Abstract
Integrins and growth factor receptors are important participants in cellular adhesion and migration. The EGF receptor (EGFR) family of tyrosine kinases and the β1-integrin adhesion receptors are of particular interest, given the implication for their involvement in the initiation and progression of tumorigenesis. We used adhesion and chemotaxis assays to further elucidate the relationship between these two families of transmembrane signaling molecules. Specifically, we examined integrin-mediated adhesive and migratory characteristics of the metastatic breast carcinoma cell line MDA-MB-435 in response to stimulation with growth factors that bind to and activate the EGFR or erbB3 in these cells. Although ligand engagement of the EGFR stimulated modest β1-dependent increases in cell adhesion and motility, heregulin-β (HRGβ) binding to the erbB3 receptor initiated rapid and potent induction of breast carcinoma cell adhesion and migration and required dimerization of erbB3 with erbB2. Pharmacologic inhibitors of phosphoinositide 3-OH kinase (PI 3-K) or transient expression of dominant negative forms of PI 3-K inhibited both EGF- and HRGβ-mediated adhesion and potently blocked HRGβ- and EGF-induced cell motility. Our results illustrate the critical role of PI 3-K activity in signaling pathways initiated by the EGFR or erbB3 to up-regulate β1-integrin function.
INTRODUCTION
The integrin family of adhesion receptors plays a pivotal role in a wide variety of events that control a cell’s communication with its environment (Diamond and Springer, 1994; Schwartz et al., 1995; Aplin et al., 1998). These include such functions as cellular adhesion, motility, survival, differentiation, and morphogenesis. The EGF receptor (EGFR) family of growth factor receptors also contributes in diverse ways to these events, and the signaling cascades governing the cellular outcomes initiated by signals from either receptor family are now being elucidated. The observation that growth factor receptors and integrins synergistically potentiate given biochemical events illustrates the interplay between these two families of cell surface receptors (Miyamoto et al., 1996; Schneller et al., 1997; Guilherme et al., 1998; Woodard et al., 1998). To better understand the normal and aberrant communications that may contribute to cellular dysregulation present in tumorigenic and metastatic cells, it is crucial to characterize the potential pathways linking growth factor receptors to integrins and the outcome of these signals on events such as cellular adhesion and metastasis.
The β1-integrin subfamily interacts with various cellular counter-receptors and extracellular matrix (ECM) components determined by the partnering of specific α subunits with β1, as well as the particular cellular context. Alterations in levels of expression of β1-integrins have been implicated in tumorigenesis (Albelda, 1993), although few consistent models have emerged to clarify how these changes contribute to cellular dysregulation in tumor cells. Recent studies using targeted disruption of the β1-integrin gene have illustrated the contribution of β1-integrins to the metastatic potential of murine lymphoma cells in vivo (Stroeken et al., 1998), and manipulation of β1 function by transgenic expression of a chimeric β1 molecule has further demonstrated the importance of β1-integrins in normal epithelial cell proliferation, apoptosis, differentiation, and maintenance of cell polarity in the developing mammary gland (Faraldo et al., 1998). However, expression levels of integrins, per se, may not necessarily translate into a commensurate functional outcome (Akiyama et al., 1990; Shimizu et al., 1990a,b), because integrin function can be rapidly and transiently regulated in response to stimulation of several cell surface receptors. This fine-tuned control of β1-integrin function in the absence of alterations in integrin levels at the cell surface is elegantly demonstrated on circulating leukocytes (Shimizu, 1994), and rapid up-regulation of β1-integrins after stimulation of receptor tyrosine kinases such as the PDGF or c-kit receptors expressed on mast cells (Kinashi and Springer, 1994; Kinashi et al., 1995; Serve et al., 1995; Vosseller et al., 1997) has also been described. However, the potential for signaling between the multisubunit EGFR family of receptors and the β1-integrins has not been extensively examined.
The EGFR family of receptor tyrosine kinases is now recognized as a multisubunit family consisting of the EGFR (erbB1), erbB2, the kinase-impaired erbB3, and erbB4. These receptors, with the exception of erbB2, are bound and activated by distinct sets of growth factors that fall broadly into three categories. The first includes EGF, amphiregulin, and TGFα, growth factors specific for the EGFR. Secondly, the heregulins (HRGs) represent a multigene family of growth factors with alternately spliced forms that bind specifically to erbB3 and erbB4. Finally, more promiscuous growth factors such as betacellulin, heparin-binding EGF, and epiregulin are capable of interacting with both the EGFR and with erbB4. Adding to the complexity of this family is the dramatic potential for signal diversity attributable to homo- and heterodimerization, as well as possible secondary dimerization (Graus-Porta et al., 1997; Huang et al., 1998) between its members. The formation of and unique biochemical properties of these ligand-driven heterodimers are now being appreciated (Lemmon and Schlessinger, 1994; Earp et al., 1995; Wallasch et al., 1995; Karunagaran et al., 1996; Cohen et al., 1996; Zhang et al., 1996; Alroy and Yarden, 1997; Graus-Porta et al., 1997; Riese and Stern, 1998). However, characterization of the unique biochemistry presented by specific heterodimers has just begun, and the possible contribution of different receptor combinations in cellular adhesion and motility has remained relatively unexplored. It is likely that the signaling pathways leading to mitogenesis are quite distinct from those of other cellular events such as motility or invasion (Chen et al., 1994; Elenius et al., 1997), and recent studies have made important contributions toward dissecting the EGF-sensitive motility responses (Chen et al., 1996; Ware et al., 1998; Xie et al., 1998; Li et al., 1999). Nonetheless, experimental systems used to assess EGF regulation of motility have often used exogenous expression of the EGFR in receptor-negative cells or cell lines with artificially high receptor levels. In addition, little experimental work has been directed at integrin-mediated events in response to HRG.
The rapid and transient up-regulation of β1 function on leukocytes (Shimizu, 1994) provides a compelling parallel with metastatic processes undertaken by aggressive tumor cells, and the lipid kinase phosphoinositide 3-OH kinase (PI 3-K) has emerged as a critical component in many of the pathways that contribute to the regulation of β1-integrin function (Shimizu and Hunt 1996). Cell surface receptors, including the EGFR family, in a host of cell types and with varying functions interact directly or indirectly with PI 3-K and stimulate its enzymatic activity, thereby generating lipid byproducts that are now believed to participate directly, both in a positive and negative manner, in pathways critical to mitogenesis, cell survival and apoptosis, adhesion, motility, and cytoskeletal reorganization (Kapeller and Cantley, 1994; Carpenter and Cantley, 1996; Klippel et al., 1997; Stokoe et al., 1997; Falasca et al., 1998). Importantly, PI 3-K has emerged as a critical enzyme in the basal motility of other breast carcinoma cell lines (Keely et al., 1997). In addition, activation of PI 3-K by the α6β4-integrin has been implicated in the enhanced migration of a breast cancer cell line expressing transfected β4-integrin (Shaw et al., 1997). Given these reports, it seems plausible to suggest that members of the EGFR family may facilitate β1-integrin-mediated adhesion and migration via activation of PI 3-K, particularly in tumor cell lines that have not been altered via transfection of integrin subunits or EGFR family members.
The role of PI 3-K in EGFR signaling and in the regulation of integrin function in the immune system suggests a potential synergy between EGFR signaling and integrin function in breast cancer. Therefore, we have dissected the contributions of members of the EGFR family of receptor tyrosine kinases to the regulation of β1-integrin function in breast cancer cells and examined the role of PI 3-K in these pathways. Our data demonstrate rapid up-regulation of β1-integrin function by ligand stimulation of the EGFR or erbB3, in a PI 3-K-dependent manner and illustrate the preferential participation of erbB2 in HRGβ- rather than EGF-stimulated adhesion and migration.
MATERIALS AND METHODS
Cell Lines
The MDA-MB-435 cell line was maintained in Leibovitz’s L-15 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% FCS (Atlanta Biologicals, Norcross, GA). The 528 hybridoma, expressing the anti-EGFR monoclonal antibody, was maintained in RPMI 1640 medium (Mediatech, Washington, DC) containing 10% FCS. Culture supernatant was harvested from confluent cultures of 528 cells and was titered for detection of the EGFR. All cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA), and all cell culture media contained additives of 2 mM l-glutamine and 50 U/ml penicillin/streptomycin (Mediatech).
Flow Cytometry
Single-color flow cytometric analysis (FACS) was performed on cells in suspension after removal from tissue culture flasks with EDTA or trypsin. Cells (5 × 105) were typically analyzed with antibodies incubated as 1 μg purified antibody, 5 μl ascites antibody, or 25 μl antibody in culture supernatant/1 × 106 cells. Antibodies in the form of ascites or culture supernatant were routinely titered for appropriate detection of cell surface receptors. Antibodies for flow cytometric analysis included the anti-EGFR monoclonal antibody 528 (ATCC), the anti-erbB2 monoclonal Ab-5 (Calbiochem, La Jolla, CA), the anti-erbB3 monoclonal antibody Ab-4, the anti-erbB4 monoclonal antibody Ab-1 (Lab Vision, Fremont, CA), the β1-integrin-specific monoclonal antibody TS2/16 (ATCC), the β2-integrin-specific monoclonal antibody TS1/18 (ATCC), the α1-integrin-specific monoclonal TS2/7 (ATCC), the α2-integrin-specific monoclonal antibody P1E6 (Life Technologies), the α3-integrin-specific monoclonal antibody P1B5 (Life Technologies), the α4-integrin-specific monoclonal antibody NIH49d-1 (a kind gift from Dr. S. Shaw, National Institutes of Health), the α5-integrin-specific monoclonal antibody P1D6 (Life Technologies), the α6-integrin-specific monoclonal antibody GoH-3 (ICN/Cappell, Cochranville, PA), and FITC-conjugated goat anti-mouse IgG or goat anti-rat IgG (Southern Biotechnology, Alabaster, AL). Cells in FACS buffer (HBSS containing 1% bovine calf serum [Hyclone Laboratories, Logan, UT]) were incubated with appropriate antibodies for 30 min on ice, washed three times in FACS buffer, and incubated for an additional 30 min with appropriately diluted FITC-conjugated secondary antibodies. After two washes in ice-cold FACS buffer, data were acquired on a Becton Dickinson (Mountain View, CA) FACScan or FACScalibur and analyzed using Cellquest software.
DNA Constructs and Transfections
The green fluorescent protein (GFP)-wild-type p85 and GFP-Δp85 constructs have been previously described (Chan et al., 1997). Transfections were carried out by electroporation in 4-mm gap cuvettes (Invitrogen, San Diego, CA). Cells (5 × 106) in 300 μl Opti-MEM (Life Technologies) were incubated with 25 μg appropriate DNA and electroporated using 250-V, 960-μF settings on a Bio-Rad (Hercules, CA) gene pulser with capacitance extension. After allowing the cells to recover for 20 min at room temperature, cells were transferred to tissue culture flasks containing 20% FCS and 80% L-15 media and were allowed to recover for 24–48 h before use in adhesion or migration assays. Typical transient expression of DNA constructs ranged from 15–35% of recovered cells.
Adhesion Assays
Standard adhesion assays were performed using cells labeled with Calcein AM (Molecular Probes, Eugene, OR) as previously described (Zell et al., 1996). ECM ligands were human type IV collagen (Sigma, St. Louis, MO), mouse Engelbreth-Holm-Swarm Sarcoma (EHS)-derived type IV collagen (Life Technologies), human merosin or EHS-derived laminin (Life Technologies), and human fibronectin (FN). For transient expression of GFP fusion proteins, adhesion was quantitated after collection of adherent cells and analysis by flow cytometry essentially as described (Chan et al., 1997; Kivens and Shimizu, 1999). Growth factor stimulation was performed with EGF (Life Technologies), betacellulin, HRGα, or HRGβ (all from R&D Systems, Minneapolis, MN). For receptor blocking studies, cells were incubated in the presence of control mouse IgG (Caltag, South San Francisco, CA), the anti-β1-integrin antibody P5D2 (a kind gift from T. LeBien, University of Minnesota, Minneapolis, MN), the anti-erbB2 Ab-16, the anti-erbB3 Ab-5, or the anti-erbB4 Ab-3 (all from Lab Vision) at 1 μg antibody/1 × 106 cells or as indicated in figure legends. Tyrphostin AG1478 (Calbiochem) was used for inhibition of the EGFR. Pharmacological inhibition of PI 3-K was performed with wortmannin (Sigma) or LY294002 (Alexis, San Diego, CA). Inhibition of mitogen-activated, ERK-activating kinase was performed using the inhibitor PD98059 (Parke-Davis, Ann Arbor, MI).
Migration Assays
Cell lines were allowed to grow to suconfluency (∼75–85%) before harvest for migration studies. Subconfluent cell cultures were placed in serum-free media for 12–24 h and harvested by releasing from flasks with 1 mM EDTA. After cells were washed free of EDTA in serum-free RPMI 1640 media, they were quantitated and assessed for viability using trypan blue. Cells at a density of 400,000 cells/ml in assay media (RPMI, 20 mM HEPES, 0.1% BSA) were added in 57 μl to the upper well of a 48-well chemotaxis chamber (Neuroprobe, Cabin John, MD), containing assay media or appropriate growth factor. Polycarbonate filters (8 μm; Osmonics, Livermore, CA) were precoated with mouse EHS-derived type IV collagen or EHS-derived laminin (Life Technologies) at 20 μg/ml in PBS overnight at 4°C and allowed to air dry before placing in chambers. Cells were allowed to migrate in the presence or absence of stimulators for 4–6 h at 37°C before disassembly of the chambers, fixing, and staining of the migrated cells. Nonmigrated cells were removed from the upper surface of the filters after placing on a microscope slide, and cell migration was quantitated by counting and taking the sum of migrated cells in four separate fields of at least three individual wells. For inhibition studies, cells were preincubated for 15 min on ice with inhibitor or appropriate control before addition to chemotaxis chambers. Although some variability in basal cell migration was observed in separate experiments, relative changes between stimulated and unstimulated migration were consistent.
For transient transfection–migration assays cells were transfected as described above. Cells were serum starved for 12 h before harvesting for migration assays. Transwell chambers (six-well size, 8-μm filters; Costar, Cambridge, MA) or 8-μm polycarbonate membrane filters for Boyden chemotaxis chambers were coated overnight at 4°C in solutions of mouse EHS-laminin or EHS-collagen at 20 μg/ml in PBS. Growth factors diluted in assay media were added to the lower wells of chemotaxis chambers or transwells, and coated filters were placed on top. Cells were then added to upper wells at ∼1 × 106 cells per well in 1.5 ml assay media. The same dilution of cells was used for addition to quadruplicate wells in 24-well plates (100 μl/well) for determination of starting cell populations. Cells were also added to the upper wells of Boyden chambers, and migration was allowed to proceed overnight in both transwells and Boyden chambers. Chemotaxis chambers were disassembled and analyzed the following morning as described above. Transwell migration chambers were disassembled, and migrated cells were removed from the lower surface of each well with 1:1 trypsin:EDTA. Dislodged cells were added to FACS tubes containing ice-cold 10% FACS buffer (HBSS and 10% bovine calf serum), spun, and resuspended in 200 μl 10% FACS buffer. Cells plated in 24-well plates were also harvested and placed into FACS tubes for approximation of cells added per well and for determining the percent efficiency of transfectants. FACS tubes containing representative starting cell populations or migrated cell populations were analyzed by flow cytometry with additions of 25,000 reference beads per tube (9.7 μm; Interfacial Dynamics, Portland, OR) to determine cell numbers present in each tube. Quantitation was done essentially as described (Chan et al., 1997). Briefly, the numbers of cell and reference bead events acquired by flow cytometric analysis were used to calculate cell numbers present in starting populations composed of GFP−, GFP+, GFP++, and GFP+++ (fluorescing between logs 0 and 1, 1 and 2, 2 and 3, or 3 and 4, respectively). Using reference beads, the number of cells in each migrated population was determined in the same way and compared directly with the starting cell population to calculate percent migration and fold change. Comparison of assays carried out for 4 versus 16 h gave similar results. The average of three wells per condition was determined for each data point (Kivens and Shimizu, 1999).
Immunoprecipitation
Cells that had been serum starved for 12–24 h were harvested from tissue culture flasks using 1 mM EDTA. Cells were washed in serum-free RPMI 1640 medium to remove EDTA and were quantitated by trypan blue exclusion. Equal aliquots of cells were added to Eppendorf tubes and stimulated in the presence or absence of growth factors for the indicated periods at 37°C. For studies assessing the effects of erbB2 blocking, cells were preincubated on ice for 15 min with mouse IgG as control or erbB2 blocking Ab-16 at 0.5 μg/1 × 106 cells before stimulation. After stimulation, cells were lysed directly in 0.5 ml 2× lysis buffer (1× = 1% Triton X-100, 1% deoxycholic acid, 158 mM NaCl, 5 mM EDTA, 10 mM Tris, pH 7.2, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM sodium orthovanadate) on ice for 20 min. Supernatants were clarified by centrifugation for 20 min at 4°C, and postnuclear supernatants were immunoprecipitated with the anti-phosphotyrosine antibody PY20 (10 μl 1:10 ascites, provided by Dr. M. Kamps, University of California, San Diego, CA), the EGFR mAb 528 (15 μl culture supernatant), the erbB2 mAb-5 (1 μg), or the erbB3 mAb4 (1 μg) overnight at 4°C. Protein-A Sepharose 4B or goat anti-mouse IgG-Sepharose 4B (Zymed, San Francisco, CA; 50 μl/tube) was added the following morning for an additional 1-h incubation, and immunocomplexes were washed twice in 1× lysis buffer containing protease inhibitors. Protein A-Sepharose– or goat anti-mouse Sepharose–bound proteins were boiled for 4 min in the presence of 2× SDS-sample buffer (125 mM Tris, pH 6.8, 4% SDS, 2 mM EDTA, 20% glycerol, 10% β-mercaptoethanol, 0.6% bromphenol blue) and were separated on 10% polyacrylamide gels by SDS-PAGE.
Western Blotting
Cell lysates or immunoprecipitates were separated by SDS-PAGE and transferred to an Immobilon-P membrane (Millipore, Bedford, MA) in transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol, 0.075% SDS) for 2 h at 400 mA. Membranes were incubated in blocking buffer (5% Carnation milk and PBS) for 1 h at room temperature or overnight at 4°C. Blots were rinsed in PBS before addition of primary antibodies diluted in blocking buffer (4G10, Upstate Biotechnology, Lake Placid, NY; anti-EGFR sc-03, Santa Cruz Biotechnology, Santa Cruz, CA; anti-p85, Upstate Biotechnology; anti-erbB3 Ab-7, Lab Vision; anti-erbB2 Ab-10, Lab Vision) for 1 h at room temperature. Blots were rinsed three times in PBS and 0.1% Tween 20 for 10 min each before addition of secondary antibodies diluted in blocking buffer (goat anti-mouse IgG-HRP; Life Technologies) or donkey anti-rabbit-IgG-HRP (Amersham, Arlington Heights, IL) for 1 h at room temperature. Blots were rinsed three times in PBS and 0.1% Tween 20, and bands were visualized using enhanced chemiluminescence (Pierce, Rockford, IL). For reprobing membranes, stripping buffer (62.5 mM Tris, pH 6.8, 2% SDS, 0.1 M β-mercaptoethanol) was used at 50°C for 30 min followed by blocking membranes in 5% milk and PBS and reprobing with appropriate antibodies.
In Vitro PI 3-Kinase Assays
PI 3-Kinase assays were performed with PY20 immunoprecipitates of 10 × 106 cells per sample as previously described (Chan et al., 1997). Phosphatidylinositol (Avanti Polar Lipids, Alabaster, AL) was used as a substrate for PY20-associated PI 3-kinase, and radioactive lipid products were separated by TLC and visualized by autoradiography. Quantification was performed by PhosphorImager analysis (Molecular Dynamics, Sunnyvale, CA) of the TLC plates. Similar results were obtained from a minimum of three independent assays.
RESULTS
Adhesion of MDA-MB-435 Breast Carcinoma Cell Lines to β1-Integrin Ligands Can Be Regulated by Stimulation of EGFR Family Members
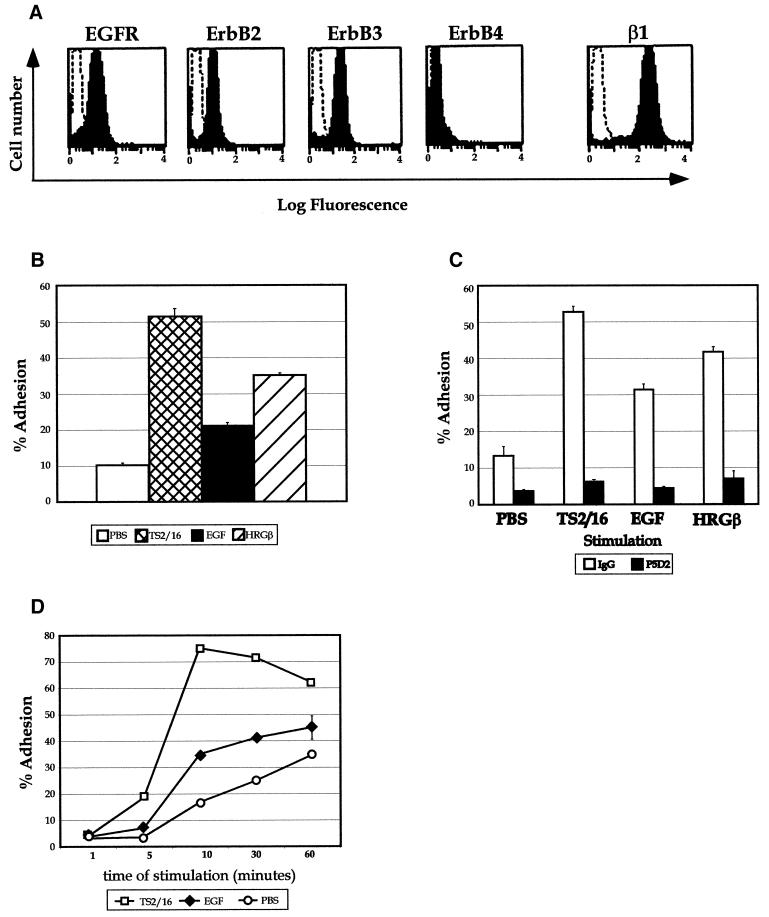
To test our hypothesis that stimulation of EGFR family receptors might up-regulate β1-integrin function in breast cancer cells, we determined the expression of these receptor tyrosine kinases on the surface of metastatic MDA-MB-435 cells and examined their ability to respond to growth factor stimulation by up-regulating adhesion to β1-integrin ECM ligands. MDA-MB-435 cells express moderate levels of the EGFR, erbB2, and erbB3 (Figure 1A), with no detectable erbB4 protein by flow cytometry (Figure 1A) or blotting methods (our unpublished results). Because erbB2 is still considered an “orphan” receptor in that no growth factor has yet been found by which erbB2 is directly bound and activated, the presence of both EGFR and erbB3 on the surface of these cells led us to investigate growth factors that specifically bind to and activate either the EGFR or erbB3. These cells exhibited inducible adhesion to several β1-integrin ligands such as FN, EHS-derived laminin (LAM), or merosin (our unpublished data), but the strongest inducible adhesion was consistently observed on type IV collagen (COLL) in response to EGF or HRGβ (Figure 1B). HRGβ-induced adhesion was typically 1.5- to 2-fold higher than that induced by EGF and was slightly less than adhesion achieved by directly activating the β1-integrin with the monoclonal antibody TS2/16 (Arroyo et al., 1992; Kovach et al., 1992). β1-integrins were the major adhesion receptors responsible for this event, as indicated by the nearly complete inhibition of both unstimulated and stimulated (TS2/16, EGF, or HRGβ) adhesion to COLL by these cells when a blocking antibody against β1 was used (Figure 1C). EGF-stimulated adhesion to COLL was induced in a rapid manner, with maximal increases over unstimulated adhesion found by 10–20 min of stimulation at 37°C (Figure 1D). Time courses of inducible adhesion to other ECM ligands examined were similar, and the maximal time of stimulated adhesion for HRGβ was similar to that of EGF (our unpublished data). Thus, adhesion assays were typically performed for 10 min at 37°C.
Figure 1.
EGF and HRGβ modulate breast carcinoma cell adhesion to ECM ligands. (A) Surface expression of the EGFR, erbB2, erbB3, erbB4, or β1-integrin on MDA-MB-435 cells was determined by flow cytometry as described in MATERIALS AND METHODS. Representative histogram profiles are shown, with open histograms representing negatively stained control cells. (B) Cells were analyzed for their ability to respond to EGF or HRGβ treatment in cell adhesion assays performed on human type IV COLL. Cells that had been serum starved for 12–24 h were harvested by release from tissue culture flasks in 1 mM EDTA. Suspended cells were than washed in serum-free media to remove excess EDTA before quantitation and labeling with Calcein AM as previously described (Zell et al., 1996). Adhesion assays were performed in 96-well plates that were precoated overnight at 4°C with COLL at 1 μg/well. Cells were then added to wells containing assay media alone (open bar) or stimulators: the activating β1-integrin-specific monoclonal antibody TS2/16 (cross-hatched bar), EGF at 100 ng/ml final concentration (shaded bar), or HRGβ at 100 ng/ml (hatched bar). (C) For blocking of β1-integrins, cells were preincubated at 4°C with control mouse IgG (open bars) or with the inhibitory β1-integrin-specific antibody P5D2 (shaded bars; a kind gift from T. LeBien) at 1 μg/1 × 106 cells for 10 min before addition to plates coated with 1 μg/well COLL. The time course of adhesion to COLL (D) was determined in the presence of 1 μg/well TS2/16 (open squares) or 100 ng/ml EGF (solid diamonds) in comparison with unstimulated adhesion (PBS; open circles). Cells were allowed to settle briefly in wells before analyzing preadherent fluorescence on a fluorescence plate reader. Cells were then stimulated at 37°C for 10 min or for designated times before removing nonadherent cells by hand washing with a syringe–manifold system. Comparison of adhesion with human type IV collagen or mouse EHS-collagen did not show significant differences (our unpublished data). Adhesion data are representative of at least three independent experiments and reflect the average of triplicate samples per condition.
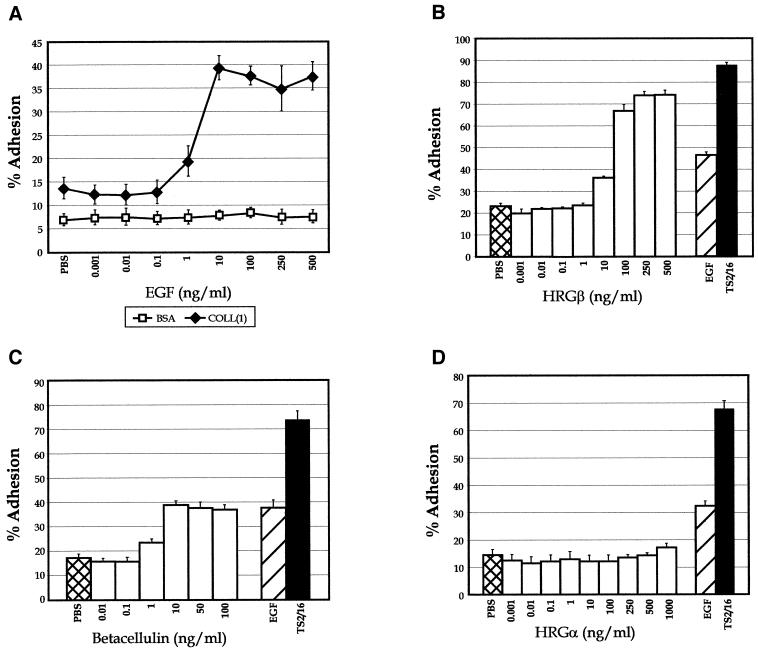
To further evaluate the stimulation of β1-integrin adhesion by EGFR family receptors, the dose–response curves for several growth factors capable of interacting with EGFR or erbB3 were assessed. EGF- and HRGβ-mediated adhesion to COLL were dose dependent, with maximal stimulation of adhesion peaking at 10 ng/ml EGF (Figure 2A) or 100 ng/ml HRGβ (Figure 2B), with a plateau of maximal adhesion at higher concentrations of growth factor. Based on these findings, we typically stimulated cells with 100 ng/ml EGF or HRGβ in cellular adhesion assays. Betacellulin stimulation of the EGFR also increased MDA-MB-435 cell adhesion to COLL in a dose-dependent manner, with maximal stimulation of adhesion paralleling that found with EGF stimulation in the same assay (Figure 2C). Examination of HRGα, a growth factor that binds only to erbB3 or erbB4, showed no effects on adhesion of this cell line at any of the concentrations of growth factor tested (Figure 2D).
Figure 2.
MDA-MB-435 cell adhesion is stimulated by multiple growth factors that bind to and activate members of the EGF receptor family. For analysis of dose response to growth factor, increasing amounts of EGF ranging from 1 pg/ml to 500 ng/ml were added to COLL-coated wells before addition of cells and stimulation at 37°C (A; solid diamonds). Adhesion to BSA-coated wells (open squares) was performed as a control. Dose responses for adhesion to COLL were determined in the presence of increasing amounts of HRGβ (B), the EGF-like growth factor betacellulin (C), or HRGα (D). Unstimulated (PBS; B–D, cross-hatched bars), EGF-stimulated (B–D, hatched bars), or TS2/16-stimulated (B–D, solid bars) adhesion was analyzed for comparison. Cells plated on BSA alone as a control for nonspecific adhesion generally showed <10% adhesion (A; our unpublished data). Data are representative of at least three separate assays.
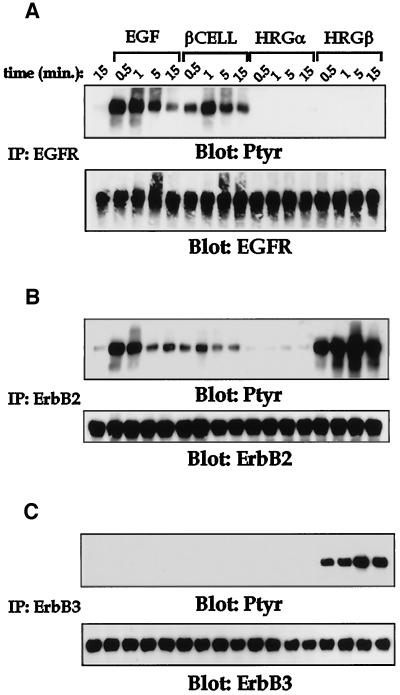
To understand the receptor biochemistry in MDA-MB-435 cells in response to these various stimuli, we analyzed receptor immunoprecipitates of the EGFR (Figure 3A), erbB2 (Figure 3B), or erbB3 (Figure 3C) after stimulation with each of the four growth factors examined in Figure 2. EGF and betacellulin stimulated intense tyrosine phosphorylation of the EGFR and erbB2 with rapid and transient kinetics. HRGβ strongly activated both erbB3 and erbB2 in a very sustained manner over the stimulation time course assessed. In contrast, HRGα had little effect on any of the EGFR family receptors in these cells, in keeping with the lack of adhesion response observed in Figure 2D. Thus, all three ligands active in these cells caused stimulation of their primary receptors, the EGFR for EGF and betacellulin and erbB3 for HRGβ, as well as phosphorylation of erbB2. These data suggest that stimulation of the EGFR with ligands such as EGF or betacellulin activates EGFR and erbB2 phosphorylation and significantly increases β1-integrin-dependent adhesion of the MDA-MB-435 cell line to type IV collagen. Although erbB3 levels were not significantly higher than EGFR on these cells (Figure 1A), HRGβ stimulation gave a much more potent induction of both erbB2 phosphorylation and β1-mediated adhesion, suggesting a qualitative difference in the pathways used by these receptors. Importantly, this up-regulation occurred in the absence of significant changes in either integrin subunit or EGFR family receptor numbers on the cell surface as assessed by flow cytometry (our unpublished data).
Figure 3.
Multiple growth factors induce tyrosine phosphorylation of the EGFR, erbB2, and erbB3 in MDA-MB-435 cells. Receptor activation was assessed after stimulation of MDA-MB-435 cells (10 × 106 cells per sample) for increasing periods at 37°C with PBS alone (first lane in each panel), EGF at 100 ng/ml, betacellulin (βCELL) at 10 ng/ml, HRGα at 100 ng/ml, or HRGβ at 100 ng/ml. Lysis buffer (2×) was added to each sample after the indicated stimulation time, and cleared lysates were immunoprecipitated for EGFR (A), erbB2 (B), or erbB3 (C). Western blotting was performed on samples after separation by SDS-PAGE and transfer to polyvinylidene difluoride membranes. Total phosphotyrosine content in each sample was assessed by probing with anti-phosphotyrosine Ab 4G10 (upper panels). Equivalent receptor loading was confirmed by stripping and reprobing each blot for the presence of the indicated receptor (lower panels). Blots were also stripped and reprobed for the presence of p85 (our unpublished data). Similar results were observed in at least three independent experiments.
Stimulation of EGFR Family Members Increases Migration of MDA-MB-435 Breast Carcinoma Cells
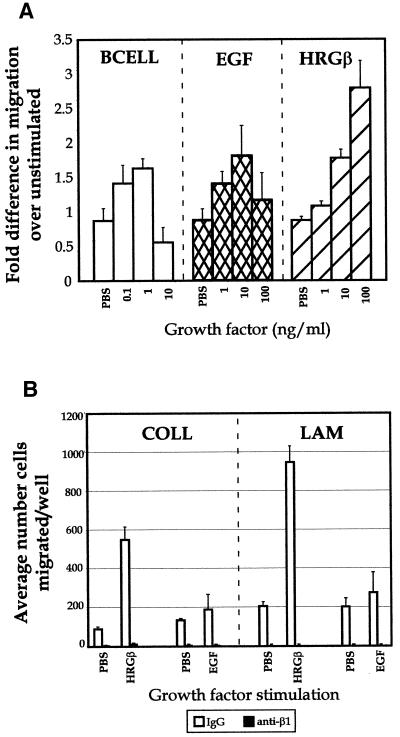
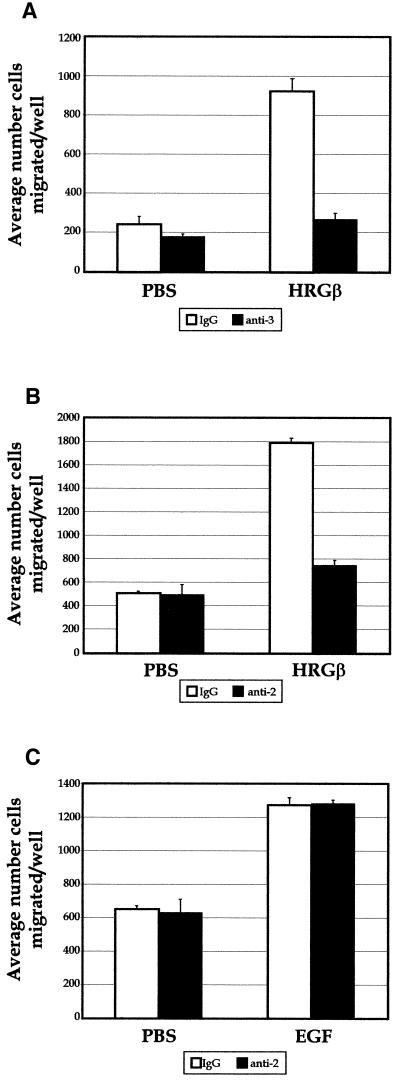
Because the MDA-MB-435 cells are highly metastatic in nude mouse models (Price et al., 1990), we also examined cellular migration and motility in vitro. Previous studies have described migration and adhesion of unstimulated MDA-MB-435 cells on COLL and LAM (Shaw et al., 1996), and our FACS analyses showed strong expression of β1-integrins capable of binding COLL and LAM (α2, α3, and α6; our unpublished data), consistent with this earlier study. Thus, we examined the migration of unstimulated and stimulated MDA-MB-435 cells on both COLL and LAM. As found in our adhesion experiments, betacellulin, EGF, and HRGβ all increased the migration of 435 cells toward LAM (Figure 4A) in a dose-dependent manner, whereas parallel assays showed similar results on COLL (our unpublished data). Although the adhesion experiments showed increasing growth factor-stimulated adhesion with a plateau response (Figure 2), betacellulin and EGF both demonstrated bell-shaped curves for stimulated migration, with maximal responses at 0.1–1 and 1–10 ng/ml for betacellulin and EGF, respectively (Figure 4A). Migration in response to HRGβ showed similar dose effects as seen for adhesion, with strong induction of migration, reaching a maximal response by 100–250 ng/ml growth factor. The stimulated migration by HRGβ was consistently much higher than that mediated by EGF, and both events were β1-integrin dependent as illustrated by the ability of an inhibitory β1-integrin-specific antibody to completely abrogate both unstimulated and EGF- or HRGβ-stimulated migration toward COLL or LAM (Figure 4B). Thus, the adhesion and migration data support our hypothesis that growth factor stimulation of the EGFR couples to β1-integrin-mediated functional events. Furthermore, we have found that erbB3, a kinase-impaired receptor in the EGFR family, mediates potent stimulation of both adhesion and cell migration by the growth factor HRGβ.
Figure 4.
Betacelluin, EGF, and HRGβ induce β1-integrin-dependent MDA-MB-435 cell migration on LAM. (A) Cells were grown to ∼75% confluency and placed in serum-free media for 16 h before harvesting as for adhesion assays. Polycarbonate filters (8 μm) were coated overnight at 4°C in PBS containing mouse EHS-LAM or EHS-COLL (our unpublished data) at 20 μg/ml. Forty-eight-well chemotaxis chambers were assembled with assay media alone or containing increasing amounts of growth factors in the lower chambers, as indicated. LAM-coated filters were placed over the lower wells and, ∼23,000 MDA-MB-435 cells were placed in the upper wells and allowed to migrate at 37°C for 4–6 h. Migrated cells in each well were quantitated on fixed and stained filters. The sum of four microscopic fields was taken for each well, and three wells were averaged for each stimulation condition. (B) Antibody blocking studies were carried out by preincubating cells with control IgG (open bars) or the inhibitory β1-integrin-specific mAb P5D2 (solid bars) at 1 μg/1 × 106 cells on ice for 10 min before adding the cells to the upper wells of chambers containing EGF or HRGβ and containing filters coated with LAM or COLL. Some variability was observed with the levels of basal migration in the data shown in A, because each growth factor titration was carried out in a separate chemotaxis chamber. However, the level of stimulation over that of basal migration for each growth factor was reproducible over at least three separate assays.
Contribution of Dimerization Partners with the EGFR and erbB3 in EGF and HRGβ Regulation of β1-Integrins
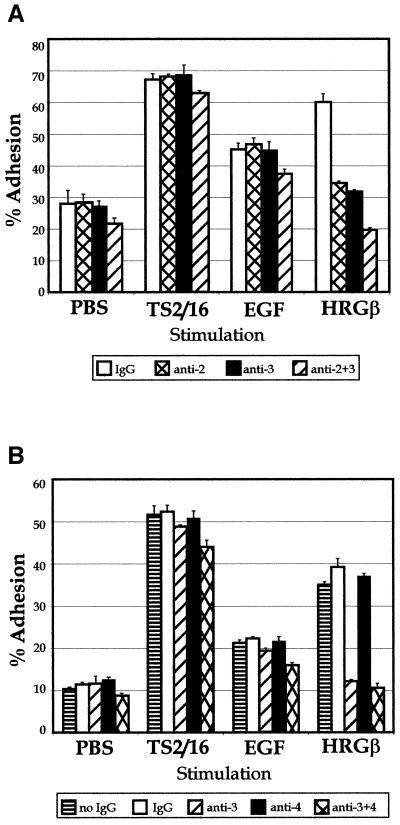
The observation that two mechanistically distinct growth factors, EGF and HRGβ, were capable of activating erbB2 phosphorylation and stimulating β1-integrin activity in MDA-MB-435 cells, coupled with the complex heterodimerization potential of the EGFR family of receptors (Lemmon and Schlessinger, 1994; Earp et al., 1995; Riese and Stern, 1998), led us to investigate the potential contribution of erbB2 as a dimerization partner with the EGFR or with erbB3 in mediating the unique effects of EGF and HRGβ on β1-integrin function. An anti-erbB3 antibody that blocks HRGβ binding (Chen et al., 1996) specifically abrogated HRGβ-induced adhesion of MDA-MB-435 cells without affecting EGF- or TS2/16-stimulated adhesion, even at high concentrations of antibody (Figure 5A and our unpublished data). Additionally, an anti-erbB2 antibody that blocks the effects of EGF or HRGβ binding to the dimerization partners of erbB2 (Klapper et al., 1998) negated HRGβ-stimulated adhesion without specifically affecting EGF or TS2/16 stimulation conditions (Figure 5A). The combination of both anti-erbB3 and anti-erbB2 antibodies gave a slightly stronger reduction in adhesion, but this decrease was consistent across unstimulated and all stimulated adhesion conditions. Although we could not detect erbB4 protein expression in our experiments with the MDA-MB-435 cells, it was possible that low but undetectable levels of erbB4 might be mediating the HRGβ effects that we observed. However, an antibody that blocks HRGβ binding to erbB4 (Chen et al., 1996) did not inhibit HRGβ-induced adhesion, even when combined with the erbB3 blocking antibody (Figure 5B). Although no specific effects of the erbB2 or erbB3 blocking antibodies were seen on EGF-mediated adhesion, only EGF-mediated adhesion was abrogated by the highly EGFR-specific inhibitor tyrphostin AG1478 (Fry et al., 1994) in a dose-dependent manner (our unpublished data). Because of the strong stimulation of cell migration initiated by HRGβ, we extended our antibody blocking studies to COLL and LAM migration assays to determine the receptor subunits contributing to these signals. Similar to the adhesion assays, anti-erbB3 and anti-erbB2 antibodies blocked HRGβ-stimulated MDA-MB-435 cell migration toward LAM (Figure 6, A and B) or COLL (our unpublished data). In addition, incubation of cells with the AG1478 tyrphostin had negligible effects on either unstimulated or HRGβ-stimulated cell migration (our unpublished data). EGF-stimulated migration of MDA-MB-435 cells was not affected by incubation with the erbB2 inhibitory antibody, as had been observed in the adhesion assays (Figure 6C).
Figure 5.
HRGβ–mediated adhesion of MDA-MB-435 cells to COLL requires heterodimerization with erbB2. Adhesion assays were performed as described for Figure 1. (A) To examine contributions by erbB2 and erbB3, control IgG (open bars) or blocking mAbs specific for erbB2 (cross-hatched bars), erbB3 (shaded bars), or a combination of anti-erbB2 and anti-erbB3 mAbs (hatched bars) were incubated with cells before addition to plates containing TS2/16, EGF, or HRGβ. (B) To examine contributions by erbB3 and erbB4, cells were incubated with no IgG (horizontally striped bars), control IgG (open bars), or blocking mAbs specific for erbB3 (hatched bars), erbB4 (solid bars), or a combination of anti-erbB3 and anti-erbB4 mAbs (cross-hatched bars) before addition to plates containing TS2/16, EGF, or HRGβ. The data reflect an average of three wells per condition and are representative of at least three separate assays.
Figure 6.
HRGβ-mediated migration of MDA-MB-435 cells on LAM requires heterodimerization with erbB2. Migration assays were carried out as described for Figure 4. To examine contributions by erbB3 (A) or erbB2 (B and C), control IgG (open bars) or blocking mAbs specific for erbB3 (A, solid bars) or erbB2 (B and C, solid bars) were incubated with cells before addition to chemotaxis chambers containing control media, EGF, or HRGβ. Cell migration was quantitated by taking the sum of four microscopic fields per well and the average of at least three wells per condition. Experiments were performed a minimum of three times.
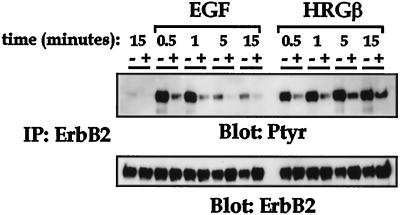
To further explore the lack of effect of the erbB2 blocking Ab-16 on EGF-stimulated adhesion and migration, we analyzed the phosphorylation status of erbB2 in response to EGF or HRGβ after exposure to Ab-16. As previously demonstrated in Figure 3, both EGF and HRGβ stimulate a time-dependent increase in the tyrosine phosphorylation of erbB2 (Figure 7), with EGF eliciting rapid and transient phosphorylation and HRGβ causing a rapid but more sustained phosphorylation of erbB2. Preincubation of cells with Ab-16 (+ lanes) dramatically reduced the activation of erbB2 phosphorylation by both EGF and HRGβ, whereas incubation with control mouse IgG (− lanes) had no effect. Taken together, these data indicate that both EGF and HRGβ stimulate their respective receptors, EGFR and erbB3, and subsequently cause increased phosphorylation of erbB2, presumably via a heterodimerization mechanism. However, although the anti-erbB2 Ab-16 functionally reduces the amount of tyrosine-phosphorylated erbB2 after stimulation with either growth factor, this antibody does not affect EGF-stimulated adhesion or migration (Figures 5 and 6), suggesting a differential role for erbB2 in EGF versus HRGβ-mediated regulation of β1-integrin function.
Figure 7.
Tyrosine phosphorylation of erbB2 after EGF or HRGβ stimulation is inhibited by the anti-erbB2 blocking Ab-16. MDA-MB-435 cells (5 × 106 per sample) were coated for 15 min on ice with either control mouse IgG (− lanes) or erbB2 Ab-16 (+ lanes) at 0.5 μg/1 × 106 cells. Cells were then stimulated for various times with 100 ng/ml EGF or HRGβ at 37°C followed by lysis in an equal volume of 2× lysis buffer. Immunoprecipitation for erbB2 was performed on cleared lysates, and samples were separated by SDS-PAGE. Immunoblotting for phosphotyrosine using 4G10 (upper panel) was performed after Western transfer. The membrane was then stripped and reprobed for erbB2 (lower panel) to confirm receptor levels. The figure shown is representative of a minimum of three independent assays.
Role of PI 3-K in EGF- and HRGβ-induced up-regulation of β1-Integrin function
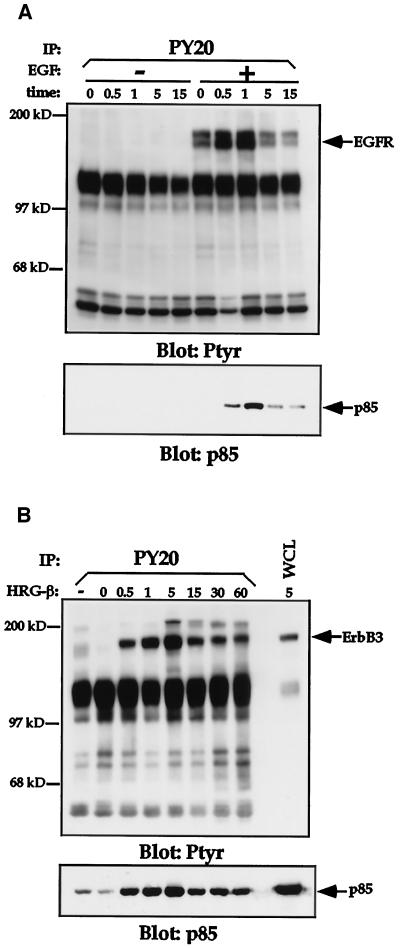
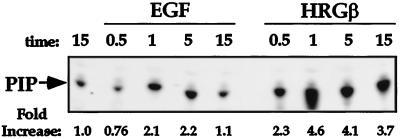
Because PI 3-K plays a role in signaling by the EGFR family members, we analyzed the contribution of this enzyme to the adhesion and migration events we had observed in the MDA-MB-435 cells. Stimulation of MDA-MB-435 cells with either EGF (Figure 8A) or HRGβ (Figure 8B) resulted in rapid recruitment of the p85 subunit of PI 3-K to the phosphotyrosine-containing cellular fraction, consistent with previous reports of growth factor-stimulated PI 3-K activation in other cell lines (Carraway et al., 1995). Analysis of in vitro PI 3-kinase activity from PY20 immunoprecipitates further demonstrated the increased activity of PI 3-K in response to EGF or HRGβ stimulation of MDA-MB-435 cells (Figure 9). Consistent with the effects of EGF and HRGβ on integrin-mediated adhesion, HRGβ induced more potent PI 3-K activity than did EGF (Figure 9).
Figure 8.
EGF and HRGβ stimulation of MDA-MB-435 cells induces recruitment of the p85 subunit of PI 3-K to the phosphotyrosine cellular fraction. Cells were serum starved for 24 h and harvested as previously described. Cells (7.5 × 106 per sample) were incubated in the absence or presence of 100 ng/ml EGF (A) or 100 ng/ml HRGβ (B) for the indicated periods at 37°C. Cells were then lysed, and phosphotyrosine-containing proteins were immunoprecipitated with the anti-phosphotyrosine mAb PY20 coupled to protein A-Sepharose beads. Washed immunocomplexes were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Western blotting was performed on membranes with the anti-phosphotyrosine mAb 4G10 to detect phosphotyrosine-containing proteins (upper panels). Blots were stripped and reprobed for EGFR or erbB3 (A and B, respectively; our unpublished data) and for p85 (lower panels). Similar data were obtained in at least two additional assays.
Figure 9.
EGF and HRGβ activate PI 3-K enzymatic activity in vitro. Cells were left unstimulated or stimulated in the presence of EGF or HRGβ (each at 100 ng/ml) for various times at 37°C. PY20 immunoprecipitates were prepared for a PI 3-K in vitro kinase assay as previously described (Chan et al., 1997). Kinase activity was detected by the in vitro phosphorylation of sonicated phosphatidylinositol, the products of which were visualized and quantitated after TLC separation, autoradiography, and phosphorimage analysis.
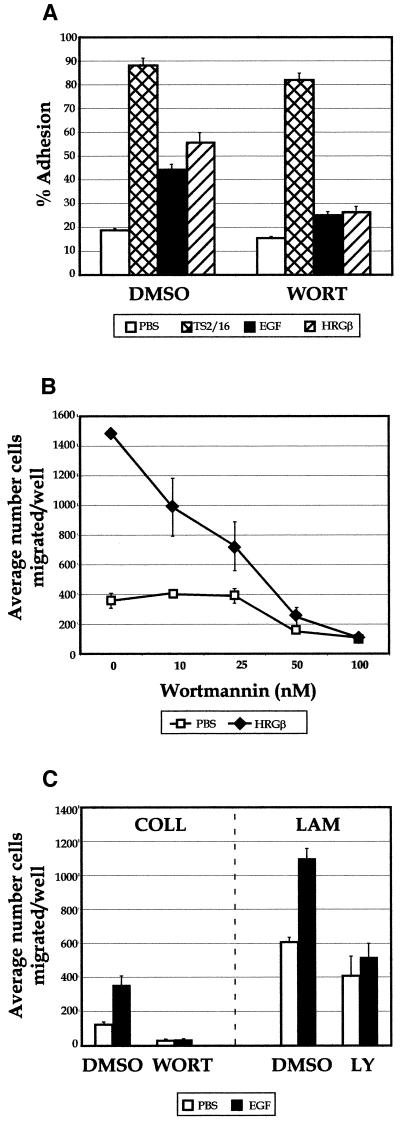
We tested the relevance of PI 3-K activation to adhesion with two pharmacologically distinct PI 3-K inhibitors, wortmannin and LY294002 (Arcaro and Wymann, 1993; Yano et al., 1993; Okada et al., 1994; Vlahos et al., 1994). When cellular adhesion assays were performed in the presence of 100 nM wortmannin (Figure 10A), we observed a significant decrease in both EGF- and HRGβ-stimulated MDA-MB-435 cell adhesion to COLL. In contrast, only small reductions in TS2/16-induced or unstimulated adhesion were observed. PI 3-K appears to contribute even more significantly to the process of migration in these cells, because wortmannin completely blocked HRGβ-mediated migration on LAM (Figure 10B) as well as COLL (our unpublished data). Similar results were obtained when adhesion and migration were assessed in the presence of 25 μM LY294002 (our unpublished data). In these experiments, unstimulated migration was also reduced, but only at the highest doses of the inhibitors. Although cells generally migrated in smaller numbers in experiments using EGF as a stimulus, motility induced by EGF on COLL and LAM was also strongly inhibited by wortmannin or LY294002 (Figure 10C).
Figure 10.
PI 3-K inhibitors block EGF- or HRGβ-mediated adhesion and migration of MDA-MB-435 cells. (A) Adhesion assays were carried out in the presence of 100 nM wortmannin (WORT) with no stimulation (open bars) or after stimulation for 10 min at 37°C with TS2/16 (cross-hatched bars), EGF (shaded bars), or HRGβ(hatched bars). (B) Migration analysis was carried out in the presence of increasing amounts of wortmannin in the presence of media only (open squares) or HRGβ (solid triangles). Similar effects were observed when dose–response analysis was carried out on COLL or when assays were performed in the presence of 25 μM LY294002 (our unpublished data). (C) Migration on COLL or LAM was performed in the presence of control DMSO, 100 nM wortmannin (WORT), or 25 μM LY294002 (LY) with no stimulation (open bars) or stimulation with 10 ng/ml EGF (solid bars). HRGβ stimulation was slightly lower than normal in the adhesion assay shown in A in comparison with EGF or TS2/16 stimulation, but the data shown are otherwise representative of at least three separate experiments.
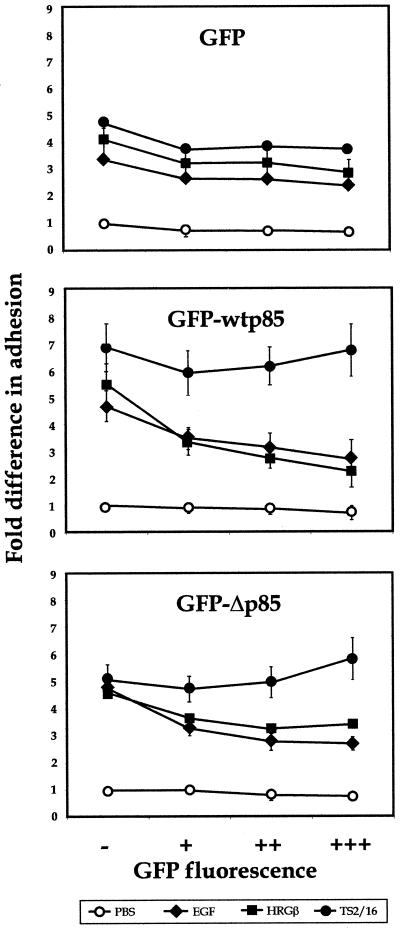
As an alternative approach, we used a transient transfection assay that allowed us to assess the adhesion and migration of untransfected as well as transfected cells by flow cytometric analysis of adherent or migrated cell populations (Chan et al., 1997; Kivens and Shimizu, 1999). Control vector expressing GFP alone or constructs expressing GFP-tagged wild-type p85 (GFP-wtp85) or a dominant negative p85 subunit (GFP-Δp85) were transiently transfected into MDA-MB-435 cells followed by analysis in a modified cell adhesion assay. As shown in Figure 11, increasing levels of GFP alone had little effect on the adhesion of MDA-MB-435 cells under any stimulation condition, whereas expression of either GFP-wtp85 or GFP-Δp85 subunits of PI 3-K decreased EGF or HRGβ-mediated adhesion by ∼50% without affecting TS2/16 or unstimulated adhesion significantly.
Figure 11.
Overexpression of the wild-type or dominant negative p85 subunit of PI 3-K inhibits EGF- or HRGβ-mediated increases in MDA-MB-435 adhesion to COLL. Control vector expressing GFP (top panel) or constructs expressing a GFP-wt p85 (middle panel) or GFP-Δp85 (bottom panel) fusion protein were transiently transfected into MDA-MB-435 cells as described in MATERIALS AND METHODS. Transfected cells were allowed to recover for 24 h and then placed in serum-free media overnight. Cells were harvested as for standard adhesion assays, except that no Calcein AM labeling was performed, and cells (∼300,000 cells per well) were added to 24-well plates coated with 6 μg/well COLL. Adhesion was analyzed in the presence of PBS alone (open circles) or containing 1 μg/well TS2/16 (solid circles), 100 ng/ml EGF (solid diamonds), or 100 ng/ml HRGβ (solid squares) for 10 min at 37°C. Nonadherent cells were washed away, and adherent cells were collected fromwells using a 1:1 trypsin:1 mM EDTA solution. Collected cells were then analyzed by flow cytometry using aliquots of preadherent cell populations to confirm cell numbers added to wells for each transfectant and to determine the percent expression of GFP in the starting cell populations. Percent adhesion was determined by gating GFP-negative (−), GFP-low (+), -middle- (++), and -high (+++)-positive cells and comparing preadherent and adherent cell numbers from each population. The data shown reflect fold differences in adhesion when compared with the GFP-negative, unstimulated cell subpopulation from each transfectant. Average percent adhesion was determined from samples examined in triplicate for each stimulation condition, and results were similar for a minimum of three independent assays.
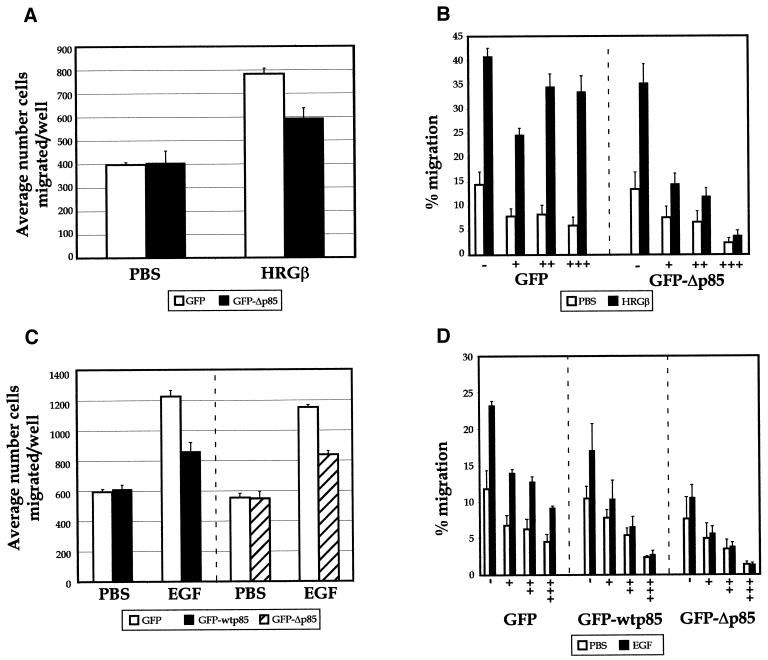
The effects of molecularly inhibiting PI 3-K function on HRGβ- or EGF-stimulated cell migration of MDA-MB-435 cells were also investigated. Comparison of control transfected or GFP-Δp85-transfected cell migration in standard Boyden chamber conditions revealed ∼25% inhibition upon expression of GFP-Δp85 in cells stimulated to migrate in the presence of HRGβ, whereas no striking inhibition of unstimulated migration was apparent (Figure 12A). However, analysis of the specific GFP-Δp85-positive cells in comparison with GFP-negative cells showed a striking inhibition of both HRGβ-stimulated and unstimulated cell migration with increasing expression of the GFP-Δp85 construct (Figure 12B), in keeping with our results using wortmannin and LY294002. Similar results were obtained when cells were transfected with GFP-wtp85 or GFP-Δp85 constructs and cell migration was stimulated with EGF (Figure 12C and D). Expression of the GFP protein alone had some inhibitory effect on overall migration, but the GFP-wtp85 or GFP-Δp85-positive cells effectively blocked EGF-stimulated migration in comparison with levels of unstimulated cell motility. Thus, as demonstrated using both pharmacological and molecular inhibition methods, both EGF- and HRGβ-stimulated pathways require functional PI 3-K for optimal β1-integrin-mediated adhesion and migration of MDA-MB-435 cells.
Figure 12.
Overexpression of the dominant negative p85 subunit of PI 3-K inhibits HRGβ- and EGF-mediated increases in MDA-MB-435 migration on LAM. Control vector expressing GFP or a construct expressing a GFP-Δp85 fusion protein were transiently transfected into MDA-MB-435 cells. Transfected cells were allowed to recover for 24 h and then placed in serum-free media for 24 h. Cells were harvested and quantitated as previously described. (A) To monitor the efficiency of stimulated migration on bulk transfected populations, standard chemotaxis analysis was performed as described in MATERIALS AND METHODS with aliquots of cells at the same concentration as used for migration in transwells (below). Total cell populations expressing GFP (open bars) or GFP-Δp85 (solid bars) were allowed to migrate in Boyden chambers in the presence or absence of HRGβ in the lower wells on LAM-coated filters overnight, and migrated cells were fixed, stained, and quantitated as described. (B) The migration of GFP-positive versus GFP-negative cells in each bulk transfectant cells was assessed specifically by allowing cells to migrate overnight in transwell chambers that had been precoated with LAM in the absence (open bars) or presence of 100 ng/ml HRGβ (solid bars). After ∼16 h, migrated cells were collected from the lower surface of LAM-coated filters with trypsin/EDTA and analyzed by flow cytometry as described for transient adhesion assays in Figure 11. Data represent the average of three wells per condition and show the percent migration of each GFP-negative or GFP-positive cell population. (C) Cells expressing GFP (open bars), GFP-wtp85 (solid bars), or GFP-Δp85 (hatched bars) were placed in the upper wells of Boyden chambers and allowed to migrate in the presence or absence of 10 ng/ml EGF on LAM-coated filters overnight, and migrated cells were fixed, stained, and quantitated as described. (D) The migration of GFP-positive transfected cells was assessed specifically by allowing cells to migrate overnight in transwell chambers that had been precoated with LAM in the absence (open bars) or presence of 10 ng/ml EGF (solid bars). After ∼16 h, migrated cells were collected from the lower surface of LAM-coated filters with trypsin/EDTA and analyzed by flow cytometry as described for transient adhesion assays in Figure 11. Data represent the average of three wells per condition and show the percent migration of each GFP-negative or GFP-positive cell population. Migration observed under standard Boyden chamber conditions (A and C) reflected increases in migration in response to stimuli comparable with those seen in the transwell analysis. Modest effects of GFP-wtp85 or GFP-Δp85 on migration were observed in the mixed cell populations in A and C but are more apparent upon single-cell analysis as shown in B and D. Comparison of assays carried out for 4 h vs. 16 h gave similar results, and the experimental data are representative of at least three independent assays.
DISCUSSION
In this study we describe the activation of the β1-integrin in breast carcinoma cells upon EGF or HRGβ growth factors binding to and activating members of the EGFR family of receptor tyrosine kinases. EGF treatment stimulated the rapid adhesion of MDA-MB-435 cells and increased cell migration toward ECM-coated surfaces in a β1-integrin-dependent manner. HRGβ, a growth factor activating erbB3 in these cells, was even more potent at stimulating both adhesion and migration of breast carcinoma cells. Both EGF and HRGβ use PI 3-K in pathways leading to increased adhesion and migration, and the more potent effects of HRGβ on adhesion and migration are associated with a greater ability of HRGβ to recruit and activate PI 3-K when compared with EGF. Furthermore, only HRGβ-mediated signals were significantly affected by an antagonistic antibody toward erbB2, indicating a preferential contribution of heterodimers containing erbB2 and erbB3 rather than erbB2 and the EGFR in eliciting these effects on adhesion and motility.
Communication between Growth Factor Receptors and Integrins
We observed significant increases in EGF- and HRGβ-mediated cell adhesion when binding was assessed on different ECM ligands, including FN, COLL, merosin, and LAM. Although the degree of HRGβ-stimulated adhesion was consistently higher than that seen for EGF, direct activation of the β1-integrin with the monoclonal antibody TS2/16 was capable of stimulating cellular adhesion on all ECM components examined, suggesting that the complexity of the EGFR family of receptors and their ligands plays a significant role in the response of these cells with respect to growth factor induction of integrin activation. Although other reports have described increases in cell adhesion upon overexpression of the EGFR (Lichtner et al., 1993; Lichtner et al., 1995; Verbeek et al., 1998), the low levels of EGFR, erbB2, and erbB3 in the MDA-MB-435 cells are sufficient to result in growth factor-mediated changes in β1-integrin-mediated adhesion and migration. Many reports have now illustrated the regulation of integrin function by cell surface receptors in a variety of cell types. Included in these are the increased adhesion through β1-integrins initiated by the PDGF (Kinashi et al., 1995) and c-kit (Kinashi and Springer, 1994; Serve et al., 1995; Vosseller et al., 1997) receptors. Although overexpression of the EGFR up-regulates adhesion of murine metastatic mammary cells (Lichtner et al., 1993, 1995), high levels of the EGFR inhibited the function of RGD-sensitive integrin receptors in human squamous carcinoma cells (Fujii, 1996). In contrast to these previous studies with overexpressed EGFR, our studies demonstrate that modest levels of the EGFR can functionally activate the β1-integrin in response to EGF or betacellulin stimulation in MDA-MB-435 breast tumor cells and further describe the potent increases in adhesion or migration initiated by HRGβ binding to erbB3. Importantly, this up-regulation occurred in the absence of significant changes in integrin or EGFR family receptors on the cell surface (our unpublished data). Furthermore, our studies illustrate an additional level of complexity in EGFR family signaling by demonstrating differences in β1-integrin activation through unique receptor pairs in response to EGF or HRGβ.
Historically, experimental data have described signaling from growth factor receptors or from integrins independent of each other. However, a cell in its native environment likely experiences multiple stimuli, both from available growth factors and from integrin-mediated interactions with its physical surroundings. Several studies recently have been reported that describe the synergistic actions of integrin and growth factor receptor stimulation. For example, activation of the αvβ3-integrin and the PDGF receptor regulates endothelial cell migration (Woodard et al., 1998) and potentiates PDGF-initiated signals (Schneller et al., 1997), and insulin (Guilherme et al., 1998) or interferon-γ (McCarthy et al., 1997) receptor signals are potentiated by cell adhesion to β1-integrin ECM ligands. Several recent reports have described cross-talk between the EGFR and integrins as assessed by activation of the MAP kinase pathway (Miyamoto et al., 1996; Moro et al., 1998; Wang et al., 1998). EGF stimulation also induces tyrosine phosphorylation of the α6β4-integrin, suppresses its association with several signaling and cytoskeletal molecules, and increases α6β4-dependent migration on LAM (Mainiero et al., 1996). Clearly, a highly regulated and complex network of signaling is beginning to emerge that uses available integrins and growth factor receptor signaling complexes on a given cell type.
Regulation of EGF- and HRGβ-stimulated Adhesion and Migration by PI 3-K
The identity of signaling molecules responsible for pathways of cellular communication between growth factor receptors and integrins has not been well characterized, but the requirement for PI 3-K activity in a variety of growth factor-regulated adhesion events has now been established, including those mediated by c-kit (Kinashi and Springer, 1994), PDGF (Kinashi et al., 1995), and insulin (Guilherme et al., 1998). Although evidence exists that PI 3-K is involved in unstimulated invasion by T47D (Keely et al., 1997) and MDA-MB-435 breast carcinoma cells (Shaw et al., 1997), our work has now demonstrated that ligand-activated EGFR family members also play an important role in augmenting β1-integrin-mediated adhesion and migration via activation of PI 3-K. Pharmacological inhibitors of PI 3-K gave strong inhibition of EGF- and HRGβ-mediated increases in adhesion, and the overexpression of dominant negative forms of p85 supported this observation, as shown by an ∼50% decrease in EGF- or HRGβ-mediated adhesion with high levels of GFP-Δp85. A significant decrease was also observed in the presence of high levels of GFP-wtp85, a result consistent with other studies demonstrating inhibition of downstream PI 3-K-dependent signaling upon overexpression of wild-type p85 (Rameh et al., 1995; King et al., 1997; Shaw et al., 1997). In contrast to our adhesion data, both pharmacological and molecular inhibition of PI 3-K in our migration assays revealed PI 3-K as an essential component of EGF- or HRGβ-driven signals to up-regulate β1-integrin-mediated migration, because treatment with wortmannin or LY294002 or overexpression of dominant negative p85 completely blocked EGF- or HRGβ-induced cell migration.
Thus, our results suggest that growth factor receptors coupled to PI 3-K can play a critical role in tumor cell adhesion and motility by augmenting β1-integrin function. It is interesting to note that increased migration of MDA-MB-435 cells also occurs upon expression of a transfected β4-integrin subunit, which activates PI 3-K much more effectively than does the β1-integrin subunit (Shaw et al., 1997). Thus, migration in untransfected MDA-MB-435 cells mediated by β1-integrins may require the activation of PI 3-K by growth factor receptors such as the EGFR family members. The cumulative activation of PI 3-K by a combination of growth factor receptors and integrins may therefore be critical to ensuring vigorous integrin-dependent migration of tumor cells. Our results are in contrast to a recent study that analyzed breast epithelial cell migration in response to EGF (Verbeek et al., 1998). In this report, transfectants overexpressing the EGFR showed a dramatic increase in EGF-stimulated cell migration in comparison with parental ZR75–1 cells. Incubation with wortmannin and LY294002 enhanced EGF-mediated migration of EGFR-overexpressing cells, whereas the MEK1 inhibitor PD98059 decreased EGF-stimulated migration. However, no effect of either inhibitor was observed on parental cells, reported to express ∼20,000 EGFRs on the cell surface compared with 1,200,000 EGFRs on transfected cells. The physiological relevance of effects of PI 3-K inhibitors on cells expressing such high levels of EGFR is currently unclear. Our results suggest that PI 3-K plays a positive role in both stimulated and unstimulated adhesion and migration of MDA-MB-435 cells, and we have noted little effect of inhibitors targeting the MAPK pathway (our unpublished data), consistent with reports by others (Shaw et al., 1997). These results are also in keeping with recent data demonstrating the wortmannin-sensitive but PD98059-insensitive regulation of EGF-stimulated suppression of membrane ruffling and increased lamellipodia formation in rat nonmetastatic mammary adenocarcinoma cells (Wyckoff et al., 1998), the HRG-inducible aggregation of breast cancer cells through a PI 3-K-dependent but MAPK-independent pathway (Tan et al., 1999), and a role for PI 3-K in EGF-stimulated bladder cancer cell motility (Theodorescu et al., 1998). These observations, and the fact that we see little effect of MAPK pathway inhibitors, are significant given the suggestion that subtle changes in levels and duration of MAPK activation might contribute to the differential outcome of specific growth factors acting on the same cell (Marshall, 1995; Pinkas-Kramarksi et al., 1998).
EGFR Family Heterodimerization and Regulation of β1-integrin Function
The importance of heterodimerization of EGFR family receptors for a variety of signaling processes is now clearly established (Earp et al., 1995; Alroy and Yarden, 1997; Riese and Stern, 1998). For example, both qualitative and quantitative differences in EGFR receptor phosphorylation and recruitment of p85 have been described after activation with EGF versus HRGβ in NIH3T3 cells expressing EGFR and erbB4 (Olayioye et al., 1998). The emerging common theme is that erbB2 recruitment into a heterodimer with other members of the EGFR family greatly intensifies the signaling capacity initiated by a given growth factor binding to its cognate receptor (Graus-Porta et al., 1995; Cohen et al., 1996; Zhang et al., 1996). Although the importance of the dimerization potential of this receptor family is now well accepted, virtually no work has been done to address the importance of these diverse signals in cellular adhesion and motility pathways. Our antibody blocking studies clearly show that the erbB2 receptor is a critical component of erbB3-mediated adhesion and migration pathways when stimulated by HRGβ in MDA-MB-435 cells. This antibody reduces diverse aspects of both EGF and HRG signaling, including ligand-dependent tumor growth, DNA synthesis, and receptor dimerization (Klapper et al., 1998). Our studies also confirm that this antibody reduces the transactivation effects of EGF and HRGβ on erbB2 as illustrated by the dramatically diminished tyrosine phosphorylation of erbB2 after preincubation with Ab-16 (Figure 7). However, to our surprise, we observed no antagonistic effects of this antibody on EGF-mediated regulation of β1-integrin function, even at 10-fold higher than normal antibody:cell ratios (our unpublished observations), as measured by changes in cell adhesion or migration. This unexpected result reinforces the notion that pathways used for cell proliferation are likely overlapping but unique from those used in cell motility and adhesion, as previously suggested by others (Chen et al., 1994). Furthermore, it suggests that, although the EGFR requires erbB2 for optimal stimulation of cellular responses such as proliferation, erbB2 may not be a critical component of EGF-stimulated changes in β1-integrin-dependent adhesion and migration on these cells.
It is intriguing that stimulation by HRGβ is much more potent than EGF at mediating adhesion and migration of MDA-MB-435 cells, particularly given the kinase-impaired status of the erbB3 receptor. Although erbB2 tyrosine phosphorylation is increased in response to both growth factors (Figures 3 and 7), our antibody blocking data clearly suggest that erbB2 plays a prominent role in erbB3- but not EGFR-mediated regulation of the β1-integrin. How this use of erbB2 differentially contributes to growth factor-mediated adhesion and migration is currently under investigation. Our data also reveal that both EGF and HRGβ require functional PI 3-K for optimal adhesion and migration responses, although erbB3 is considered to be a more effective direct recruiter of the p85 subunit of PI 3-K, bearing multiple optimal p85 binding sites in its carboxyl-terminal tail (Hellyer et al., 1998). It is possible that subtle differences in the mechanism of p85 activation and/or recruitment may contribute to the difference in strength of signals we observe between EGF and HRGβ. Indeed, our immunoprecipitation analysis suggests a strong and more sustained recruitment of p85 to the phosphotyrosine cellular fraction upon HRGβ stimulation compared with EGF (Figure 8). Furthermore, although p85 can be found in both erbB3 and erbB2 receptor immunoprecipitates after HRGβ stimulation, we have not detected its coassociation with the EGFR after EGF activation in MDA-MB-435 cells (our unpublished observations). Other differences in recruitment of signaling molecules to or physical regulation of dimer complexes may also be involved. For example, the cbl molecule is primarily recruited to the EGFR and not to other members of the EGFR family (Levkowitz et al., 1996), and the EGFR is significantly down-regulated from the cell surface in response to ligand binding, whereas erbB2, erbB3, and erbB4 are not regulated in the same manner (Baulida et al., 1996; Pinkas-Kramarksi et al., 1996). In further contrast, erbB3 does not bind to phospholipase Cγ or GAP GTPase-activating protein (Fedi et al., 1994), molecules commonly recruited to the ligand-activated EGFR. Given the current complexity of the EGFR family of receptors and their roles in the various cellular processes contributing to tumor cell formation and metastatic growth, it will be important to more clearly delineate the dimerization events undertaken by members of the EGFR family after the binding of specific ligands in a way that is informative for the signals generated by those ligands to up-regulate β1-integrins. This complexity is exemplified by the fact that varying reports have described mitogenic, growth-inhibitory, or differentiative effects of HRG stimulation depending on the growth factor isotype, the concentration used, and the cell line studied (Graus-Porta et al., 1995; Ram et al., 1995; Hamburger and Yoo, 1997; Xu et al., 1997). The consistent strength of response to HRGβ stimulation in our studies suggests that this growth factor may play a significant role in the regulation of mammary tissue growth and development, as well as processes regulating cellular transformation in this tissue, through pathways communicating to β1-integrins. Recent studies performed in a separate noninvasive breast cell line have also demonstrated the importance of PI 3-K as well as erbB2 recruitment in HRG-induced cytoskeletal reorganization and cell migration (Adam et al., 1998). Thus, our current understanding of erbB3 and erbB4 signaling upon HRG binding suggests a prominent role in regulating both noninvasive and metastatic breast cells.
In summary, our data clearly demonstrate the ability of EGF-family growth factors to rapidly up-regulate β1-integrin-mediated adhesion and enhance migration. We also have shown that EGF and betacellulin, ligands that activate the EGFR, and HRGβ, a member of the neuregulin family of ligands that bind to and activate erbB3 and erbB4, induce dose- and time-dependent adhesion of MDA-MB-435 cells to COLL through mechanisms requiring functional PI 3-K. EGF and HRGβ also up-regulate β1-integrin-mediated migration on COLL and LAM, again using PI 3-K-dependent pathways. Studies with blocking antibodies further suggest a differential use of erbB2 by the erbB3 receptor rather than the EGFR upon growth factor binding. These results identify a novel functional outcome for stimulation by EGFR ligands and a critical role for EGFR signaling via PI 3-K in regulating integrin-dependent tumor cell adhesion and motility.
ACKNOWLEDGMENTS
We thank B. Vacchani for technical assistance, N.J. Maihle for critical reading of the manuscript, and S. Shaw, T. LeBien, and M. Kamps for providing antibody reagents. This work was supported by Department of Defense grant DAMD17-97-1-7228. Y.S. is the Harry Kay Professor of Cancer Research at the University of Minnesota.
Abbreviations used:
- COLL
collagen
- ECM
extracellular matrix
- EGFR
EGF receptor
- FACS
flow cytometric analysis
- FN
fibronectin
- GFP
green fluorescent protein
- HRG
heregulin
- LAM
laminin
- PI 3-K
phosphoinositide 3-OH kinase
REFERENCES
- Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- Akiyama SK, Larjava H, Yamada KM. Differences in the biosynthesis and localization of the fibronectin receptor in normal and transformed cultured human cells. Cancer Res. 1990;50:1601–1607. [PubMed] [Google Scholar]
- Albelda SM. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993;68:4–17. [PubMed] [Google Scholar]
- Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Howe A, Alahari SK, Juliani RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Sanchez-Mateos P, Campanero MR, Martin-Padura I, Dejana E, Sanchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the β1 subunit. J Cell Biol. 1992;117:659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- Carraway KL, III, Soltoff SP, Diamonti AJ, Cantley LC. Heregulin stimulates mitogenesis and phosphatidylinositol 3-kinase in mouse fibroblasts transfected with erbB2/neu and erbB3. J Biol Chem. 1995;270:7111–7116. doi: 10.1074/jbc.270.13.7111. [DOI] [PubMed] [Google Scholar]
- Chan ASH, Mobley JL, Fields GB, Shimizu Y. CD7-mediated regulation of integrin adhesiveness on human T cells involves tyrosine phosphorylation-dependent activation of phosphatidylinositol 3-kinase. J Immunol. 1997;159:934–942. [PubMed] [Google Scholar]
- Chen P, Gupta K, Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol. 1994;124:547–555. doi: 10.1083/jcb.124.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Xie H, Wells A. Mitogenic signaling from the EGF receptor is attenuated by a phospholipase C-γ/protein kinase C feedback mechanism. Mol Biol Cell. 1996;7:871–881. doi: 10.1091/mbc.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, Leitner O, Ratzkin BJ, Bacus SS, Yarden Y. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–7629. [PubMed] [Google Scholar]
- Cohen BD, Kiener PA, Green JM, Foy L, Fell P, Zhang K. The relationship between human epidermal growth-like factor receptor and cellular transformation in NIH3T3 cells. J Biol Chem. 1996;271:30897–30903. doi: 10.1074/jbc.271.48.30897. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Earp HS, Dawson TL, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat. 1995;35:115–132. doi: 10.1007/BF00694752. [DOI] [PubMed] [Google Scholar]
- Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of β1-integrin function alters the development of murine mammary gland. EMBO J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedi P, Pierce JH, Di Fiore PP, Kraus MH. Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase Cγ or GTPase-activating protein, distinguished ErbB-3 signaling from that of other ErbB/EGFR family members. Mol Cell Biol. 1994;14:492–500. doi: 10.1128/mcb.14.1.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DW, Kraker AJ, McMichael A, Ambrosa LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- Fujii K. Ligand activation of overexpressed epidermal growth factor receptor results in loss of epithelial phenotype and impaired RGD-sensitive integrin function in HSC-1 cells. J Invest Dermatol. 1996;107:195–202. doi: 10.1111/1523-1747.ep12329606. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, Beerli RR, Hynes NE. Single-chain antibody-mediated intracellular retention of erbB-2 impairs neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol. 1995;15:1182–1191. doi: 10.1128/mcb.15.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme A, Torres K, Czech MP. Cross-talk between insulin receptor and integrin α5β1 signaling pathways. J Biol Chem. 1998;273:22899–22903. doi: 10.1074/jbc.273.36.22899. [DOI] [PubMed] [Google Scholar]
- Hamburger AW, Yoo JY. Phosphatidylinositol 3-kinase mediates heregulin-induced growth inhibition in human epithelial cells. Anticancer Res. 1997;17:2197–2200. [PubMed] [Google Scholar]
- Hellyer NJ, Cheng K, Koland JG. ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J. 1998;333:757–763. doi: 10.1042/bj3330757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GC, Ouyang Y, Epstein RJ. Proxy activation of protein erbB2 by heterologous ligands implies a heterotetrameric mode of receptor tyrosine kinase interaction. Biochem J. 1998;331:113–119. doi: 10.1042/bj3310113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller R, Cantley LC. Phosphatidylinositol 3-kinase. BioEssays. 1994;16:565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- Karunagaran D, Tzahar E, Beerli RR, Chen X, Graus-Porta D, Ratzkin BJ, Seger R, Hynes NE, Yarden Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 1996;15:254–264. [PMC free article] [PubMed] [Google Scholar]
- Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Kinashi T, Escobedo JA, Williams LT, Takatsu K, Springer TA. Receptor tyrosine kinase stimulates cell-matrix adhesion by phosphatidylinositol 3 kinase and phospholipase C-γ1 pathways. Blood. 1995;86:2086–2090. [PubMed] [Google Scholar]
- Kinashi T, Springer TA. Steel factor and c-kit regulate cell-matrix adhesion. Blood. 1994;83:1033–1038. [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivens WJ, Shimizu Y. Tracking integrin-mediated adhesion using green fluorescent protein and flow cytometry. In: Guan J-L, editor. Signaling through Cell Adhesion Molecules. Boca Raton, FL: CRC Press; 1999. pp. 219–234. [Google Scholar]
- Klapper LN, Vaisman N, Hurwitz E, Pinkas-Kramarksi R, Yarden Y, Sela M. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks cross-talk with growth factor receptors. Oncogene. 1998;14:2099–2109. doi: 10.1038/sj.onc.1201029. [DOI] [PubMed] [Google Scholar]
- Klippel A, Kavanaugh WM, Pot D, Williams LT. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach NL, Carlos TM, Yee E, Harlan JM. A monoclonal antibody to β1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J Cell Biol. 1992;116:499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994;19:459–463. doi: 10.1016/0968-0004(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Klapper LN, Tzahar E, Freywald A, Sela M, Yarden Y. Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene. 1996;12:1117–1125. [PubMed] [Google Scholar]
- Li J, Lin ML, Wiepz GJ, Guadarrama AG, Bertics PJ. Integrin-mediated migration of murine B82L fibroblasts is dependent on the expression of an intact epidermal growth factor receptor. J Biol Chem. 1999;274:11209–11219. doi: 10.1074/jbc.274.16.11209. [DOI] [PubMed] [Google Scholar]
- Lichtner RB, Kaufmann AM, Kittmann A, Rohde-Schulz B, Walter J, Williams L, Ullrich A, Schirrmacher V, Khazaie K. Ligand mediated activation of ectopic EGF receptor promotes matrix protein adhesion and lung colonization of rat mammary adenocarcinoma cells. Oncogene. 1995;10:1823–1832. [PubMed] [Google Scholar]
- Lichtner RB, Wiedemouth M, Noeske-Jungblut C, Schirrmacher V. Rapid effects of EGF on cytoskeletal structures and adhesive properties of highly metastatic rat mammary adenocarcinoma cells. Clin Exp Metastasis. 1993;11:113–125. doi: 10.1007/BF00880072. [DOI] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Yeon M, Ren YL, Giancotti FG. The intracellular functions of α6β4 integrin are regulated by EGF. J Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- McCarthy JB, Vachhani B, Wahl SM, Finbloom DS, Feldman GM. Human monocyte binding to fibronectin enhances IFN-γ-induced early signaling events. J Immunol. 1997;159:2424–2430. [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Sakuma L, Fukui Y, Hazeki O, Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:3563–3567. [PubMed] [Google Scholar]
- Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol. 1998;18:5042–5051. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkas-Kramarksi R, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- Pinkas-Kramarksi R, et al. ErbB tyrosine kinases and the two neuregulin families constitute a ligand-receptor network. Mol Cell Biol. 1998;18:6090–6101. doi: 10.1128/mcb.18.10.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- Ram TG, Kokeny KE, Dilts CA, Ethier SP. Mitogenic activity of neu differentiation factor/heregulin mimics that of epidermal growth factor and insulin like growth factor-I in human mammary epithelial cells. J Cell Physiol. 1995;163:589–596. doi: 10.1002/jcp.1041630320. [DOI] [PubMed] [Google Scholar]
- Rameh LE, Chen CS, Cantley LC. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI 3-kinase association with tyrosine-phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- Riese DJ, II, Stern DF. Specificity within the EGF family ErbB receptor family signaling network. BioEssays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Serve H, Yee NS, Stella G, Sepp-Lorenzino L, Tan JC, Besmer P. Differential roles of PI 3-kinase and kit tyrosine 821 in kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO J. 1995;14:473–483. doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Chao C, Wewer UM, Mercurio AM. Function of the integrin α6β1 in metastatic breast carcinoma cells assessed by expression of a dominant negative receptor. Cancer Res. 1996;56:959–963. [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HHF, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. Signaling to and from T cell integrins. In: Takada Y, editor. The Integrin: The Biological Problems. Boca Raton, FL: CRC Press; 1994. pp. 101–132. [Google Scholar]
- Shimizu Y, Hunt SW., III Regulating integrin-mediated adhesion: one more function for PI 3-kinase? Immunol Today. 1996;17:565–573. doi: 10.1016/s0167-5699(96)10061-x. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Regulated expression and binding of three VLA (β1) integrin receptors on T cells. Nature. 1990a;345:250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Roles of adhesion molecules in T cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990b;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PRJ, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Stroeken PJM, Van Rijthoven EAM, Van der Valk MA, Roos E. Targeted disruption of the β1 integrin gene in a lymphoma cell line greatly reduces metastatic capacity. Cancer Res. 1998;58:1569–1577. [PubMed] [Google Scholar]
- Tan M, Grijalva R, Yu DH. Heregulin β1-activated phosphatidylinositol 3-kinase enhances aggregation of MCF-7 breast cancer cells independent of extracellular signal-regulated kinase. Cancer Res. 1999;59:1620–1625. [PubMed] [Google Scholar]
- Theodorescu D, Laderoute KR, Gulding KM. Epidermal growth factor receptor-regulated human bladder cancer motility is in part a phosphatidylinositol 3-kinase-mediated process. Cell Growth Differ. 1998;9:919–928. [PubMed] [Google Scholar]
- Verbeek BS, Adriaansen-Slot SS, Vroom TM, Beckers T, Rijksen G. Overexpression of EGFR and c-erbB2 causes enhanced cell migration in human breast cancer cells and NIH3T3 fibroblasts. FEBS Lett. 1998;425:145–150. doi: 10.1016/s0014-5793(98)00224-5. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Vosseller K, Stella G, Yee NS, Besmer P. c-Kit receptor signaling through its phosphatidylinositide-3′-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol Biol Cell. 1997;8:909–922. doi: 10.1091/mbc.8.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware MF, Wells A, Lauffenburger DA. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J Cell Sci. 1998;111:2423–2432. doi: 10.1242/jcs.111.16.2423. [DOI] [PubMed] [Google Scholar]
- Woodard AS, García-Cardeña G, Leong M, Madri JA, Sessa WC, Languino LR. The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J Cell Sci. 1998;111:469–478. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Insel L, Khazaie K, Lichtner RB, Condeelis JS, Segall JE. Suppression of ruffling by the EGF receptor in chemotactic cells. Exp Cell Res. 1998;242:100–109. doi: 10.1006/excr.1998.4093. [DOI] [PubMed] [Google Scholar]
- Xie H, Pallero MA, Gupta K, Chang P, Ware MF, Witke W, Kwiatkowski DJ, Lauffenburger DA, Murphy-Ullrich JE, Wells A. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCγ signaling pathway. J Cell Sci. 1998;111:615–624. doi: 10.1242/jcs.111.5.615. [DOI] [PubMed] [Google Scholar]
- Xu F-J, Stack S, Boyer C, O’Briant K, Whitaker R, Mills GB, Yu YH, Bast RC., Jr Heregulin and agonistic antip185c-erbB2 antibodies inhibit proliferation but increase invasiveness of breast cancer cells that overexpress p185c-erbB2: increased invasiveness may contribute to poor prognosis. Clin Cancer Res. 1997;3:1629–1634. [PubMed] [Google Scholar]
- Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- Zell T, Hunt SW, III, Finkelstein LD, Shimizu Y. CD28-mediated up-regulation of β1 integrin-mediated adhesion involves phosphatidylinositol 3-kinase. J Immunol. 1996;156:883–886. [PubMed] [Google Scholar]
- Zhang K, Sun J, Liu N, Wen D, Chang D, Thomason A, Yoshinage SK. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J Biol Chem. 1996;271:3884–3890. [PubMed] [Google Scholar]