10.1016/0042-6822(75)90402-x. [DOI] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Thelander L., Akerman M. Ribonucleotide reductase from calf thymus. Purification and properties. Biochemistry. 1979 Jul 10;18(14):2941–2948. doi: 10.1021/bi00581a004. [DOI] [PubMed] [Google Scholar]
- Francke B. Cell-free synthesis of herpes simplex virus DNA: conditions for optimal synthesis. Biochemistry. 1977 Dec 27;16(26):5655–5664. doi: 10.1021/bi00645a001. [DOI] [PubMed] [Google Scholar]
- Francke U., Francke B. Requirement of the human chromosome 11 long arm for replication of herpes simplex virus type 1 in nonpermissive Chinese hamster x human diploid fibroblast hybrids. Somatic Cell Genet. 1981 Mar;7(2):171–191. doi: 10.1007/BF01567656. [DOI] [PubMed] [Google Scholar]
- Huszar D., Bacchetti S. Partial purification and characterization of the ribonucleotide reductase induced by herpes simplex virus infection of mammalian cells. J Virol. 1981 Feb;37(2):580–588. doi: 10.1128/jvi.37.2.580-588.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Bjursell G. Deoxyribonucleoside triphosphate pools in herpes simplex type 1 infected cells. J Gen Virol. 1976 Apr;31(1):101–113. doi: 10.1099/0022-1317-31-1-101. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Schimke R. T. Amplification and loss of dihydrofolate reductase genes in a Chinese hamster ovary cell line. Mol Cell Biol. 1981 Dec;1(12):1069–1076. doi: 10.1128/mcb.1.12.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier Y., Buttin G. Characterization of ribonucleotide reductase induction in BHK-21/C13 Syrian hamster cell line upon infection by herpes simplex virus (HSV). J Gen Virol. 1981 Nov;57(Pt 1):21–31. doi: 10.1099/0022-1317-57-1-21. [DOI] [PubMed] [Google Scholar]
- Lankinen H., Gräslund A., Thelander L. Induction of a new ribonucleotide reductase after infection of mouse L cells with pseudorabies virus. J Virol. 1982 Mar;41(3):893–900. doi: 10.1128/jvi.41.3.893-900.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. H., Wright J. A. Genetic characterization of hydroxyurea-resistance in Chinese hamster ovary cells. J Cell Physiol. 1978 Oct;97(1):73–85. doi: 10.1002/jcp.1040970108. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Wright J. A. Ribonucleotide reductase from wild type and hydroxyurea-resistant chinese hamster ovary cells. J Cell Physiol. 1978 Oct;97(1):87–97. doi: 10.1002/jcp.1040970109. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Nii S., Rosenkranz H. S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. 3. Effect of hydroxyurea. J Virol. 1968 Oct;2(10):1163–1171. doi: 10.1128/jvi.2.10.1163-1171.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon M., Eisenberg R. J., Cohen G. H. Ribonucleotide reductase from herpes simplex virus (types 1 and 2) infected and uninfected KB cells: properties of the partially purified enzymes. J Gen Virol. 1977 Jul;36(1):163–173. doi: 10.1099/0022-1317-36-1-163. [DOI] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Eriksson S., Akerman M. Ribonucleotide reductase from calf thymus. Separation of the enzyme into two nonidentical subunits, proteins M1 and M2. J Biol Chem. 1980 Aug 10;255(15):7426–7432. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Swanstrom R. I., Stafford M. G. Transcription of the herpes simplex virus genome in human cells. J Virol. 1972 Oct;10(4):675–682. doi: 10.1128/jvi.10.4.675-682.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


