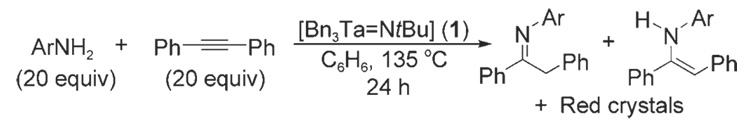Hexanuclear transition-metal clusters are well-known, but examples displaying closely packed organic substituents are scarce.[1] The terminal oxo ligands in hexamolybdate and hexatungstate have been replaced to varying degrees by imido[2] or hydrazido[3] groups, and cyclopentadienyl-functionalized hetero-hexanuclear clusters have been prepared.[4] Still, incorporation of organic groups into the bridging sites has been more challenging.[5] Herein we report a high-yielding synthesis of neutral, oxygen-centered hexatantalum clusters with all other metal valencies occupied by terminal and µ3 arylimido ligands. Although chemically inert in their neutral form,[6] these “nearly homoleptic” tantalum imido species possess electronic structures which may facilitate interesting redox-promoted transformations.
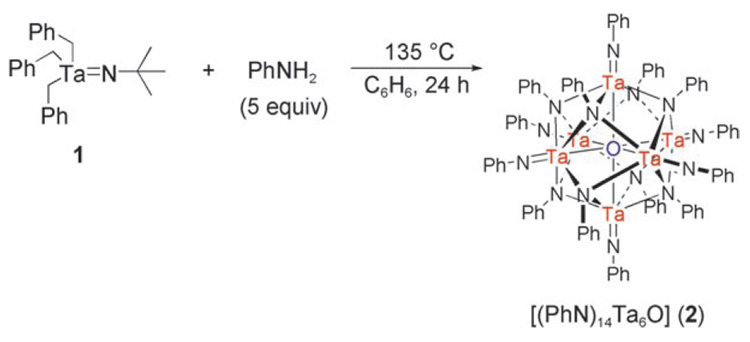
The discovery of these new compounds occurred serendipitously during our investigation of the hydroamination of alkynes by anilines with the imido catalyst [Bn3Ta=NtBu] (1).[7] Reaction mixtures often developed a red color during the course of the reaction, and catalyst deactivation was always observed within 24 hours regardless of the amount of 1 added (Scheme 1). A control experiment revealed that treatment of 1 with excess aniline at 135°C resulted in the precipitation of a sparingly soluble, highly air- and moisture-sensitive red crystalline material in low and erratic yield. Subsequent characterization showed that this material was the octahedral cluster [(PhN)14Ta6O] (2; Scheme 2).
Scheme 1.
Scheme 2.
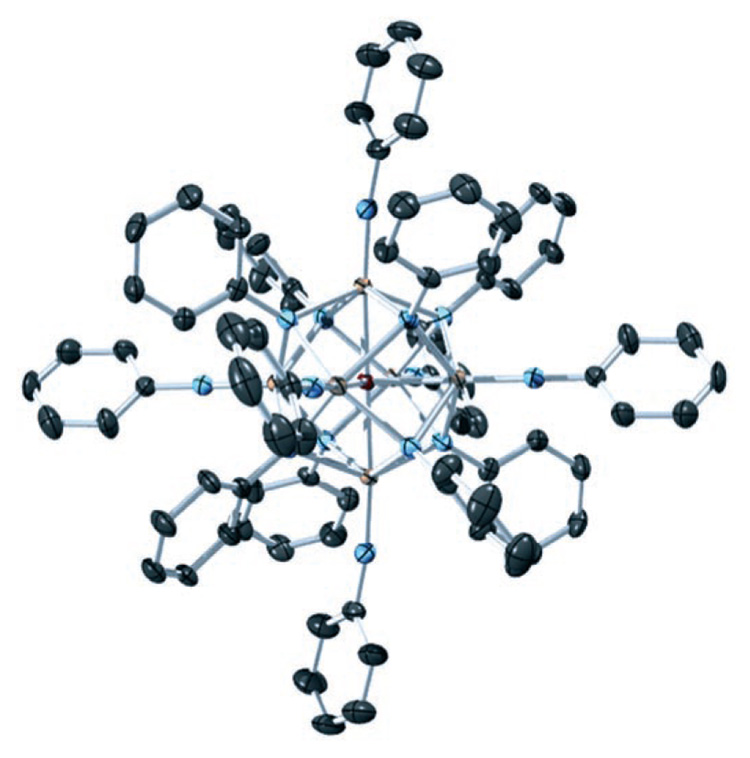
Single-crystal X-ray diffraction analysis[8] of 2 showed a centrosymmetric hexatantalum cluster[9] with six terminal and eight facially bridging phenylimido ligands (Figure 1). Whereas only two nonequivalent sets of phenyl resonances are observed in the 1H NMR spectrum of 2, in the solid state the cluster core is slightly distorted from Oh symmetry and the phenylimido ligands pack so that the potential D2d symmetry is not realized. A central atom was located that was initially assumed to be nitrogen[10] on the basis of precedent from known nitride-centered polyimido clusters.[11] However, the structure was refined equally well with oxygen in this position, and thus alternate methods of characterization were employed to resolve this issue (see below).
Figure 1.
ORTEP diagram of [(PhN)14Ta6O] (2) at 50% probability. Hydrogen atoms have been removed for clarity. Selected bond lengths (Å): Ta-O 2.2035(5), 2.2079(4), 2.2119(4); Ta-Nterminal 1.790(3), 1.791(4), 1.793(4). C gray, N blue, O red, Ta orange.
Irreproducible yields obtained in the synthesis of 2 led us to suspect the adventitious presence of a necessary reactant. Whereas oxygen sources such as peroxides, pyridine N-oxide, or phosphine oxides failed to yield 2, the addition of water prior to heating of the reaction mixture resulted in consistent yields of up to 57%. Surprisingly, tantalum oxide was also moderately effective as an oxygen source. This observation provides an explanation for the variable yield without added water: tantalum oxide may have remained on the surface of the glassware from one reaction to the next and may not have been inert as was assumed.
The reaction is not limited to aniline itself. Substituted anilines p-toluidine and p-anisidine provided [(p-MeC6H4N)14Ta6O] (3) and [(p-MeOC6H4N)14Ta6O] (4) in 48% and 49% yield, respectively. Although p-chloro-, p-fluoro-, and p-trifluoromethylaniline did not furnish isolable products, m-chloroaniline gave [(m-ClC6H4N)14Ta6O] (5) in modest yield (15%). High-quality crystals of 5 were obtained from a reaction mixture, and structural elucidation[12] revealed a configuration analogous to that of 2.
A screening of alternate metal sources showed that commercially available pentakis(dimethylamido)tantalum provided yields comparable to those obtained by employing 1 and allowed for the preparation of 2 and 3 on a much larger scale. Thus, it is likely that many reported reactions involving addition of an arylamine to tantalum coordination complexes have resulted in the formation, but not the detection, of these clusters.
The presence of oxide in the cluster core was confirmed by electrospray mass spectrometry studies on 3 and 4 using THF as the matrix. Owing to extensive fragmentation and subsequent reaction with the matrix, none of the observed fragments corresponded to combinations of the cluster core and arylimido fragments. However, many of the high-molecular-weight fragments (up to ca. 2000 Da) possessed masses that were 16 Da greater for 4 (MeO) than for 3 (Me). Therefore, these fragments apparently each retained one imido ligand. Samples of 3 and 4 that had been prepared using 17O- and 18O- enriched water yielded spectra in which each high-mass fragment from the nonlabeled cluster was accompanied by fragments that were one and two Da heavier, including those that were inferred to still possess an imido ligand (see the Supporting Information). These data provide strong evidence for assignment of these compounds as oxide-centered clusters, with the oxide deriving from added water.
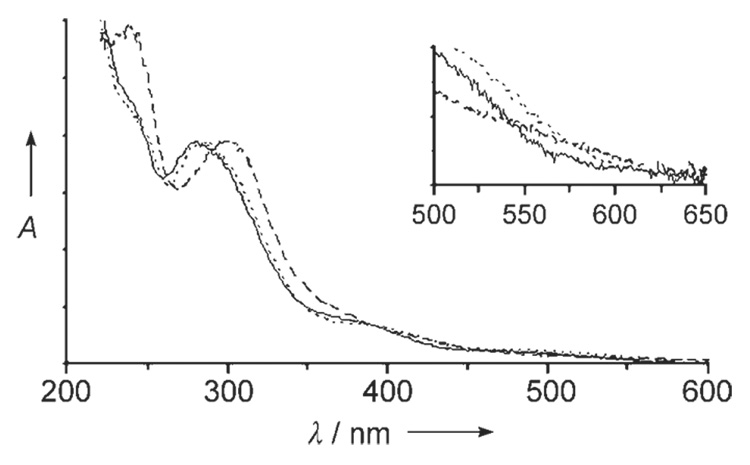
Compounds 3 and 4 are soluble in benzene to about 10 mm and were recrystallized without decomposition. Both are significantly more air-sensitive than 2, decomposing in solution over about two days if stored in polypropylene-capped glass vials inside an inert-atmosphere glove box. Nonetheless, a sample of 3 in C6D6 showed no decomposition after it was heated at 135°C for two weeks under rigorously air-free conditions. Compound hues vary from red/orange (5) to red/black for the more electron-rich 4 (Figure 2, insert). Compounds 2, 3, and 4 exhibit intense UV absorptions quite similar in energy to those of the anilines from which they are derived (Figure 2). Fluorescence measurements, however, detected no emission between 300 and 1000 nm. The large number and density of vibrational modes accessible to these clusters probably makes thermal relaxation the dominant excited-state decay pathway.
Figure 2.
UV/Vis absorption spectra of 2 (solid trace), 3 (dotted), and 4 (dashed) in THF. Inset: visible-wavelength absorption onsets.
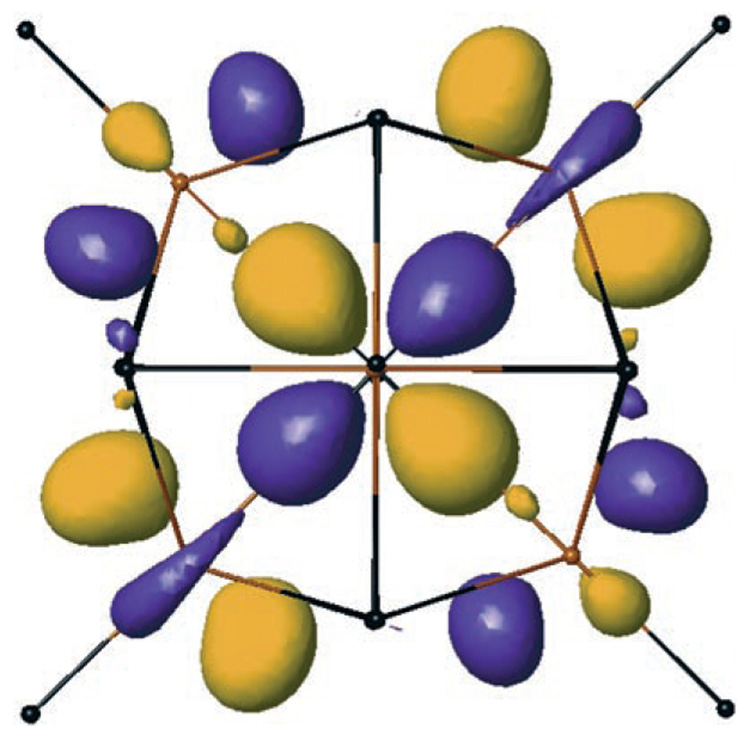
Results of orbital calculations[13] (X3LYP/LACVP*)[14] on Ci-optimized 2 agree with the expected bonding scheme wherein all occupied frontier orbitals are ligand-centered. Both the geometry and orbital structure of optimized 2 are very similar to those calculated from the crystallographically determined coordinates.
The calculated LUMO of 2 is delocalized over all six tantalum centers and is primarily nonbonding (Figure 3). This bonding scheme is consistent with the observed optical properties in that ligand-to-metal charge-transfer processes should be very facile. Furthermore, the computational results suggest that the title compounds may exhibit interesting redox behavior. Indeed, cursory reactivity studies have shown that although 2 and 3 are inert towards unsaturated small molecules, they are easily reduced to stable anions. Preliminary data suggest that further study of this novel family of compounds will yield new insights into the nature of polynuclear metal–imido bonding.
Figure 3.
LUMO of Ci-optimized 2 (X3LYP/LACVP*) viewed along the fourfold axis of the cluster core (nonbonding, localized on six tantalum centers). Ph groups have been removed for clarity.
Experimental Section
[(ArN)14Ta6O] (method 1): Compound 1 (100 mg, 0.190 mmol, 1 equiv) was dissolved in benzene (1 mL). The aniline (0.950 mmol, 5 equiv) was treated with degassed water (0.6 µL, 0.03 mmol, 0.16 equiv) by microsyringe and then dissolved in benzene (0.5 mL). The two solutions were then combined. Hexane (4 mL) was added and the mixture was heated at 135°C for 24 h in a sealed vessel, during which time the product precipitated as microcrystalline material. After cooling to room temperature, the product was washed with Et2O (2 × 2 mL), then pentane (2 × 2 mL). Isotopically enriched samples were prepared in an identical fashion using 17O/18O-enriched water.
2: Material collected from the reaction mixture was analytically pure. Yield: 43.3 mg, 0.018 mmol, 57%; 1H NMR (500 MHz, [D8]THF, 22°C, TMS): δ=7.68 (dd, J(H,H)=8.5 Hz, 1.0 Hz, 16H), 7.13 (m, 16H), 6.98 (m, 12 H), 6.92 (m, 8H), 6.71 (m, 6H), 6.07 ppm (dd, J(H,H)=8.5 Hz, 1.0 Hz, 12H); 13C{1H} NMR (125.8 MHz, [D8]THF, 22°C, TMS): δ=157.84, 156.83, 129.80, 129.20, 128.35, 126.87, 125.27, 124.86 ppm; UV/Vis (THF): λmax(onset)=280 nm (580 nm). Elemental analysis (%) calcd for C84H70N14OTa6 : C 42.09, H 2.94, N 8.18; found: C 42.23, H 2.89, N 8.86.
3: Material collected from the reaction mixture was analytically pure. Yield: 39.2 mg, 0.015 mmol, 48%; 1H NMR (500 MHz, C6D6, 22°C, TMS): δ=8.03 (d, J(H,H)=8.5 Hz, 16H), 6.87 (d, J(H,H)=8.0 Hz, 12H), 6.81 (d, J(H,H)=8.5 Hz, 16H), 6.47 (d, J(H,H)=8.0 Hz, 12H), 2.08 (s, 18 H), 1.96 ppm (s, 24H); 13C{1H} NMR (125.8 MHz, C6D6, 22°C, TMS): δ=155.49, 155.13, 134.25, 133.68, 130.18, 128.91, 126.78, 125.12, 21.17, 20.75 ppm; UV/Vis (THF): λmax(onset)=285 nm (600 nm). Elemental analysis (%) calcd for C98H98N14OTa6 : C 45.74, H 3.84, N 7.62; found: C 46.10, H 4.08, N 7.87.
4: Yield: 43.4 mg, 0.016 mmol, 49%; 1H NMR (500 MHz, C6D6, 22°C, TMS): δ=8.19 (d, J(H,H)=9.0 Hz, 16H), 6.74 (d, J(H,H)=9.0 Hz, 16H), 6.63 (s, 24 H), 3.12 (s, 18H), 3.09 (s, 24H); 13C{1H} NMR (125.8 MHz, C6D6, 22°C, TMS): d=157.53, 157.45, 151.70, 151.63, 127.96, 126.22, 114.90, 113.75, 55.34, 55.17 ppm; UV/Vis (THF): λmax(onset)=300 nm (630 nm). The product was recrystallized by vapor diffusion of pentane into a benzene solution of 4 prior to submission for elemental analysis. Elemental analysis (%) calcd for C98H98N14O15Ta6 : C 42.07, H 3.53, N 7.01; found: C 42.62, H 3.74, N 6.90.
5: After heating, the solvent was removed under vacuum. The orange oil was dissolved in toluene (1 mL), into which pentane was then allowed to diffuse. The resulting crystalline material was insoluble in nonreactive solvents. Yield: 13.4 mg, 0.0047 mmol, 15%. Elemental analysis (%) calcd for C84H56Cl14N14OTa6 : C 35.28, H 1.97, N 6.86; found: C 34.94, H 2.17, N 6.81.
2 (method 2): Pentakis(dimethylamido)tantalum (1.00 g, 2.49 mmol, 1 equiv) was dissolved in toluene (5 mL). A solution of aniline (1.41 g, 15.14 mmol, 6.08 equiv) in toluene (10 mL) was added, followed by water (7.5 µL, 0.42 mmol, 0.16 equiv). The mixture was heated at reflux for 4 days, after which time the flask was cooled to room temperature and the supernatant was removed. The solid was washed with Et2O (2 × 10 mL), then extracted with THF (2 × 20 mL). The extracts were combined and the solvent removed under vacuum. Yield: 380 mg, 0.158 mmol, 38%.
3 (method 2): p-Toluidine (1.36 g, 12.7 mmol, 5.10 equiv) and water (7.5 µL, 0.42 mmol, 0.16 equiv) were combined and dissolved in toluene (5 mL). Pentakis(dimethylamido)tantalum (1.00 g, 2.49 mmol, 1 equiv) was then added, followed by n-octane (20 mL). The mixture was heated at reflux for 2 days, after which time the flask was cooled to room temperature and the supernatant was removed. The solid was washed with Et2O (2 × 10 mL), then extracted with hot toluene (2 × 20 mL). The extracts were combined and the solvent removed under vacuum. Yield: 320 mg, 0.124 mmol, 30%.
General experimental, crystallographic, and computational procedures, as well as coordinates for Ci-optimized 2 and mass spectrometry data, can be found in the Supporting Information.
Supplementary Material
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Footnotes
We are indebted to Dr. Fred Hollander and Dr. Allen Oliver at the UC Berkeley CHEXRAY X-ray crystallographic facility and Dr. Kathleen Durkin at the Molecular Graphics Facility (NSF grant CHE-0233882) for crystallographic and computational consultation, respectively, as well as Dr. John Greaves at UC, Irvine for mass spectrometry data. We also thank Dr. Alexandr Shafir for providing a preliminary structure of 2 obtained by using MoKα radiation. This work was supported by NIH grant GM-25459 to R.G.B. and DOE funding to J.A.
Contributor Information
Jamin L. Krinsky, Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA), Fax: (+1) 510-642-7714
Laura L. Anderson, Department of Chemistry, University of California, Irvine, Irvine, CA 92697-2025 (USA)
John Arnold, Email: arnold@berkeley.edu, Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA), Fax: (+1) 510-642-7714.
Robert G. Bergman, Email: rbergman@berkeley.edu, Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA), Fax: (+1) 510-642-7714.
References
- 1.(a) Pope MT, Mueller A. Angew. Chem. 1991;103:56–70. Angew. Chem. Int. Ed. Engl. 1991, 30, 34 – 48. [Google Scholar]; (b) Gouzerh P, Proust A. Chem. Rev. 1998;98:77–111. doi: 10.1021/cr960393d. [DOI] [PubMed] [Google Scholar]
- 2.(a) Strong JB, Yap GPA, Ostrander R, Liable-Sands LM, Rheingold AL, Thouvenot R, Gouzerh P, Maatta EA. J. Am. Chem. Soc. 2000;122:639–649. [Google Scholar]; (b) Clegg W, Errington RJ, Fraser KA, Holmes SA, Schaefer A. J. Chem. Soc. Chem. Commun. 1995:455–456. [Google Scholar]
- 3.Kang H, Zubieta J. J. Chem. Soc. Chem. Commun. 1988:1192–1193. [Google Scholar]
- 4.(a) Che TM, Day VW, Francesconi LC, Fredrich MF, Klemperer WG, Shum W. Inorg. Chem. 1985;24:4055–4062. [Google Scholar]; (b) Harper JR, Rheingold AL. J. Am. Chem. Soc. 1990;112:4037–4038. [Google Scholar]
- 5.Kessler VG, Seisenbaeva GA. Inorg. Chem. Commun. 2000;3:203–204. [Google Scholar]
- 6.Wigley DE. Progress in Inorganic Chemistry. Vol. 42. New York: Wiley; 1994. pp. 239–482. [Google Scholar]
- 7.Anderson LL, Arnold J, Bergman RG. Org. Lett. 2004;6:2519–2522. doi: 10.1021/ol0492851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crystal data and structure refinements for 2: C84H70N14OTa6·4C6H6, Mr=2689.67 gmol−1, crystal dimensions 0.05 × 0.05 × 0.04 mm3, triclinic, space group P1̅, a=14.137(3), b=14.262(3), c=14.343(3) Å, α=76.192(4), β=72.097(4), γ=60.491(4)°, V=2381(9) Å3, Z=1, ρcalcd=1.875 gcm−3, µ=8.59 mm−1, F(000)=1286, 32552 reflections measured, 13506 unique [R(int)=0.0600], 2θ=3.60–67.34°, 583 refined parameters, R1=0.0501 (12494 observations where I> 2σ(I)), wR2=0.1359, GoF=1.026. Data were collected at 193(2) K on a Bruker Platinum 200 CCD diffractometer (APEX2 v.2.02, Area-Detector Software Package, Bruker AXS, Madison, WI, 1995–99) with Si-〈111〉 channel-cut crystal-monochromated synchrotron radiation (λ=0.77490 Å, Advanced Light Source beamline 11.3.1, Lawrence Berkeley National Laboratory, operated under DOE contract DE-AC03-76SF00098) using ω scans (0.3° per 1.5-s frame). All non-hydrogen atoms were refined anisotropically; hydrogen atoms were included in calculated positions but not refined. The maximum and minimum peaks on the final difference Fourier map corresponded to 2.820 and −2.150 e−Å−3, respectively, and were located near the tantalum atoms. General methods for integration, solution, and refinement can be found in the Supporting Information. CCDC-615636 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9.Possesses the minimal cluster core of hexatantalate; see: Pickhard F, Hartl H. Z. Anorg. Allg. Chem. 1997;623:1311–1316.
- 10.Possibly deriving from the tBuN moiety. An undetected proton could provide charge balance.
- 11.Banaszak Holl MM, Wolczanski PT. J. Am. Chem. Soc. 1992;114:3854–3858. [Google Scholar]
- 12.Crystal data and structure refinements for 5: C84H56Cl14N14OTa6·2C4H11N·2C6H6, Mr=3161.92 gmol−1, crystal dimensions 0.22 × 0.14 × 0.06 mm3, triclinic, space group P1̅, a=13.148(1), b=13.796(1), c=16.005(1) Å, α=108.687(1), β=96.277(1), γ=94.847(1)°, V=2711.5(3) Å3, Z=1, ρcalcd=1.936 gcm−3, µ=6.43 mm−1, F(000)=1510, 15509 reflections measured, 10598 unique [R(int)=0.0196], 2θ=4.62–52.78°, 617 refined parameters, R1=0.0303 (8490 observations where I> 2σ(I)), wR2=0.0713, GoF=0.997. Data were collected at 168(2) K on a Bruker APEX diffractometer (SMART v.5.631, Bruker AXS, Madison, WI, 1995–99) with graphite-monochro-mated radiation (λ=0.71073 Å) using ω scans (0.3° per 10-s frame). All non-hydrogen atoms were refined anisotropically except those of tBuNH2, while hydrogen atoms were included in calculated positions but not refined (no hydrogen atoms were included for tBuNH2). The occupancies of disordered Cl2 were also refined. The maximum and minimum peaks on the final difference Fourier map corresponded to 1.309 and −1.065 e− Å−3, respectively, and were located near the tantalum atoms. CCDC-615637 contains the supplementary crystallo-graphic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13.Jaguar . v.6.5. New York, NY: Schrodinger, LLC; 2005. [Google Scholar]
- 14.Extension of B3LYP by Xu and Goddard to include the Perdew–Wang 1991 gradient exchange correction.Xu X, Goddard WA., III Proc. Natl. Acad. Sci. USA. 2004;101:2673–2677. doi: 10.1073/pnas.0308730100.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.