Abstract
The small intestine has been shown to be an extra-pituitary site of TSH (thyroid stimulating hormone) production, and previous in vivo studies have shown that TSH synthesis localizes within areas of enteric virus infection within the small intestine; however, the cellular source of intestinal TSH has not been adequately determined. In the present study, we have used the murine MODE-K small intestinal epithelial cell line to demonstrate both at the transcriptional level and as a secreted hormone, as measured in a TSHβ-specific enzyme-linked assay, that epithelial cells in fact respond to infection with reovirus serotype 3 Dearing strain by upregulating TSH synthesis. Moreover, sequence analysis of a PCR-amplified TSHβ product from MODE-K cells revealed homology to mouse pituitary TSHβ. These findings have direct functional implications for understanding a TSH immune-endocrine circuit in the small intestine.
Keywords: Immune-endocrine, sequence, gene, transcript, realtime PCR
Under normal physiological conditions, TSH (thyroid stimulating hormone) is produced by the anterior pituitary, whereupon it is used to regulate the synthesis and release of thyroid hormones. Although knowledge of an immune-endocrine network involving hormones of the hypothalamus-pituitary-thyroid axis dates back a number of years [Ajjan et al., 1996; Fabris et al., 1971; Pierpaoli and Besedovsky, 1975; Pierpaoli et al., 1977; Pierpaoli and Sorkin, 1972], many gaps in information exist as to the nature of this network. Tissues other than the pituitary are known to be capable of producing TSH [Kruger and Blalock, 1986; Kruger et al., 1989; Smith et al., 1983; Wang et al., 2003], and studies from our laboratory have described a TSH circuit in the small intestine that functions to regulate the differentiation and/or function of IELs (intestinal intraepithelial lymphocytes) [Wang and Klein, 1994; Wang and Klein, 1995; Wang et al., 1997]. Additionally, we recently demonstrated by immunocytochemical staining that mice infected with rotavirus, a member of the reoviridae family [Scofield et al., 2005], produced TSH in areas that co-localized with sites of infection. TSH levels are similarly upregulated in the intestine following reovirus infection [Klein, 2006]. However, because of the extensive heterogeneity of intestinal tissues in vivo, it was impossible to determine the cellular source of intestinal TSH in the context of virus infection. This is an important issue for elucidating TSH-mediated interactive mechanisms within that intestinal immune-endocrine pathway.
To resolve this, we have used the murine MODE-K small intestinal epithelial cell line to assess the ability of intestinal epithelial cells to produce TSH following infection with T3D reovirus (reovirus serotype 3 Dearing strain). The findings reported here unambiguously demonstrate that virus infection leads to an increase in TSH synthesis by intestinal epithelial cells, and we show that TSH produced by intestinal epithelial cells is homologous to the mRNA sequence of mouse pituitary TSH.
MATERIALS AND METHODS
Virus Stocks and Infection
Reovirus serotype 3 Dearing strain was purchased from the American Type Culture Collection (Manassas, VA). Virus stocks were grown in L929 (American Type Culture Collection; cat. No. CCL-1) cell monolayers in RPMI-1640 supplemented with FCS (10% v/v), 100 U/ml penicillin-streptomycin, 2 mM L-glutamine, and 5 × 10−5 M 2-ME (all from Sigma-Aldrich, St. Louis, MO). Tissue supernatants were collected from monolayers showing 50-60% cytopathic effect; supernatants were clarified by centrifugation and virus titers were calculated to be 2×108.5 pfu/ml using a virus plaque assay with L929 cells. C57BL/6 mice were infected by oral gavage with T3D reovirus as previously described [Montufar-Solis et al., 2006]. Four days post-infection, mice were euthanized, small intestine tissues were washed of fecal material with cold PBS, and tissues were used for RNA extraction for reovirus and TSH realtime PCR analysis as described below. Animals were used in accord with University of Texas Animal Welfare Protocols.
Realtime PCR, Agarose Gel Electrophoresis, and Sequence Analysis
RNA was isolated using an RNeasy Protect Mini Kit with DNase treatment (Qiagen; Valencia, CA) from 5×106 non-infected or 1.6×107.5 pfu T3D reovirus infected MODE-K cells. RNA concentrations were estimated spectrophotometrically at A260. Forward and reverse primers (Integrated DNA Technologies; Coralville, IA) for T3D reovirus were based on the design reported by others [Uchiyama and Besselsen, 2003], for a target amplicon of 72 bp using a forward primer sequence (5′-TGATTTCCATTACTTCTGCTGCTT-3′) in nucleotide position 1085-1108, and a reverse primer sequence (5′-TCCTGTTCACGATTCCATCAGAT-3′) in nucleotide position 1156-1134 (GenBank Accession no. M27262) as previously reported from our laboratory [Montufar-Solis et al., 2006]. Two mouse TSHβ (GenBank Accession no. NM_009432) primer sets were used. The first consisted of TSHβ primers purchased from SuperArray Bioscience Corporation (Marietta, GA) (cat no. PPM30787A) for a 159 bp amplicon with reference position of 332-353 of mouse TSHβ mRNA. The second TSHβ primer set consisted of an upstream primer 5′-TTGTATGACACGGGATATCAA-3′ and a downstream primer 5′-ACAGCCTCGTGTATGCAGTC-3′ for a 210 nucleotide amplicon spanning TSHβ exons 4 and 5, with the upstream primer located across the exon 4/5 junctional site in a translated region of the TSHβ open reading frame. The TSHα primer set consisted of an upstream primer 5′-TTGCTTCTCCAGGGCATATC-3′ and a downstream primer 3′-GTAGGGAGGAGGTGGTGACA-3′. The primer set for GAPDH (GenBank Accession no. NM_001001303) (cat. no. PPM02946A) with a 128 bp amplicon with reference position 970-991 of mouse GAPDH mRNA was purchased from SuperArray Bioscience Corp.
SYBR Green I realtime PCR was done using a Bio-Rad Mini-Opticon Instrument (Bio-Rad; Hercules, CA) with 200 ng RNA with an iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad). The amplicon generation rate was determined by monitoring SYBR Green fluorescence on a continuous basis in a 48-well thin-wall plate with a protocol of 10 min at 50°C for generation of cDNA; 5 min at 95°C for reverse transcriptase inactivation; and 45 cycles of 95°C for 10 sec and 55°C for 30 sec for the data collection. Melt curve analysis was performed using a protocol of increasing the temperatures in 0.5°C increments starting at 55°C for 80 cycles of 10 sec each. The presence of a single PCR product was verified by a single melting temperature peak signifying a unique product. Data for reovirus and TSHβ gene expression were normalized to GAPDH levels of respective samples and the relative amplification values were calculated by the 2−ΔΔCt method [Livak and Schmittgen, 2001]. PCR products were electrophoresed through a 2% agarose gel to confirm the amplicon size. For sequencing, the PCR product was cut from the gel, extracted using a Zymoclean Gel DNA Recovery Kit (Zymoresearch; Orange, CA), and sequenced with the amplification primers by SeqWright DNA Technology Services (Houston, TX).
Immunocytochemical Staining
MODE-K cells were grown to 80-90% confluency in serum-supplemented DMEM on glass microscope slides. Cells were infected with 107.5 pfu T3D reovirus for 1 hr in serum-free DMEM after which medium was replaced with serum-supplemented DMEM. 24 hrs later virus-infected and non-infected tissue slides were fixed in acetone for 10 min, air dried, and hydrated in PBS. Tissues were incubated at room temperature for 10 min with avidin blocker (Zymed Laboratories; South San Francisco, CA), washed in PBS, and incubated for 10 min with biotin blocker (Zymed). Tissues were washed in PBS and incubated for 10 min with rat anti-mouse CD16/CD32 Fc block (BD-PharMingen; San Diego, CA). Following PBS washing , tissues were reacted overnight at 4°C with biotin-labeled anti-TSHβ mAb 1B11 [Zhou et al., 2002]. Tissues were washed in PBS and incubated with streptavidin-FITC (BD- PharMingen) for 1 hr at room temperature. After PBS washing, tissues were mounted and examined using an Olympus BH-2 immunofluorescence microscope. Numbers of TSH+ cells per high-dry field were counted in 25 fields of non-infected and T3D reovirus infected MODE-K cells.
Enzyme-Linked Immunoassay (EIA)
The EIA used in this study was patterned after a procedure developed and published by our laboratory [Zhou et al., 2002]. Briefly, high binding type I EIA/RIA strip plates (Costar; Corning, NY) were coated overnight at 4°C with 1:3 dilutions of MODE-K cell-free supernatants. Positive control wells received dilutions (1,860 – 0.060 ng/ml) of purified recombinant mouse TSHβ generated in our laboratory. The following day, wells were washed with a Multi-Wash Advantage Microplate Washer (Tricontinent; Grass Valley, CA). Wells were blocked with PBS/2% BSA for 1 hr at 4°C, washed, and biotin-labeled anti mouse TSHβ antibody [Zhou et al., 2002] was added for 2 hr at room temperature. Wells were washed and 1:1000 streptavidin-HRP (PharMingen; San Diego, CA) was added for 30 min at room temperature. Wells were washed and O-phenylenediamine dihydrochloride (OPD) substrate was added for 15 min. Colorimetric changes were recorded at 490 nm using an automated EIA reader (Molecular Devices; Sunnyvale, CA).
Statistical Analyses
Analyses of realtime PCR data and immunocytochemical staining experiments were done by ANOVA. Spearman correlation analysis was used to compare transcript levels for TSH and reovirus in intestinal tissues of T3D virus infected mice.
RESULTS AND DISCUSSION
TSHβ Gene Expression is Elevated in Small Intestinal Tissues following T3D Reovirus Infection
Previous studies in our laboratory demonstrated that oral infection of mice with rotavirus [Scofield et al., 2005] or reovirus [Klein, 2006] induces the local synthesis of TSH within the intestinal epithelium. However, because immunocytochemistry has low sensitivity for identifying virus and TSH, and because it is not possible quantify data using that method, we have assessed levels of gene expression for reovirus and TSHβ by realtime PCR in small intestinal tissues of T3D reovirus-infected mice. Three tissue specimens were recovered from the small intestine of mice four days after oral 3TD reovirus infection. Realtime PCR was done using reovirus- and TSHβ-specific primers. Gene expression values were normalized to GAPDH and were compared to that of non-infected mice using established protocols [Livak and Schmittgen, 2001]. The results of these experiments are shown in Fig. 1, where the data are displayed in an order of increasing virus gene levels to facilitate analysis. Although there was variation in the relative level of reovirus gene expression in the three tissue samples, transcript levels for TSHβ increased proportionally according to the amount of virus present. That pattern was confirmed by Spearman correlation analyses, which revealed a strong positive association (r = 0.8752) between virus and TSHβ transcript levels (Fig. 1), thus demonstrating a direct relation between virus infection and TSHβ gene expression.
Fig. 1.

Realtime gene expression for reovirus and mouse TSHβ genes from three small intestine tissues four days after oral T3D reovirus infection. Expression values reflect an increase over that of intestinal tissues from non-infected animals. Data from the three samples are arbitrarily displayed in an increasing order of gene activity to emphasize the relationship between virus and TSHβ gene expression. Numbers above bars indicate gene expression levels. Spearman correlation analysis indicated a strong positive association (r = 0.8752) between reovirus and TSHβ transcript levels.
TSHβ Gene Expression is Upregulated in MODE-K Cells following T3D Reovirus Infection
To study the effects of virus infection on TSH synthesis in MODE-K cells, it was necessary to confirm that MODE-K cells were susceptible to T3D reovirus infection. Monolayers of MODE-K cells were infected with 107.5 pfu of T3D reovirus; mock-infected cells received neat medium (designated as day 0 post-infection). On days 0, 1 and 2 post-infection, RNAs were isolated and realtime PCR was conducted for reovirus gene expression relative to non-infected tissues. As shown in Fig. 2A, there was a statistically-significant increase in T3D reovirus gene expression on days 1 and 2 post-infection, with a marked increase in virus gene expression on day 2 relative to day 1 post-infection, thus confirming that MODE-K cells were highly susceptible to reovirus infection.
Fig. 2.
(A) Relative T3D reovirus gene expression levels in MODE-K cells on days 1 and 2 post-infection. Note the statistically-significant increase (p<0.01) in gene expression at each day relative to non-infected cells and on day 2 post-infection relative to cells on day 1 post-infection. (B) Quantification of TSHβ and (C) TSHα gene expression in MODE-K cells on days 1 and 2 post-infection relative to expression in non-infected MODE-K cells. Note the statistically-significant difference of TSHβ (p<0.05) and TSHα (p<0.01) gene expression on day 2 post-infection compared to that of non-infected cells. Values are means ± SEM of three experiments.
Realtime PCR was done using non-infected and T3D reovirus-infected MODE-K cells to determine whether virus infection would alter TSHβ gene expression. To quantify the change in TSHβ gene expression following infection, RNAs from non-infected (day 0 post-infection) and infected cells were used for realtime PCR analyses with TSHβ-specific primers. As shown in Fig. 2B, there was a modest increase in TSHβ gene expression on day 1 post-infection, and a statistically-significant increase in TSHβ expression on day 2 post-infection relative to that of non-infected (day 0) cells. That pattern also held true for TSHα gene expression (Fig. 2C).
Numbers of TSH-Producing MODE-K Cells Increase following T3D Reovirus Infection and TSH is Secreted at Higher Levels from Virus-Infected Cells
Based on the above findings, we sought to determine if the TSH protein was actively produced by MODE-K cells. Non-infected and T3D reovirus infected MODE-K cells were stained after 24 and 48 hrs of infection using an anti-mouse TSHβ-specific mAb [Zhou et al., 2002]. Numbers of TSH+ cells were enumerated by counting cells at high-dry magnification in virus-infected MODE-K cell cultures and non-infected cells. Typical staining of non-infected (Fig. 3A and B) and T3D reovirus-infected (Fig. 3C and D) cells is shown in overlaid phase contrast images (Fig. 3B and D). When enumerated from multiple high-dry fields, there was a statistically-significant increase (p<0.001) in the number of TSH+ cells among virus-infected MODE-K cultures compared to non-infected cultures on day 1 post-infection, demonstrating that virus infection caused an increase in TSH synthesis in MODE-K cells. On day 2 post-infection, there was a slight but not statistically-significant increase in the number of TSH+ cells in infected cultures relative to non-infected cells. The lack of a continual increase in the number of TSH+ cells through day 2 may be due to cell death from virus infection. Moreover, the increase in the number of TSH+ cells between days 1 and 2 of culture for non-infected cells is consistent with the fact that some normal MODE-K cells produce TSH (see Fig. 2B and C). However, when levels of secreted TSHβ were measured by EIA, there was a statistically-significant increase in TSHβ in supernatants from infected cells by day 2 of culture (Fig. 3C), indicating that production of TSH was greatest in virus-infected cultures.
Fig. 3.
(A) Immunocytochemical staining of (A and B) non-infected and (C and D) T3D reovirus-infected MODE-K cells 48 hr post-infection using an anti-mouse TSHβ mAb [Zhou et al., 2002]. Panels A and C are phase contrast micrographs of cell monolayers; panels B and D are immunocytochemical staining overlays showing the presence of TSHβ producing cells. Images have been enlarged to permit visualization of cells. (E) Numbers of cells per high-dry field were quantified in monolayers of non-infected and T3D reovirus-infected MODE-K cells at day 1 and 2 post-infection. There was a statistically-significant increase (p<0.001) in the number of TSH-producing cells at day 1 post infection compared to non-infected cells. By day 2 post-infection, there was a slight but not statistically-significant increase in the number of TSH-producing cells in T3D reovirus-infected cultures compared to non-infected cells. Values are means ± SEM of numbers of TSHβ+ cells in 7-25 high-dry fields. (F) Levels of secreted TSHβ in cell-free supernatants from non-infected and T3D reovirus-infected MODE-K cells on days 0, 1, and 2 of culture. Note the statistically-significant (p<0.05) increase in TSHβ in supernatants from T3D reovirus-infected cultures on day 2 post-infection compared to non-infected MODE-K cells. Data are derived from five replicate values; similar findings were obtained in 3 experiments.
Sequence Analysis of Virus-Induced TSHβ in MODE-K Cells Reveals Identity to Pituitary TSHβ
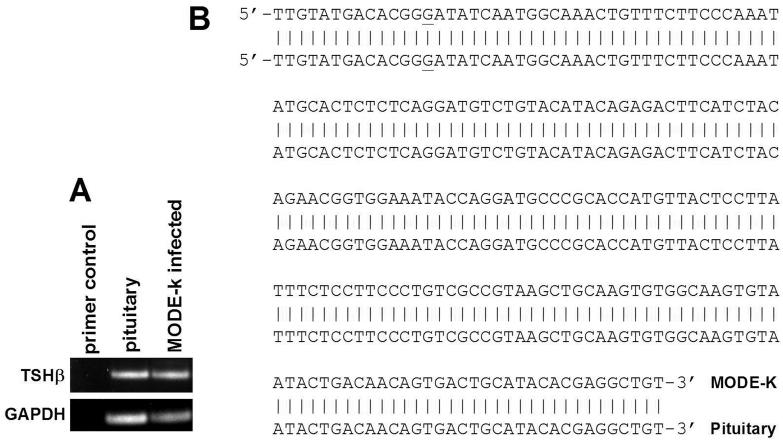
To confirm that PCR-amplified product from MODE-K cells was TSHβ, sequence analysis was done using an upstream primer that spanned the junctional site of exons 4 and 5, in order to eliminate products generated from genomic DNA. The sequence obtained from that MODE-K PCR product consisted of the anticipated amplicon size, 210 nucleotides, and it matched identically with that of mouse pituitary TSHβ mRNA (Fig. 4B).
Fig. 4.
(A) Agarose gel of PCR products of TSHβ from pituitary and MODE-K RNA. (B) The MODE-K PCR product was recovered from the agarose gel as described in the Experimental procedures. Sequence data were compared to know sequences using an NIH NCBI mouse BLAST search, which revealed 100% identity to mouse pituitary mRNA within the amplified portion. The GAT nucleotide triplet beginning at position 14 from the 5' end is the first codon of exon 5; the upstream primer used for PCR amplification spanned the exon 4/exon 5 junction.
CONCLUDING REMARKS
By using an intestinal epithelial cell line that can be grown devoid of other cell types, we have definitively demonstrated that intestinal epithelial cells are not only a source of TSH, but that they respond in a regulated fashion by producing TSH following infection with an enteric mouse virus. Previous studies from our laboratory using congenitally-athymic nude mice and neonatally-thymectomized (NTX) mice [Wang and Klein, 1994; Wang and Klein, 1995], and hyt/hyt mice with defective TSH receptor [Wang et al., 1997], have shown that TSH is involved in the differentiation of intestinal IELs. This was particularly true for the local extrathymic development of intestinal IELs within the TCRαβ CD8αβ lineage as revealed by experiments in which extrathymic development of that lineage occurred in NTX mice following in vivo administration of TSH (or TRH as an inducer of local TSH) [Wang and Klein, 1994; Wang and Klein, 1995], thus defining a role for TSH in IEL maturation. Failure to replicate that pattern in hyt/hyt, which have a congenital defective in the TSH receptor [Biesiada et al., 1996], and in congenitally-athymic nude mice, which have a defect in TSH binding [Wang et al., 1997], confirmed the involvement of TSH in the IEL developmental/differentiation process [Wang et al., 1997].
The present study, therefore, supports a model in which intestinal TSH is produced by epithelial cells following immune challenge. Although TSH is known to be produced within the intestine during enteric virus infection [Scofield et al., 2005], the extent to which TSH participates in the host immune response remains to be defined. However, the functional advantage of TSH utilization might be to rapidly modulate the local immune response though IEL differentiation as described above, thereby curtailing the spread of virus at the site of entry. This would occur through intestinal IELs that preferentially express the TSH receptor. Previous studies from our laboratory demonstrated IELs express TSH receptor gene [Wang et al., 1997] as well as surface TSH receptor on CD4−CD8+ and the CD4+CD8+ IEL subsets [Scofield et al., 2005]. Although B cells have been shown to express the TSH receptor and to respond to TSH [Blalock et al., 1984; Coutelier et al., 1990], the role of TSH-responsive B cells in the intestine is unknown. Current studies in our laboratory are aimed at understanding how TSH receptor-positive cells respond functionally to TSH during virus infection.
ACKNOWLEDGMENTS
This work was supported by NIH Grant DK035566. We thank Dr. Dominique Kaiserlian for generously providing the MODE-K cell line.
REFERENCES
- Ajjan RA, Watson PF, Weetman AP. Cytokines and thyroid function. Adv Neuroimmunol. 1996;6:359–86. doi: 10.1016/s0960-5428(97)00027-7. [DOI] [PubMed] [Google Scholar]
- Biesiada E, Adams PM, Shanklin DR, Bloom GS, Stein SA. Biology of the congenitally hypothyroid hyt/hyt mouse. Adv Neuroimmunol. 1996;6:309–46. doi: 10.1016/s0960-5428(97)00028-9. [DOI] [PubMed] [Google Scholar]
- Blalock JE, Johnson HM, Smith EM, Torres BA. Enhancement of the in vitro antibody response by thyrotropin. Biochem Biophys Res Commun. 1984;125:30–4. doi: 10.1016/s0006-291x(84)80329-0. [DOI] [PubMed] [Google Scholar]
- Coutelier JP, Kehrl JH, Bellur SS, Kohn LD, Notkins AL, Prabhakar BS. Binding and functional effects of thyroid stimulating hormone on human immune cells. J Clin Immunol. 1990;10:204–10. doi: 10.1007/BF00918653. [DOI] [PubMed] [Google Scholar]
- Fabris N, Pierpaoli W, Sorkin E. Hormones and the immunological capacity. 3. The immunodeficiency disease of the hypopituitary Snell-Bagg dwarf mouse. Clin Exp Immunol. 1971;9:209–25. [PMC free article] [PubMed] [Google Scholar]
- Klein JR. The immune system as a regulator of thyroid hormone activity. Exp Biol Med (Maywood) 2006;231:229–36. doi: 10.1177/153537020623100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger TE, Blalock JE. Cellular requirements for thyrotropin enhancement of in vitro antibody production. J Immunol. 1986;137:197–200. [PubMed] [Google Scholar]
- Kruger TE, Smith LR, Harbour DV, Blalock JE. Thyrotropin: an endogenous regulator of the in vitro immune response. J Immunol. 1989;142:744–7. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Montufar-Solis D, Garza T, Teng BB, Klein JR. Upregulation of ICOS on CD43+ CD4+ murine small intestinal intraepithelial lymphocytes during acute reovirus infection. Biochem Biophys Res Commun. 2006;342:782–90. doi: 10.1016/j.bbrc.2006.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W, Besedovsky HO. Role of the thymus in programming of neuroendocrine functions. Clin Exp Immunol. 1975;20:323–8. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W, Kopp HG, Muller J, Keller M. Interdependence between neuroendocrine programming and the generation of immune recognition in ontogeny. Cell Immunol. 1977;29:16–27. doi: 10.1016/0008-8749(77)90271-4. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W, Sorkin E. Hormones, thymus and lymphocyte functions. Experientia. 1972;28:1385–9. doi: 10.1007/BF01965362. [DOI] [PubMed] [Google Scholar]
- Scofield VL, Montufar-Solis D, Cheng E, Estes MK, Klein JR. Intestinal TSH production is localized in crypt enterocytes and in villus ‘hotblocks’ and is coupled to IL-7 production: evidence for involvement of TSH during acute enteric virus infection. Immunol Lett. 2005;99:36–44. doi: 10.1016/j.imlet.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Phan M, Kruger TE, Coppenhaver DH, Blalock JE. Human lymphocyte production of immunoreactive thyrotropin. Proc Natl Acad Sci U S A. 1983;80:6010–3. doi: 10.1073/pnas.80.19.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama A, Besselsen DG. Detection of Reovirus type 3 by use of fluorogenic nuclease reverse transcriptase polymerase chain reaction. Lab Anim. 2003;37:352–9. doi: 10.1258/002367703103051903. [DOI] [PubMed] [Google Scholar]
- Wang HC, Dragoo J, Zhou Q, Klein JR. An intrinsic thyrotropin-mediated pathway of TNF-α production by bone marrow cells. Blood. 2003;101:119–23. doi: 10.1182/blood-2002-02-0544. [DOI] [PubMed] [Google Scholar]
- Wang J, Klein JR. Thymus-neuroendocrine interactions in extrathymic T cell development. Science. 1994;265:1860–2. doi: 10.1126/science.8091211. [DOI] [PubMed] [Google Scholar]
- Wang J, Klein JR. Hormonal regulation of extrathymic gut T cell development: involvement of thyroid stimulating hormone. Cell Immunol. 1995;161:299–302. doi: 10.1006/cimm.1995.1040. [DOI] [PubMed] [Google Scholar]
- Wang J, Whetsell M, Klein JR. Local hormone networks and intestinal T cell homeostasis. Science. 1997;275:1937–9. doi: 10.1126/science.275.5308.1937. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang HC, Klein JR. Characterization of novel anti-mouse thyrotropin monoclonal antibodies. Hybrid Hybridomics. 2002;21:75–9. doi: 10.1089/15368590252917674. [DOI] [PubMed] [Google Scholar]