Abstract
Aims
The purpose of this prospective study was to investigate whether internet-based remote monitoring offers a safe, practical, and cost-effective alternative to the in-office follow-up visits of patients with an implantable cardioverter defibrillator (ICD).
Methods and results
Forty-one patients (62 ± 10 years, range 41–76, 83% male) with previously implanted ICD were followed for 9 months. One-hundred and nineteen scheduled and 18 unscheduled data transmissions were performed. There were no device-related adverse events. Over 90% of the patients found the system easy to use. Physicians reported the system as being ‘very easy’ or ‘easy’ to use and found the data comparable to traditional device interrogation in 99% of the cases. They were able to address all unscheduled data transmissions remotely. Compared with the in-office visits, remote monitoring required less time from patients (6.9 ± 5.0 vs. 182 ± 148 min, P < 0.001) and physicians (8.4 ± 4.5 vs. 25.8 ± 17.0 min, P < 0.001) to complete the follow-up. Substitution of two routine in-office visits during the study by remote monitoring reduced the overall cost of routine ICD follow-up by 524€ per patient (41%).
Conclusion
Remote monitoring offers a safe, feasible, time-saving, and cost-effective solution to ICD follow-up.
Keywords: Implantable cardioverter defibrillator, Remote monitoring, Usability, Cost-effectiveness
Introduction
The clinical use of implantable cardioverter defibrillators (ICDs) has been increasing rapidly since the results of several randomized trials confirmed the efficacy of ICDs in the secondary and primary prevention of sudden cardiac death.1–3 Patients with ICD require high-quality care and intense follow-up to ensure safe and effective device performance. According to international guidelines, ICD patients should be followed at 1- to 4-month intervals, depending on the device model and the patient’s clinical status.2 Given the expanding indications for use and the complexity of these devices, there is an urgent need to develop new means of ICD follow-up, so as to optimize patient safety and the use of healthcare resources. It has been suggested that an internet-based remote-monitoring system could provide a practical substitute to time-consuming and expensive in-office visits.4,5 Although the initial experience with these systems has been favourable,4,6–8 many practical issues remain. In particular, more information is required on the usability and safety of remote monitoring for patient-initiated transmissions and cost-effectiveness of the system as a substitute for routine in-office visits during long-term follow-up.
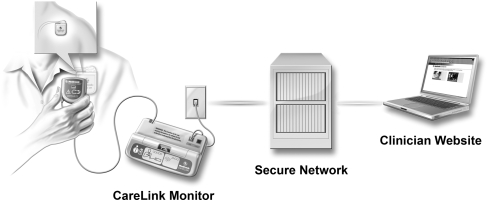
In this study, we report the first experiences in Europe with the Medtronic CareLink™ system. The system consists of a portable patient monitor, a central database in a secure server, and a password-protected website, where clinicians can view and analyse patient's device data (Figure 1). Our aim was to provide comprehensive data on the safety, clinical practicability, and cost-effectiveness of remote ICD monitoring in an area characterized by long travelling distances to the device clinic. The economic impact of remote ICD monitoring was evaluated by comparing the direct and indirect costs of remote follow-up to those of standard clinical practice.
Figure 1.
Medtronic CareLink remote-monitoring system. Adapted from Schoenfeld et al.6 The system consists of a portable patient monitor, central database in secure server, and a password-protected website, where clinician can view and analyse patient device data.
Methods
Study design and patient population
This prospective, non-randomized single-centre study was conducted between May 2005 and October 2006 in the Oulu University Hospital, Oulu, Finland. The primary objective of the study was to evaluate whether an internet-based remote-monitoring service offers a safe alternative to office visits in ICD follow-up. The secondary objectives were to assess: (i) the ease of use, satisfaction and acceptance of data interrogation and transmission by the patients, (ii) the ease of use and satisfaction of the clinicians with respect to reviewing device data via the website, and (iii) the travel burden on the patients and the workload of the clinic in order to calculate the economic impact of remote ICD monitoring.
The study was conducted according to the Declaration of Helsinki concerning medical research. The protocol was approved by the Local Medical Ethics Committee. All patients with a previously implanted ICD that was supported by the Medtronic CareLink remote-monitoring service were asked to participate in the study. Only patients with no access to a standard analogue telephone line or who had a hearing or other physical or mental problem hindering the use of the system were excluded from the study. The patients were medically treated at the discretion of the physician. After giving written informed consent for the study, each patient was provided with a unique monitor to self-interrogate the ICD and transmit all data within device memory (e.g. programmed and measured parameters, diagnostic information including all stored intracardiac electrograms and a 10 s real-time sample of the presenting rhythm) to the central database via a standard analogue phone line.
Study protocol
According to the study protocol, the first remote data transmission (test) was performed 1–2 weeks after inclusion of the patient into the study, and a scheduled remote data transmission at 3 and 6 months substituted for the generally recommended in-office ICD follow-up visits. If the subject forgot the data transmission, this was remedied through a call from a study nurse. A regular follow-up visit was performed in the hospital at 9 months. The patients were able to initiate additional remote interrogations due to symptoms by calling the study nurse or doctor. Likewise, the physicians were able to increase the frequency of remote interrogations to monitor elective replacement indicators or if the patient or device were otherwise considered as requiring more intensive follow-up.
All remote transmissions were evaluated by two experienced electrophysiologists, who had access to the device information by logging onto a password-protected and encrypted study-specific website. Cross-checking of the data analysis was performed if either of the electrophysiologists wanted a second opinion. There was no disagreement between the electrophysiologists in the data analysis.
Assessment of the system safety and performance
The percentage of successful transmissions that did not require a troubleshooting phone call was calculated for all data transmissions. Each of the troubleshooting calls was initially evaluated by the clinical personnel. The complexity of the calls was analysed by whether the clinical personnel triaged the phone call to a support centre representative. In addition, detailed information on all technical problems was collected, and the observations were reviewed by an independent Adverse Event Advisory Committee (AEAC).
Assessment of the usability of the system
The ease of use, satisfaction with, and acceptance of the remote-monitoring system were assessed by questionnaires completed by the patients and hospital staff after each data transmission/evaluation (at test, 3, and 6 months) and in-office visit (baseline and 9 months). The patients were also asked to rate their overall satisfaction with the remote-monitoring system and whether they preferred in-office visits or remote monitoring. The clinicians’ questionnaire focused on the usability of the website. In addition, the physicians were asked to evaluate whether the remote data were comparable to those obtained during traditional device interrogation.
Assessment of the time burden of the patients and the device clinic
The effect of remote monitoring on the time burden of the patients was assessed by comparing the time used for the in-office visits (travel time plus time in the hospital) to the time required to complete the ICD self-interrogation and data transmission. Similarly, data with regard to time consumption were analysed and compared for the remote transmissions and in-office visits. The time used for training of the patients by the study nurses was included in the data analysis.
Assessment of the economic impact of remote implantable cardioverter defibrillator monitoring
Both the direct and indirect expenditures of ICD follow-up were analysed. The municipalities were responsible for the direct cost of regular ICD follow-up of their residents except for a 22€ fee per visit paid by the patient. The indirect cost reimbursed by the Social Insurance Institution of Finland (KELA) consisted of travel and accommodation costs and sickness allowance for the patients and accompanying persons. All travel costs of the patients and necessary escorts in excess of a fixed co-payment (9.25€ per one-way trip) were refunded by the KELA. If the patients or the accompanied persons had to make an overnight stop due to reasons associated with the disease, they were also entitled to an accommodation allowance. Sickness allowance represents a compensation for income lost due to temporary incapacity for work. In this study, the average reimbursement paid by the KELA (44.00€/day) was used in the calculations, although the actual cost for the employers may have been greater.
The direct cost of the remote follow-up to the municipalities consisted of the payments to the hospital. During the course of the study, the use of the CareLink network and data evaluation by the physicians was free of charge. Therefore, currently effective prices of these services were used in the economic calculations. At Oulu University Hospital, the fee for the remote follow-up service is covered by the remote transmission evaluation fee, which is paid completely by the municipalities.
Statistical analysis
Descriptive statistics were used to summarize a patient's satisfaction with the monitor. Missing answers were reported in the tables/figures as ‘missing’. All data are shown as mean ± SD or frequencies (with percentages). To assess subject safety with respect to the use of the CareLink network, the primary endpoint of the study was to calculate the upper one-sided 90% exact confidence interval (CI) for the rate of unanticipated serious adverse device effects (USADEs). USADEs were identified from the adverse event information. It was calculated that if no subject out of 30 patients experienced any adverse events the USADE rate with 90% CI would be < 7.5%. It was conservatively estimated that 25% of the subjects would not complete the protocol. Therefore, a minimum of 40 subjects were to be enrolled into the study.
Results
Subject demographics
Forty-one patients (34 males and 7 females) with a previously implanted ICD supported by the Medtronic CareLink remote-monitoring service were included in the study. The mean age of the patients was 62 ± 10 years (range 41–76 years). The indication for the ICD implantation was secondary prevention of sudden cardiac death in 37 (90%) patients and primary prevention in 4 (10%) patients. Thirty patients (73%) had prior myocardial infarction and 5 (12%) had dilated cardiomyopathy. Most of the patients were in stable clinical condition as only one patient (2%) had New York Heart Association III symptoms and no patients were in class IV (Table 1). The most common symptoms at study inclusion included occasional dyspnoea (32%) and palpitations (34%). The implanted devices consisted of Medtronic GEM family VR (n = 21) and DR (n = 3), Marquis VR (n = 6), Maximo DR (n = 3) and VR (n = 6), and biventricular InSync ICD (n = 2).
Table 1.
Clinical characteristics of the patients
| Age (years) | 62 ± 19 (range 41–76) |
| Sex (male/female) | 34 (83%)/7 (17%) |
| ICD indication | |
| Primary prevention | 4 (10%) |
| Secondary prevention | 37 (90%) |
| LVEF (%) | 43 ± 15 |
| NYHA class (I/II/III/IV) | 15/25/1/0 |
| Prior MI | 30 (73%) |
| Prior CABG | 19 (46%) |
| Non-ischaemic cardiomyopathy | 5 (12%) |
| LQTS | 2 (5%) |
The numbers are mean ± SD. ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; MI, myocardial infarction; CABG, coronary artery bypass grafting; LQTS, long QT syndrome.
System safety and performance
No device-related adverse events and four technical observations were reported during the study. All independent AEAC members agreed that these observations were not adverse events. Hence, the rate of USADEs was <7.5%, and the safety objective was met.
Over 90% (95% CI: 85–95) of the data transmissions were performed without troubleshooting phone call. All troubleshooting calls occurred during the test transmissions. Ten of 12 troubleshooting calls were easily resolved by the patient, the hospital, or the telephone operator. In two cases, the patient monitor was replaced because of problems in connecting the monitor to the phone line. None of the problems remained unresolved after contact with the system helpline representative, and the data were always correctly displayed on the website.
Ease of use and satisfaction
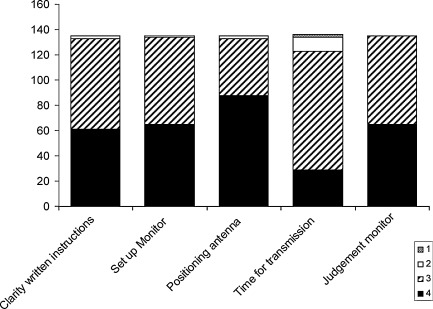
Patients’ ease of use and satisfaction are depicted in Figure 2. There were no statistically significant differences in the distribution of the patients' replies at test, 3, 6 months, and unscheduled visits. Therefore, the values in Figure 2 represent the data for all remote transmissions. The majority of the patients found the clarity of the written instructions ‘very clear’ or ‘clear’. Likewise, positioning of the antenna and setting up the monitor were ‘very easy’ or ‘easy’ for most patients, and 80% of the remote-monitoring sessions were performed by the patients without any assistance. The overall judgement was that the use of the patient monitor was better than expected in 40% of the cases and as expected in 54%.
Figure 2.
Patient's satisfaction with the use of the system. Answers are represented at a scale from zero to four. Clarity of written instructions: 4 = very clear, 3 = clear, 2 = unclear, 1 = very unclear; setup of CareLink monitor/positioning of the antenna/overall judgement of monitor: 4 = very easy, 3 = easy, 2 = difficult, 1 = very difficult; time needed for transmission: 4 = very short, 3 = short, 2 = long, 1 = very long.
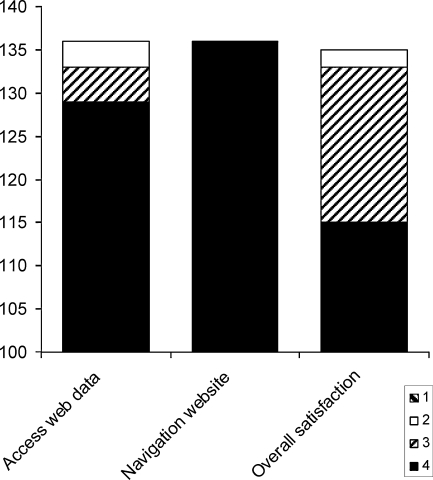
Clinicians’ ease of use and satisfaction are depicted in Figure 3. As there were no statistically significant differences in the distribution of the clinicians replies at the various transmission times, the values in Figure 3 represent the data for all remote transmissions. Almost all transmissions (97%) were reported by the physicians as being ‘very easy’ or ‘easy’ to access on the website, while website navigation was always found to be ‘very easy’. Physicians were satisfied with the performance of the system and found the data comparable to traditional device interrogation in the majority of the cases. In 2 of 137 cases, the physicians felt that an in-office visit would have provided more detailed information of the device function, because it was not possible to measure the pacing threshold remotely.
Figure 3.
Physician satisfaction with the use of the system. Answers are represented at a scale from zero to four. Access to web data/Navigation CareLink website: 4 = very easy, 3 = easy, 2 = difficult, 1 = very difficult; overall satisfaction about CareLink monitor: 4 = very satisfied, 3 = satisfied, 2 = unsatisfied, 1 = very unsatisfied.
Effect of remote monitoring on the time burden of the patients and the device clinic
The time needed by the patients for remote data transmission (6.9 ± 3.7 min, range 2.3–17.5 min) was significantly shorter than the duration (travel time + time in the hospital) of an in-office visit 391 ± 282 min (range 41–1346 min), P < 0.001. The average one-way distance and travel time to the hospital were 130 ± 95 km (range 3–350) and 182 ± 148 min (range 10–670 min), respectively. Most patients (90%) classified the time needed for the remote data transmission for all follow-ups as very short (21%) or short (69%).
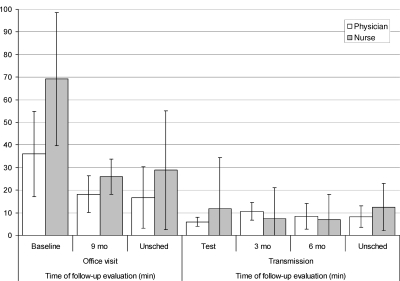
The impact of remote monitoring on the time burden of the physicians and additional hospital staff is presented in Figure 4. The time needed by the physician for reviewing device data on the secured website (8.4 ± 4.5 min, range 2–30 min) was significantly shorter than the time needed for completing an in-office follow-up (25.8 ± 17.0 min, range 5–90 min), P < 0.001. In keeping with this, it was more time-consuming also for the additional hospital staff to complete an in-clinic visit than remote monitoring (45.3 ± 30.6 min vs.9.3 ± 15.9 min, P < 0.001).
Figure 4.
Time burden for the hospital staff. The graph represents the mean time in minutes ± SD that is needed for the clinician and nurse to do the follow-up of a patient in the office vs. the time needed to evaluate a transmission.
Economic impact of remote monitoring
During the study, two generally recommended in-office visits were substituted by remote data transmission. A routine ICD follow-up, including clinical and device evaluation by a cardiologist, at Oulu University Hospital costs 210€. The use of the monitor was free of charge to the patients and they called a toll-free number. The fee to the municipality was 55€ per transmission evaluation (i.e. the same as for a paper consultation). Thus, replacement of an in-office visit by a remote data transmission reduced the direct costs of ICD follow-up to the healthcare providers by 155€. In addition, the patients saved 22€ because they did not have to pay the fee for an outpatient visit. Accordingly, compared with the generally recommended ICD follow-up scheme, remote monitoring reduced the direct cost ICD follow-up among the study population from 38 048€ to 23 534€ (38%) (Table 2).
Table 2.
Comparison between the cost of ICD follow-up according to the generally applied follow-up scheme and the study protocol among our population (n = 41)
| Generally applied follow-up scheme | Study protocol | Savings | |
|---|---|---|---|
| Number of scheduled visits | |||
| In-office visits* | 164 | 82 | |
| Remote data transmission** | 0 | 82 | |
| Direct cost | |||
| In-office visit (210€ per visit) | 34 440.00€ | 17 220.00€ | 17 220.00€ |
| Remote monitoring (55€ per visit) | 0.00€ | 4510.00€ | −4510.00€ |
| Patient fee (22€ per in-office visit) | 3608.00€ | 1804.00€ | 1804.00€ |
| Indirect cost | |||
| Traveling (77.68€ per in-office visit) | 12 195.04€ | 6097.52€ | 6097.52€ |
| Accommodation (20.18€/night) | 40.36€ | 20.18€ | 20.18€ |
| Sickness allowance (44€/day) | 1672.00€ | 836.00€ | 880.00€ |
| Total costs | 51 955.40€ | 30 487.70€ | 21 467.70€ |
| Total costs per patient | 1267.20€ | 743.60€ | 523.60€ |
*According to the generally applied follow-up scheme and the study protocol, every patient (n = 41) would have had four and two in-office visits during the 9 months follow-up period, respectively.
**According to the study protocol, every patient conducted two remote transmissions during the study period, whereas no remote transmissions would have been conducted according to the generally applied follow-up scheme.
The average distance to the device clinic was 130 ± 95 km (range 3–350). The majority of the patients used their own car to travel to the device clinic (66 visits). Other transportation modes included taxi (seven visits) and public transportation (six visits).The KELA reimbursed patients for travelling by public transportation or for using their own car at 0.20€/km, by a taxi at 1.16€/km, plus a starting fee of 4.50€. The average travelling cost of the patients and the accompanying persons was 74.36 ± 103.88€ (range 1.20–797.80€) per outpatient visit. In contrast, no travelling expenses were caused by remote data transmissions. One patient had to make an overnight stop when visiting the device clinic. The accommodation allowance paid by the KELA was 20.18€ per night, and the total cost of accommodation among the study population was 20.18€.
In nine instances the patient (11%) and in 10 instances an accompanying person (12.5%) had to be on sick-leave because of the routine in-office visits. By using the average value of daily sickness allowance (44.00€/day), it was calculated that the cost for the sickness allowance during the study period was 836€. No subjects were on sick leave due to remote monitoring. Eliminating the need for travelling and sickness allowance during remote monitoring reduced the indirect cost of ICD follow-up by 6954€. In summary, among the study population, substitution of two in-office visits during the 9-month follow-up period by remote monitoring accounted for a savings of 21 468€ (41%) in the total cost (Table 2).
There were 18 unscheduled patient- or physician-initiated data transmissions during the study period. In all of these cases, the physicians were able to address the problems remotely and there was no need for additional travelling and daily sickness allowance. An example of a symptom- initiated data transmission is shown in Figure 5. If the patients had had to visit the hospital for reassurance, the additional cost to the city would have been 2790€ (18 × 155€), 1060.10€ to the KELA, and a total of 990.60€ to the patients. The cost for patients consists of fee for travelling and in-office visits. The cost for the KELA consists of the remainder of the travel cost (average travel distance 187.5 ± 111.6 km) and sickness allowance of 2 days (one for the patient and one for the accompanying person).
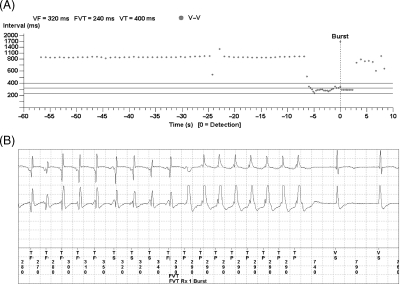
Figure 5.
Interval (V–V) plot (A) and intracardiac ECG (B) obtained during an unscheduled data transmission. The patient had occasional palpitation about once a month. The remote data transmission revealed a fast VT episode that was appropriately treated by the device with single burst pace therapy and there were no need for an in-office visit.
Discussion
Our data indicate that remote ICD monitoring with the Medtronic CareLink system provides a safe, practical, and cost-effective alternative to time-consuming in-office visits. The system was easy to use and both the patients and hospital staff were satisfied with it. In addition, compared with the generally recommended routine ICD follow-up scheme, remote monitoring diminishes the annual cost of ICD follow-up by 524€ (41%) per patient in an area characterized by long travelling distances to the device clinic.
Safety and practicability of remote implantable cardioverter defibrillator monitoring
According to international guidelines, patients with ICDs should be followed at ∼3-month intervals, depending on the device model and the patient’s clinical status.2 As no adverse events were reported during the 9-month study period, remote monitoring with the Medtronic CareLink service was proven safe. This indicates that at least two of four routine in-office ICD follow-up visits can be replaced by remote monitoring without compromising patient safety.
In line with the results of previous studies, both the physicians and patients were satisfied with the performance of the remote-monitoring system in routine ICD follow-up.5,6 Since all unscheduled data transmissions were resolved using the remote-monitoring system, our data suggest that remote monitoring can be safely used to evaluate symptoms of the patients and to detect potential problems with the device (e.g. depletion of the battery). This eliminates the need for unwarranted trips to the emergency room and device clinic, which is like to alleviate the anxiety of the patients as remote monitoring provides a prompt response to their concerns. Recently, remote monitoring with automatic data transmission has also been shown to improve the early detection of device malfunction and asymptomatic arrhythmias such as atrial fibrillation.6,9,10 This enables proactive device- and medical therapy and allows better monitoring of the efficacy of therapeutic interventions among high-risk patients. In our study, antiarrhythmic medication was optimized in six cases under remote surveillance.
Time burden of the patients and device clinics
As all data gathered during a normal in-office device interrogation can be sent remotely to the device clinic for evaluation, the patients and accompanying persons save time by using the CareLink network. The average timesaving was ∼3 h per patient per visit and it was directly related to the travelling distance to the device clinic.
In our study, two of four in-office visits were substituted by remote monitoring. This means that during the study period, the physicians would have had ∼45 min and the other hospital staff ∼90 min more time for other activities per patient, respectively. Although it is obvious that the time saved will release resources for other activities, the economic value of this remains to be established.
Clinical usability of the CareLink network and comparison with other remote implantable cardioverter defibrillator monitoring systems
Our analysis reveals that remote monitoring can reduce the number of routine follow-up visits by at least half without compromising patient safety. Like other investigators,5,6,11 we demonstrated that remote transmission of ICD data is not only safe but also extremely convenient for patients and clinicians. One of the major benefits of the CareLink network is that practically all Medtronic ICDs that can be interrogated with the programmer can also transmit data through the network, whereas some of the other available systems operate only with the latest ICD models with a specific transmitter inside the device.4,12
However, we also identified limitations for the clinical usability of the system. The main problem of the present CareLink network is that device data must be transmitted via a standard analogue phone line. In Finland, there are currently ∼5.7 million cellular phones (108 per 100 inhabitants) and only 1.9 million analogue phone lines (http://www.stat.fi/til/tvie/tau_en.html). Thus, it is not surprising that presently ∼50% of the patients in our hospital district cannot use the system. Therefore, a system that can send data automatically via a mobile phone network4,12 would be a more attractive alternative in our area, especially if it can also support older devices. During this study, patient co-operation was essential for data transmission. In contrast, the latest version of the CareLink network supports automatic wireless data transmission from the device to the patient monitor, making the system even more user-friendly for patients implanted with an ICD supporting automatic wireless transmission and alerts. Finally, it should be emphasized that remote programming of the device is not possible in any of the commercially available remote follow-up systems. In the current study, we found problems in two patients in that it was not possible to measure the pacing threshold remotely. In the latest ICD model, the threshold measurement and adjustment are done automatically, which, in conjunction with automatic data transmission, is likely to improve the safety of pacemaker-dependent patients.
Cost-effectiveness of remote implantable cardioverter defibrillator monitoring
As a result of expanding indication for use and complexity of the devices, the costs associated with ICD follow-up have risen sharply over the past several years. We calculated that the substitution of two scheduled routine in-office visits by remote monitoring reduced the total expenditure of ICD follow-up by 524€ per patient during the 9-month study period. In addition, an average of 100€ per patient was saved, because all unscheduled data transmissions (n = 18) during the study period were solved remotely and the patient did not need to come to the hospital for reassurance. Thus, depending on the number of unscheduled visits, it can be calculated that the annual saving of remote monitoring in our hospital district is 524–749€ per patient. This suggests that even in centres with shorter distances to the device clinic and longer than generally recommended follow-up intervals, the cost of the ICD follow-up would be reduced if every other routine in-office visit is substituted by remote monitoring, and the majority of the symptom-related episodes were solved remotely.
The major indirect cost driver in the ICD follow-up is travelling to the hospital. Therefore, the greatest cost benefit is expected among patients who live far away from the device clinic and are still actively working (not retired). Fauchier et al.13 calculated recently that remote monitoring reduced the overall cost of ICD follow-up when the distance between home and the device clinic was >100 km. The saving is obvious also among patients with primary prevention indication for ICD implantation.14 In Western Europe, ∼160 ICDs per million inhabitants are implanted annually. On the basis of our results, it can be calculated that if remote monitoring were to be applied to all the patients with new ICDs, the annual saving for the healthcare system would be 16–23 million euros. In addition, remote monitoring gives physicians extra time to counsel patients with critical conditions, ensuring medical efficiency, and better overall patient management, which is expected to reduce the cost of the treatment even further.
Limitations
Defining medical costs is a complicated process, because medical prices are typically based upon a patient’s needs and/or the relationship with a particular insurance firm or healthcare provider. Therefore, our results cannot be directly extrapolated to other countries and healthcare systems. Nevertheless, it is likely that remote monitoring would save a substantial amount of time and money, regardless of the economic system. As the primary aim of this study was to evaluate the safety and feasibility of remote ICD monitoring, further prospective randomized studies are needed to identify patients who would benefit most from proactive device follow-up and improved monitoring of the efficacy of therapeutic interventions.
Conclusions
The launch of remote monitoring is an important milestone in the management of ICD patients. It provides a tremendous convenience for patients and clinicians and reduces the cost of follow-up. Although the technology is not intended to replace direct patient contacts completely, it can indeed release resources for other activities and help to maintain proactive patient care.
Funding
Funding to pay the Open Access publication charges for this article was provided by Medtronic Inc.
Acknowledgements
The authors thank research nurses Eija Niemelä and Outi Keskitalo for their expert assistance during the study.
Conflict of interest: M.M.E. van Ginneken and J.P.G. Janssen are employees of Medtronic Bakken Research Center B.V.
References
- 1.Raj SR, Sheldon RS. The implantable cardioverter-defibrillator: does everybody need one? Prog Cardiovasc Dis. 2001;44:169–94. doi: 10.1053/pcad.2001.29146. [DOI] [PubMed] [Google Scholar]
- 2.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: the task force for cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2007;28:2256–95. doi: 10.1093/eurheartj/ehm305. [DOI] [PubMed] [Google Scholar]
- 3.Seidl K, Senges J. Geographic differences in implantable cardioverter defibrillator usage. J Cardiovasc Electrophysiol. 2002;13:S100–5. doi: 10.1111/j.1540-8167.2002.tb01961.x. [DOI] [PubMed] [Google Scholar]
- 4.Theuns DA, Res JC, Jordaens LJ. Home Monitoring in ICD therapy: future perspectives. Europace. 2003;5:139–42. doi: 10.1053/eupc.2002.0302. [DOI] [PubMed] [Google Scholar]
- 5.Brugada P. What evidence do we have to replace in-hospital implantable cardioverter defibrillator follow-up? Clin Res Cardiol. 2006;95:III3–9. doi: 10.1007/s00392-006-1302-x. [DOI] [PubMed] [Google Scholar]
- 6.Schoenfeld MH, Compton SJ, Mead RH, Weiss DN, Sherfesee L, Englund J, et al. Remote monitoring of implantable cardioverter defibrillators: a prospective analysis. Pacing Clin Electrophysiol. 2004;27:757–63. doi: 10.1111/j.1540-8159.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 7.Deharo JC, Djiane P. Home monitoring: what can we expect in the future? Clin Res Cardiol. 2006;95:III36–9. doi: 10.1007/s00392-006-1307-5. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30:s2–12. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 9.Scholten MF, Thornton AS, Theuns DA, Res J, Jordaens LJ. Twiddler’s syndrome detected by home monitoring device. Pacing Clin Electrophysiol. 2004;27:1151–2. doi: 10.1111/j.1540-8159.2004.00599.x. [DOI] [PubMed] [Google Scholar]
- 10.Res JC, Theuns DA, Jordaens L. The role of remote monitoring in the reduction of inappropriate implantable cardioverter defibrillator therapies. Clin Res Cardiol. 2006;95:III17–21. doi: 10.1007/s00392-006-1304-8. [DOI] [PubMed] [Google Scholar]
- 11.Joseph GK, Wilkoff BL, Dresing T, Burkhardt J, Khaykin Y. Remote interrogation and monitoring of implantable cardioverter defibrillators. J Interv Card Electrophysiol. 2004;11:161–6. doi: 10.1023/B:JICE.0000042356.52369.89. [DOI] [PubMed] [Google Scholar]
- 12.Toft E. Implantable electrocardiographic monitoring—clinical experiences. J Electrocardiol. 2006;39:S47–9. doi: 10.1016/j.jelectrocard.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Fauchier L, Sadoul N, Kouakam C, Briand F, Chauvin M, Babuty D, et al. Potential cost savings by telemedicine-assisted long-term care of implantable cardioverter defibrillator recipients. Pacing Clin Electrophysiol. 2005;28:S255–9. doi: 10.1111/j.1540-8159.2005.00071.x. [DOI] [PubMed] [Google Scholar]
- 14.Elsner C, Sommer P, Piorkowski P, Taborsky M, Neuser H, Bytesnik J, et al. A prospective multicenter comparison of Home Monitoring against regular follow-up in MADIT III patients: additional visits and cost impact. Comput Cardiol. 2006;33:241–4. [Google Scholar]