Abstract
Cell cycle progression is controlled by the sequential functions of cyclin-dependent kinases (cdks). Cdk activation requires phosphorylation of a key residue (on sites equivalent to Thr-160 in human cdk2) carried out by the cdk-activating kinase (CAK). Human CAK has been identified as a p40MO15/cyclin H/MAT1 complex that also functions as part of transcription factor IIH (TFIIH) where it phosphorylates multiple transcriptional components including the C-terminal domain (CTD) of the large subunit of RNA polymerase II. In contrast, CAK from budding yeast consists of a single polypeptide (Cak1p), is not a component of TFIIH, and lacks CTD kinase activity. Here we report that Cak1p and p40MO15 have strikingly different substrate specificities. Cak1p preferentially phosphorylated monomeric cdks, whereas p40MO15 preferentially phosphorylated cdk/cyclin complexes. Furthermore, p40MO15 only phosphorylated cdk6 bound to cyclin D3, whereas Cak1p recognized monomeric cdk6 and cdk6 bound to cyclin D1, D2, or D3. We also found that cdk inhibitors, including p21CIP1, p27KIP1, p57KIP2, p16INK4a, and p18INK4c, could block phosphorylation by p40MO15 but not phosphorylation by Cak1p. Our results demonstrate that although both Cak1p and p40MO15 activate cdks by phosphorylating the same residue, the structural mechanisms underlying the enzyme-substrate recognition differ greatly. Structural and physiological implications of these findings will be discussed.
INTRODUCTION
Cell cycle transitions are controlled by cyclin-dependent kinases (cdks; Morgan, 1995), protein kinases that are dependent on a cyclin regulatory subunit for activity (see below). Four of the cdks function in cell cycle progression: cdc2 (cdk1), cdk2, cdk4, and cdk6. The activities of the cdks are regulated by multiple mechanisms, including protein-protein interactions (with cyclins, inhibitors, and assembly factors), protein degradation, transcriptional control, subcellular localization, and multiple phosphorylations (Nasmyth, 1993; Morgan, 1995; Pines, 1995; Sherr and Roberts, 1995; King et al., 1996; Solomon and Kaldis, 1998).
Activation of cdks requires binding of cdks to a cyclin subunit. The cyclins are unstable proteins that are transcribed and synthesized periodically during the cell cycle (Evans et al., 1983; Sherr, 1994; Pines, 1995). The transcriptional control limits cyclin synthesis to specific phases of the cell cycle. The ubiquitin-mediated proteolysis of cyclins (King et al., 1996) ensures the irreversible and abrupt inactivation of the associated cdk.
Full activation of cdk/cyclin complexes requires dephosphorylation of inhibitory sites (Thr-14 and Tyr-15) by the dual-specificity phosphatase, Cdc25, and phosphorylation of a particular threonine (Thr-160 in human cdk2). The site of this activating phosphorylation is located in the “T-loop” of cdks (Johnson et al., 1996) and is phosphorylated by the cdk-activating kinase (CAK; for review see Solomon and Kaldis, 1998). The first reported putative CAKs are composed of a catalytic subunit, p40MO15 (or cdk7; Fesquet et al., 1993; Poon et al., 1993; Solomon et al., 1993); a regulatory subunit, cyclin H (Fisher and Morgan, 1994; Mäkeläet al., 1994); and an assembly factor, MAT1 (Devault et al., 1995; Fisher et al., 1995; Tassan et al., 1995). In vitro, this enzyme can phosphorylate and activate cdc2 (Fesquet et al., 1993; Solomon et al., 1993), cdk2 (Fesquet et al., 1993; Poon et al., 1993, 1994; Solomon et al., 1993; Fisher and Morgan, 1994), cdk3 (Labbéet al., 1994), cdk4 (Kato et al., 1994a; Matsuoka et al., 1994; Blain et al., 1997), and cdk6 (Aprelikova et al., 1995; Iavarone and Massagué, 1997). p40MO15, cyclin H, and MAT1 are also subunits of transcription factor IIH where they phosphorylate the C-terminal domain (CTD) of the large subunit of RNA polymerase II (Feaver et al., 1994; Roy et al., 1994; Serizawa et al., 1995; Shiekhattar et al., 1995; Adamczewski et al., 1996). CAK can also phosphorylate the retinoic acid receptor-α (Rochette-Egly et al., 1997), octamer transcription factors (Oct-1; Inamoto et al., 1997), and p53 (although the specific site of phosphorylation is in dispute [Ko et al., 1997; Lu et al., 1997]). None of these substrates contains a consensus cdk phosphorylation site or a T-loop. p40MO15 is itself phosphorylated on serine 164 and threonine 170 (in human p40MO15); phosphorylation of Ser-164 is involved in cyclin H binding, whereas phosphorylation of Thr-170 is involved in the activation of p40MO15 (Martinez et al., 1997). Phosphorylation of Thr-170 activates the dimeric p40MO15/cyclin H complex, although the trimeric p40MO15/cyclin H/MAT1 complex is active independent of the phosphorylation state of Thr-170 (Fisher et al., 1995). The assembly factor MAT1 increases the CTD kinase activity of p40MO15/cyclin H relative to its CAK activity (Rossignol et al., 1997; Yankulov and Bentley, 1997). At least some of the cdk inhibitors (p21CIP1, p27KIP1, and p18INK4c) can block phosphorylation of cdks by p40MO15 in addition to inhibiting the cdks directly, thereby creating a “double block” to their activation (Kato et al., 1994b; Polyak et al., 1994b; Aprelikova et al., 1995).
Recently, we and others have identified the CAK from budding yeast (Espinoza et al., 1996; Kaldis et al., 1996; Thuret et al., 1996). Cak1p shares only 20–25% identity to its closest protein kinase homologues. Although Cak1p belongs to the cdk subfamily of protein kinases, it lacks the GxGxxG motif known to be involved in nucleotide binding (Hanks and Quinn, 1991). Cak1p is active as a monomer (Kaldis et al., 1996) and phosphorylates yeast Cdc28p as well as human cdk2 in the presence or absence of cyclin (Espinoza et al., 1996; Kaldis et al., 1996; Thuret et al., 1996). Temperature-sensitive mutants of CAK1 arrest in G2 and G1 at the restrictive temperature with negligible CAK activity, low Cdc28p activity, and an unphosphorylated threonine 169 in Cdc28p (Kaldis et al., 1996, Thuret et al., 1996). These experiments demonstrate that Cak1p is the physiological CAK in budding yeast. In addition, expression of p40MO15 in yeast fails to rescue cak1–22 cells at the restrictive temperature (Kaldis et al., 1996).
Active cdk/cyclin complexes can be inhibited by a number of usually small proteins called cdk inhibitors (CKI or CDI; for review see Sherr and Roberts, 1995). Two families of inhibitors have been identified. The CIP/KIP family contains p21CIP1/WAF1 (El-Deiry et al., 1993; Gu et al., 1993; Harper et al., 1993), p27KIP1 (Polyak et al., 1994a,b; Toyoshima and Hunter, 1994), and p57KIP2 (Lee et al., 1995; Matsuoka et al., 1995) and the INK4 family contains p15INK4b/MTS2 (Hannon and Beach, 1994), p16INK4a/MTS1 (Serrano et al., 1993), p18INK4c (Guan et al., 1994; Hirai et al., 1995), and p19INK4d (Chan et al., 1995; Hirai et al., 1995). The CIP/KIP inhibitors can associate with a wide variety of cdks, but are more active toward cdk2 than cdk4 or cdc2 (Toyoshima and Hunter, 1994; Blain et al., 1997). The CIP/KIP inhibitors bind to both the cdk and the cyclin subunits (Lin et al., 1996; Chen et al., 1996a; Chen et al., 1996b). The crystal structure of the p27KIP1/cdk2/cyclin A complex confirmed that the same p27 molecule binds to both subunits (Russo et al., 1996a), suggesting that a single CKI molecule is sufficient to inhibit one cdk/cyclin complex. The N terminus of p27 lies in the catalytic cleft of the cdk/cyclin complex, precluding the cdk from phosphorylating substrates. The INK4 inhibitors are specific for cdk4 and cdk6 and bind predominately to the cdk subunit (Serrano et al., 1993; Guan et al., 1994; Hannon and Beach, 1994; Chan et al., 1995; Hirai et al., 1995).
Despite their common enzymatic function, human p40MO15 and yeast Cak1p share few physical properties. To determine whether these differences have functional implications, we compared the substrate specificities of these enzymes in vitro using highly purified proteins. We used the well defined substrate cdk2/cyclin A and the less characterized substrates, cdk4 and cdk6, in complex with cyclins D1, D2, and D3. The presence of cyclins had inverse effects on cdk phosphorylation by these CAKs, increasing phosphorylation by p40MO15 and decreasing phosphorylation by Cak1p. We also found that cdk inhibitors (p21, p27, p57, p16, and p18) blocked phosphorylation of cdks by p40MO15, but not by Cak1p. Thus, although Cak1p and p40MO15 specifically phosphorylate the same residue in cdks, the structural mechanisms underlying this phosphorylation are very different. These results have implications for the pathways by which cdks are activated and predict properties for a potential human Cak1p-like CAK.
MATERIALS AND METHODS
Protein Expression and Purification
Wild-type human cdk2 was expressed in insect cells (Russo, 1997), and cyclin A173–432 (Russo, 1997), human HA-cdk2 (Connell-Crowley et al., 1993), mutants of HA-cdk2 (Connell-Crowley et al., 1993), and glutathione S-transferase (GST)-Cak1p (Kaldis et al., 1996) were expressed in bacteria and purified as described.
Human GST-cdk4, GST-cdk6, GST-cdk6T177A (all constructs were a kind gift of Y. Xiong, University of North Carolina at Chapel Hill), and GST-Rb605–829 (construct was a kind gift of N. Grammatikakis, Tufts University, Boston) were expressed in Escherichia coli (Aprelikova et al., 1995). After growth to A600 of 0.6–0.8 in LB, 2-l cultures were induced for 17–20 h at 20°C with 0.4 mM IPTG. Bacteria were harvested and resuspended in 15 ml PBS, 1 mM DTT, and 0.2× protease inhibitors (1× protease inhibitors corresponds to 10 μg/ml each of leupeptin, chymostatin, and pepstatin [Chemicon, Temecula, CA]) per liter of culture. Cells were lysed using a French press (Power Laboratory Press, American Instruments, Silver Spring, MD) at 10,000 psi, and GST-fusion proteins were purified as described (Solomon et al., 1990) using PBS as the buffer (except for GST-Rb605–829, which was left on the glutathione agarose beads, aliquoted, and stored at −80°C until use). The yield of each protein was only in the microgram range since most of each GST-fusion protein was insoluble.
Recombinant baculoviruses encoding human His6-cyclin H, p40MO15, and MAT1 were kindly provided by Robert Fisher and David Morgan. Recombinant baculoviruses containing human cyclin D1, D2, and D3 were generated by inserting PCR fragments encoding six histidines N-terminal to cyclin D-coding regions into transfer vectors pVL1392 and pVL1393. Recombinant baculoviruses containing GST-tagged cdk4, cdk4D158N, cdk6, and cdk6D163N were generated by first inserting the cdk-coding sequences together with a GST-encoding fragment of pGEX2T into transfer vector pVL1392 and coinfected into Sf9 cells with BaculoGold DNA (Pharmingen, San Diego, CA). Hi Five insect cells (Invitrogen, San Diego, CA) were infected with the indicated recombinant baculoviruses (cyclin viruses were always in excess of cdk viruses) and harvested 48 h postinfection. Recombinant proteins were purified through the GST-tags with glutathione agarose beads (Sigma Chemical, St. Louis, MO). Cyclin D1, D2, and D3 monomers and cyclin H-containing complexes (with p40MO15 or p40MO15 and MAT1), were purified through His6-tags by nickel metal-affinity chromatography.
p21
Recombinant human p21-inhibitory domain, corresponding to residues 6–96, was expressed in E. coli BL21 using the pET3d vector (Novagen) according to standard procedures. The insoluble fraction of the E. coli lysate was resuspended in 8 M urea and bound to C4 resin on HPLC. The resin was washed with 0.1% trifluoroacetic acid (TFA) and eluted with 60% acetonitrile and 0.1% TFA, lyophilized to dryness, and resuspended in 0.1% TFA. The p21 was then applied to a C4 reverse phase column and eluted with an acetonitrile gradient, lyophilized, resuspended in 25 mM Tris (pH 7.5), 200 mM NaCl, 5 mM DTT, and further purified by gel filtration chromatography.
p27
Recombinant human p27 with an N-terminal His6-tag was expressed in E. coli BL21 using the pET21a vector (Novagen) according to standard procedures. The cells were lysed in a solution containing 8 M urea, 50 mM Tris-HCl (pH 7.4), 20 mM imidazole and clarified by centrifugation. Proteins were bound to nickel-agarose and the column was washed with a reverse gradient of urea from 8 M to zero in Tris-HCl (pH 7.4), 20 mM imidazole, 200 mM NaCl, 5 mM DTT. Elution was achieved by the same buffer containing 200 mM imidazole. The p27 protein was further purified by ion exchange and gel filtration chromatographies (see also Polyak et al., 1994b).
p57
Recombinant human p57-inhibitory domain, corresponding to residues 13–93, with an N-terminal His6-tag was expressed in E. coli BL21 according to standard procedures using the pET28b vector (Novagen). The insoluble fraction of the E. coli lysate was solubilized in 8 M urea and bound to nickel-agarose. The nickel-agarose was washed with 25 mM Tris (pH 7.5), 100 mM NaCl. The His6-p57 was eluted with 200 mM imidazole and dialyzed against 25 mM Tris (pH 7.5), 100 mM NaCl, 2 mM DTT. The His6-tag was then removed by thrombin cleavage. The protein was then applied onto a Mono Q column in 25 mM Tris (pH 7.5). The p57 fragment was eluted with a NaCl gradient, concentrated by ultrafiltration, and further purified by gel filtration chromatography.
p16
Recombinant human p16 was expressed as an N-terminal GST fusion protein in E. coli BL21. The bacterial lysate was clarified over a Q Sepharose column, the flow through of which was applied to a glutathione Sepharose column. The GST-p16 was eluted with 10 mM glutathione and applied to a Source 15Q column and eluted with a NaCl gradient. The GST tag was then removed by thrombin cleavage, and the p16 was further purified by ion-exchange and gel- filtration chromatography.
GST-p18
Recombinant human GST-p18 (Aprelikova et al., 1995; construct was a kind gift of Y. Xiong, University of North Carolina at Chapel Hill) was expressed in E. coli and purified as described for GST-cdk4/6.
Cdk Activation Assay
CAK assays were performed essentially as described (Cismowski et al., 1995): 5 μl of CAK (7.5 ng GST-Cak1p, 0.1 μg p40MO15/cyclin H complexes, or 0.1 μg p40MO15/cyclin H/MAT1 complexes) were incubated for 30 min at room temperature with 5 μl of substrate mix containing 0.1 μg cdk2 (or GST-cdk6), 0.075 μg cyclin A173–432 (or His6-cyclin D1, D2, D3), 1 mM ATP, 10 mM MgCl2, 50 μg/ml creatine kinase, and 35 mM phosphocreatine in EB (80 mM β-glycerophosphate [pH 7.3], 20 mM EGTA, 15 mM MgCl2, 10 mM DTT, 1 mg/ml ovalbumin, and 10× protease inhibitors [see above]). The reaction was terminated by adding 40 μl EB; 10 μl were assayed for histone H1 kinase activity in the presence of [γ-32P]ATP. Histone H1 kinase assays were performed as described previously (Solomon et al., 1990), run on 10% SDS-PAGE, and analyzed by phosphorimaging (Molecular Imager GS-250; Bio-Rad, Richmond, CA) and autoradiography. In some experiments the cyclin concentration was varied as indicated in the figure legends.
Retinoblastoma (Rb) Kinase Assay
Activity was determined as above for histone H1 kinase activity except that buffer A (100 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mg/ml ovalbumin, 10 mM DTT, 1× protease inhibitors [see above]) was used instead of EB and samples were not diluted before their Rb kinase activity had been assayed. Each sample (10 μl) was incubated with 1.5 μCi [γ-32P]ATP, 1 mM ATP, and 5 μl GST-Rb605–829 (see above) bound to glutathione agarose beads in buffer A (final volume, 16 μl). After incubation for 15 min at room temperature, reactions were terminated by the addition of 7 μl 5× SDS-PAGE sample buffer. Samples were processed as described above.
Phosphorylation of cdks
The quantities of CAK indicated above were incubated with 0.1 μg cdk2 and 0.3 μg cyclin A173–432 or with 0.1–0.5 μg GST-cdk4 or cdk6 in the presence of 5 μCi [γ-32P]ATP, 10 μM ATP, and 20 mM MgCl2 in EB (final volume 16 μl). The reactions were terminated after 30 min at room temperature by the addition of 7 μl of 5× SDS-PAGE sample buffer and analyzed as described above for the CAK assay. In some experiments the cyclin concentration was varied as indicated in the Figure legends.
CTD Phosphorylation
CTD kinase assays were performed essentially as described (Cismowski et al., 1995). Briefly, a 10-μl sample (containing the same amount of the CAKs as described under “Cdk Activation Assay”) was incubated in the presence of 3 μCi [γ-32P]ATP, 3.75 μM ATP, and 4 μg CTD peptide [(YSPTSPS)4] in buffer A (see above). Reactions were terminated after 30 min at room temperature by the addition of 7 μl of 5× SDS-PAGE sample buffer. Samples were processed as described above for the CAK assay.
Phosphorylation of cdks in the Presence of cdk Inhibitors
Cdk inhibitors (CKIs) at the indicated concentrations were preincubated for 10 min at room temperature with either 0.3 μg cdk2/0.225 μg cyclin A173–432 or 0.3 μg GST-cdk6D163N/0.225 μg cyclin A173–432. Samples were then subjected to phosphorylation by CAK as described above for the phosphorylation of cdks. The amounts of the CKIs used were 3 μg, 1.5 μg, 0.75 μg, 0.25 μg, 0.083 μg, 0.027 μg, and 0.009 μg.
Inactivation by 5′-p-Fluorosulfonlybenzoyl-Adenosine (FSBA)
CAK (0.5 μg GST-Cak1p or 1.8 μg p40MO15/cyclin H) was diluted into 18 μl buffer B (50 mM HEPES [pH 7.5], 50 mM NaCl, 10 mM MgCl2, 1 mg/ml ovalbumin, and 1× protease inhibitors [see above]). Two microliters of 10 mM FSBA in DMSO (Sigma, St. Louis, MO) were added and incubated for 15 min (p40MO15) or 60 min (Cak1p) at room temperature. DTT (5 mM) was added to inactivate the FSBA before the samples were assayed for phosphorylation of cdk2/cyclin A173–432 (see above). As a control, FSBA was first inactivated by 5 mM DTT and then added to CAK as above.
RESULTS
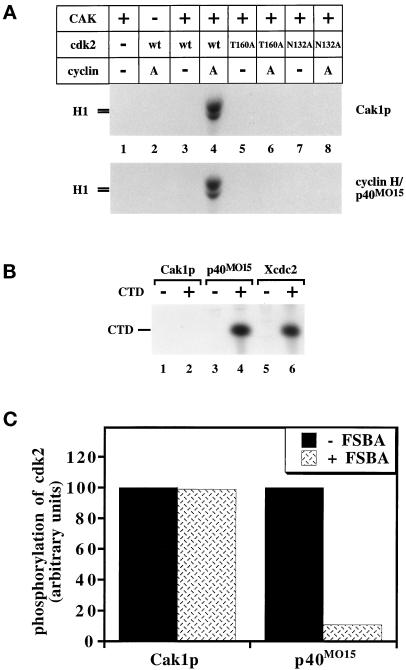
First we verified the basic activities of our purified preparations of CAKs. Cak1p and p40MO15 were originally purified by monitoring their phosphorylation and activation of either cdc2- or cdk2-containing complexes as substrates (Fesquet et al., 1993; Poon et al., 1993; Solomon et al., 1993; Fisher and Morgan, 1994; Espinoza et al., 1996; Kaldis et al., 1996). We repeated this assay by incubating CAK, cdk2, and cyclin A in the presence of unlabeled ATP. The activated cdk2 was then added to a second reaction containing the cdk2 substrate, histone H1, and radiolabeled ATP to monitor the activity of cdk2. Cak1p and p40MO15 activated wild-type cdk2 only in the presence of cyclin A (Figure 1A, compare lanes 3 and 4). Cdk2 proteins containing a mutation of the activating threonine (to alanine, “T160A,” lanes 6) or a mutation of a residue essential for catalysis (“N132A,” lanes 8) could not be activated by either CAK. Nevertheless, the N132A mutant of cdk2 could still be phosphorylated, presumably on Thr-160 (see below, Figure 2, lanes 7 and 8). p40MO15-containing enzymes have also been shown to phosphorylate the C-terminal domain (CTD) of the large subunit of RNA polymerase II (Feaver et al., 1994; Roy et al., 1994; Serizawa et al., 1995; Shiekhattar et al., 1995). We tested the ability of each CAK to phosphorylate a synthetic CTD peptide. Cak1p was unable to phosphorylate the CTD peptide, whereas both p40MO15- and cdc2-containing complexes phosphorylated this substrate efficiently (Figure 1B). In addition, we found that Cak1p and p40MO15 cannot phosphorylate each other (our unpublished results).
Figure 1.
Basic properties of yeast and human CAKs. (A) The activation of wild-type cdk2 expressed in insect cells and of mutant forms of cdk2 expressed in bacteria by CAK in the presence or absence of cyclin A173–432 was monitored by the ability of cdk2 to phosphorylate histone H1. The T160A mutation alters the site of activating phosphorylation, whereas the N132A mutation renders cdk2 catalytically inactive. (B) Phosphorylation of the CTD peptide by GST-Cak1p (lanes 1 and 2), p40MO15/cyclin H (lanes 3 and 4), and Xenopus cdc2/cyclin B complexes (lanes 5 and 6). The CTD peptide was present in the even-numbered lanes. The same amounts of Cak1p and of p40MO15 were used as in panel A. (C) GST-Cak1p or p40MO15/cyclin H was preincubated with the irreversible inhibitory ATP-analog 5′-p-fluorosulfonylbenzoyladenosine (FSBA) before assaying for phosphorylation of cdk2/cyclin A173–432 (mass ratio, 1:0.75). As a control, FSBA was inactivated by incubation with DTT before addition to CAK (−FSBA lanes). Lanes represent the averages of three and five measurements for GST-Cak1p and p40MO15/cyclin H, respectively.
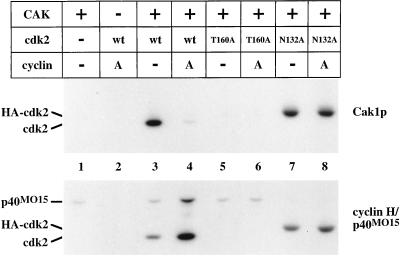
Figure 2.
Phosphorylation of cdk2. Wild-type cdk2 expressed in insect cells and mutant forms of cdk2 expressed in bacteria were incubated in the presence of radiolabeled ATP with GST-Cak1p or p40MO15/cyclin H. Samples were analyzed on 10% SDS-PAGE for incorporation of radiolabeled phosphate by autoradiography. T160A contains a mutation of the activating phosphorylation site (lanes 5 and 6), whereas N132A renders cdk2 catalytically inactive (lanes 7 and 8). Cyclin A173–432 was present in the indicated lanes in a threefold mass excess over cdk2. The mobility of cdk2N132A is decreased due to the presence of an N-terminal influenza hemagglutinin (HA) epitope tag (lanes 7 and 8). A low level of phosphorylation of p40MO15 by active cdk2 complexes is apparent in lane 4 (see also Fisher et al., 1995).
Two of the surprising sequence differences between p40MO15 and Cak1p are the absence of a canonical GxGxxG nucleotide-binding motif in Cak1p and its insensitivity to mutations at the conserved Lys-31 (equivalent to Lys-41 in human p40MO15 [Thuret et al., 1996; Chun and Goebl, 1997]; Enke, Kaldis, and Solomon, unpublished results), a residue that helps to position the ATP phosphates (Zheng et al., 1993; Jeffery et al., 1995). Other ATPases and kinases are irreversibly inhibited by the ATP analog FSBA, which covalently modifies the equivalent lysine residue (Zoller and Taylor, 1979). We found that FSBA readily inhibited p40MO15 (Figure 1C; see also Solomon et al., 1993) but that it had no effect on the ability of Cak1p to phosphorylate cdk2/cyclin A (Figure 1C).
Phosphorylation of cdk2
Both phosphorylation on Thr-160 by CAK and binding to a cyclin are required for full cdk2 activation. We investigated the effect of mutations in cdk2 and the presence of cyclin on the phosphorylation of Thr-160 in a direct phosphorylation assay. Surprisingly, Cak1p preferentially phosphorylated cdk2 in the absence of cyclin and displayed only very low activity toward cdk2 in the presence of excess cyclin A (Figure 2, upper panel, compare lanes 3 and 4). In contrast, p40MO15 phosphorylated cdk2 in the presence of cyclin A much more efficiently than cdk2 alone (Figure 2, lower panel, compare lanes 3 and 4). Neither enzyme could phosphorylate cdk2 containing an alanine mutation of the activating threonine (“T160A,” Figure 2, lanes 5 and 6). Both Cak1p and p40MO15 could phosphorylate a catalytically inactive mutant of cdk2 (“N132A”), although this phosphorylation was less sensitive to the presence of cyclin (Figure 2, lanes 7 and 8; see also below).
Effect of Cyclin on Phosphorylation by CAK
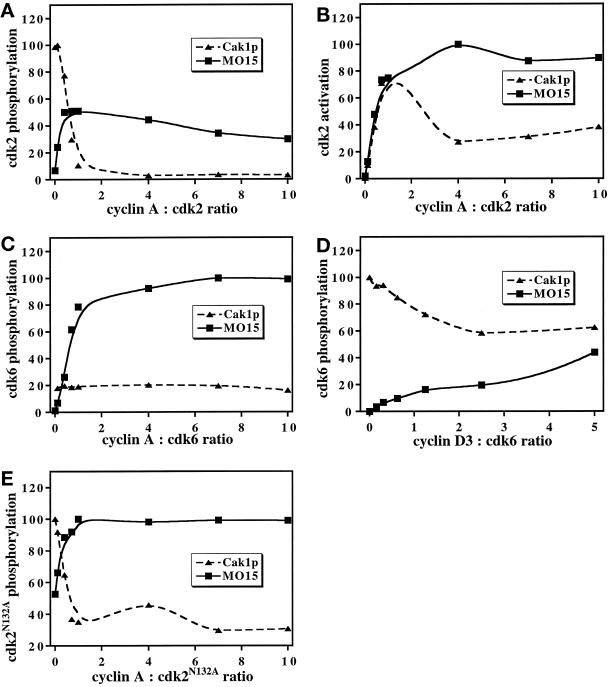
To investigate the effects of cyclin on cdk phosphorylation by CAKs in more detail, the cyclin concentration was varied over a wide range while keeping the cdk concentration constant (Figure 3). We used a fragment of cyclin A consisting of amino acids 173–432 (cyclin A173–432) that binds to cdk2 and promotes kinase activity, but that lacks the destruction box at the N terminus (Jeffery et al., 1995). Cyclin A173–432 was used because it is highly soluble and has been used successfully for crystallographic studies (Jeffery et al., 1995; Russo et al., 1996a,b). The presence of cyclin A173–432 reduced the phosphorylation of cdk2 by Cak1p by ∼90% at a 1:1 ratio of cyclin A173–432 to cdk2 and by 96% at a 10:1 ratio (Figure 3A, triangles). In contrast, a 1:1 ratio of cyclin A173–432 to cdk2 increased the phosphorylation of cdk2 by p40MO15 by more than sevenfold (Figure 3A, squares). The ability of cyclin A173–432 to increase cdk2 phosphorylation by p40MO15 indicates that its inverse effect on Cak1p is specific and not an artifact. The actual activation of cdk2 toward its substrate histone H1 will be a function both of the extent of activating phosphorylation by CAK and of the binding of cyclin. Initially, increasing cyclin A173–432:cdk2 ratios yielded increased activity of the complexes phosphorylated both by Cak1p and by p40MO15 (Figure 3B). However, at higher cyclin concentrations, cdk2 incubated with Cak1p showed reduced activity (Figure 3B, triangles), reflecting the lower level of phosphorylation of cdk2 under these conditions (see Figure 3A).
Figure 3.
Effect of cyclins on phosphorylation of cdks by Cak1p and p40MO15/cyclin H. Phosphorylation (A) and activation (B, measured as the phosphorylation of histone H1 by cdk2) of wild-type cdk2 or phosphorylation of cdk2N132A (E) by GST-Cak1p (triangles) and by p40MO15/cyclin H (squares) were determined in the presence of increasing concentrations of cyclin A173–432. Phosphorylation of cdk6 expressed in insect cells was analyzed in a similar manner in the presence of various concentrations of cyclin A173–432 (C), which binds cdk6 very weakly, or of His6-cyclin D3 (D). Note that His6-cyclin D3 binds less tightly to cdk6 in vitro (Kato et al., 1994a) than does cyclin A173–432 to cdk2, resulting in less pronounced effects on cdk phosphorylation. Data were analyzed by phosphorimaging and are expressed in arbitrary units. The cyclin:cdk ratios are mass ratios of the indicated proteins.
To address whether cyclin A173–432 affected the CAK enzymes directly or whether these effects were due to its binding to cdk2, we took advantage of the fact that cyclin A173–432 does not bind stably to cdk6 (Russo and Pavletich, unpublished results) and cannot activate cdk6 (our unpublished results). Cak1p phosphorylated cdk6 equally well in the presence and in the absence of cyclin A (Figure 3C, triangles), thus excluding a direct effect of the cyclin on Cak1p. Surprisingly, phosphorylation of cdk6 by p40MO15 increased more than 44-fold in the presence of cyclin A173–432 (Figure 3C, squares). Although we do not completely understand this effect, we believe that transient binding of cyclin A173–432 to cdk6 induces a conformation of cdk6 that favors phosphorylation by p40MO15 (see DISCUSSION). However, the lack of stable binding of cyclin A173–432 to cdk6 precludes it from activating cdk6 (our unpublished results) or from blocking cdk6 phosphorylation by Cak1p. The same general effects on phosphorylation by Cak1p and p40MO15 were observed for cyclin D3 (Figure 3D) as for cyclin A173–432 (Figure 3A), although they were less pronounced due to the low binding affinity of D-type cyclins to cdk6 in vitro (Kato et al., 1994a).
We further explored the relative cyclin-insensitivity of cdk2N132A phosphorylation observed in Figure 2. In principle, this effect could be due to reduced binding of cyclin A173–432 or to an altered conformation of cdk2N132A reflecting its inability to bind ATP. In a cyclin titration experiment, the effect of cyclin A173–432 on the phosphorylation of cdk2N132A by Cak1p (Figure 3E, triangles) was similar to its effect on phosphorylation of wild-type cdk2 (Figure 3A). In contrast, monomeric cdk2N132A was a surprisingly good substrate for p40MO15 (Figure 3E, squares). The presence of modest amounts of cyclin A173–432 increased phosphorylation of cdk2N132A by p40MO15 twofold. As with wild-type cdk2, most of the effects of cyclin A173–432 were manifested by a 1:1 ratio, indicating that its binding to cdk2N132A was approximately normal. We infer that a conformational alteration of cdk2N132A renders its phosphorylation less sensitive to the presence of cyclin A173–432.
Phosphorylation and Activation of cdk6
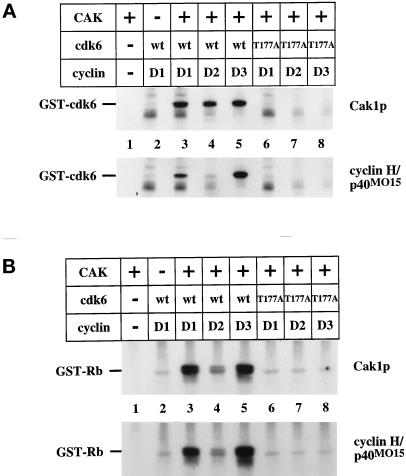
We used a bacterially produced GST-cdk6 protein to examine the activation of cdk6 by the two CAK enzymes. We chose this reagent because it is not already phosphorylated on Thr-177 (the site of activating phosphorylation in cdk6) and because a previous study showed that it could be phosphorylated by p40MO15 (Aprelikova et al., 1995). Wild-type GST-cdk6, but not a T177A mutant, was phosphorylated by both Cak1p and p40MO15 (Figure 4A, compare lanes 3–5 to 6–8). Each enzyme could phosphorylate wild-type cdk6 in the presence of cyclin D1, D2, and D3 (Figure 4A, lanes 3–5). Phosphorylation by either CAK stimulated the Rb kinase activity of wild-type GST-cdk6 in the presence of cyclin D1, D2, or D3 (Figure 4B, lanes 3–5). The T177A mutant of cdk6 could not be activated by either CAK (Figure 4B, compare lanes 6–8 to 3–5). We noticed that phosphorylation of cdk6 (Figure 4A, lanes 4) and the resulting Rb kinase activity (Figure 4B, lanes 4) were consistently lower in the presence of cyclin D2 compared with cyclin D1 and D3 (Figure 4B, compare lane 4 to lanes 3 and 5). We do not know whether this difference in activity is due to a property of D2-type cyclins in general or to a lower quality of this particular reagent (see DISCUSSION).
Figure 4.
Cdk6 phosphorylation and activation by CAK. (A) Wild-type and mutant forms of GST-cdk6 expressed in bacteria were incubated in the presence of radiolabeled ATP with GST-Cak1p (upper panel) or p40MO15/cyclin H (lower panel). T177A contains a mutation of the activating phosphorylation site (lanes 6, 7, and 8). Purified His6-cyclin D1, D2, or D3 was present in the indicated lanes. (B) Activation of cdk6 by CAKs. Wild-type and mutant forms of GST-cdk6 expressed in bacteria were incubated with the indicated D-type cyclins and GST-Cak1p (upper panel) or p40MO15/cyclin H (lower panel) in the presence of unlabeled ATP. The reaction was then incubated with GST-Rb605–928 as a substrate in the presence of radiolabeled ATP. Samples were analyzed by 10% SDS-PAGE and autoradiography. His6-cyclin D1, D2, and D3 proteins were expressed in insect cells and purified via their His6-tags, whereas GST-cdk6 was expressed in E. coli.
D-Type Cyclin Complexes as Substrates for CAKs
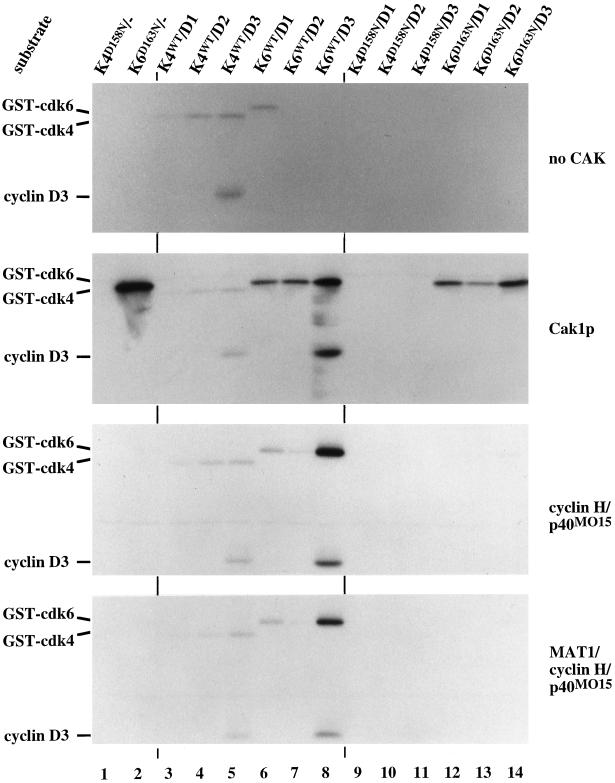
We investigated the phosphorylation of cdk4 and cdk6 bound to D-type cyclins in a more systematic manner, looking first at phosphorylation by Cak1p. GST-cdk4 and GST-cdk6 were purified from insect cells infected with viruses for these proteins alone or in the presence of excess virus encoding a D-type cyclin. The latter enzymes will be referred to as cdk/cyclin D complexes even though some free cdk is likely to be present as well. We observed that all cdk4 complexes as well as cdk6/cyclin D1 complexes autophosphorylated to a significant extent (Figure 5 top panel, lanes 3–6) and displayed considerable Rb kinase activity even without incubation with CAK (our unpublished results), presumably due to activating phosphorylation in vivo. We do not know whether this autophosphorylation occurs in cis or trans and which sites are phosphorylated. Cak1p phosphorylated monomeric GST-cdk6D163N, wild-type GST-cdk6 complexes, and catalytically inactive cdk6D163N complexes (K6D163N, Figure 5, second panel from top, compare lanes 6–8 and 12–14). Complex formation with the D-type cyclins significantly reduced phosphorylation of cdk6 by Cak1p (Figure 5, second panel from top, compare lane 2 to lanes 6–8 and 12–14; see also Figure 3D).
Figure 5.
Phosphorylation of cdk4 and cdk6-cyclin D complexes by CAK. Monomeric and cyclin-bound GST-cdk4 and GST-cdk6 complexes were purified from baculovirus-infected insect cells and used as substrates for autophosphorylation (upper panel) or for direct phosphorylation by GST-Cak1p (second panel), p40MO15/cyclin H (third panel), and p40MO15/cyclin H/MAT1 (bottom panel) in the presence of radiolabeled ATP. Samples were analyzed by 10% SDS-PAGE and autoradiography. Lanes 3–8 contained wild-type cdks whereas lanes 1, 2, and 9–14 contained catalytically inactive enzymes. The positions of the phosphorylated cdks and of cyclin D3 are indicated at the left. Note that the GST-cdk4/cyclin D complexes undergo significant autophosphorylation (top panel, lanes 3–5) and display Rb kinase activity (our unpublished results).
In contrast, neither p40MO15/cyclin H nor p40MO15/cyclin H/MAT1 could phosphorylate monomeric cdk6 and only efficiently phosphorylated wild-type cdk6 bound to cyclin D3 (Figure 5, bottom two panels, lanes 8). Neither form of p40MO15 phosphorylated the catalytically inactive mutant of cdk6, suggesting that the wild-type conformation of cdk6 contributes to phosphorylation by p40MO15, but not to phosphorylation by Cak1p. We do not believe this effect results from activation of p40MO15 by wild-type cdk6 in a feedback process. First, there was much less phosphorylation of p40MO15 following incubation with cdk6 (our unpublished results; and see faint band between cyclin D3 and GST-cdk4 label in Figure 5) than by cdk2/cyclin A complexes (see Figure 2, lower panel, lane 4), which are known to phosphorylate and activate p40MO15/cyclin H complexes in vitro (Fisher et al., 1995). Preincubation of p40MO15/cyclin H with cyclin B/cdc2 had no effect on the subsequent phosphorylation of cdk6D163N by p40MO15 (our unpublished results). Second, MAT1 is known to fully activate even unphosphorylated p40MO15/cyclin H complexes (Fisher et al., 1995), yet this ternary complex failed to phosphorylate the catalytically inactive form of cdk6D163N (Figure 5, bottom panel, lanes 12–14). These results also indicate that MAT1 does not contribute to the substrate specificity of p40MO15 toward these cdk/cyclin complexes under our experimental conditions. We obtained identical results using p40MO15 immunoprecipitated from HeLa cell extracts (our unpublished results).
None of our purified CAK preparations could phosphorylate any form of cdk4 we tested (Figure 5 and our unpublished results), although p40MO15 immunoprecipitated from HeLa cell extracts phosphorylated purified cdk4N158D/cyclin D2 (our unpublished results). In addition, coinfection of insect cells with GST-cdk4/cyclin D viruses and either p40MO15/cyclin H or Cak1p viruses did not elevate the Rb kinase activity of the purified cdk4/cyclin D complexes (our unpublished results).
Cdk Inhibitors Prevent cdk Phosphorylation by p40MO15 but Not by Cak1p
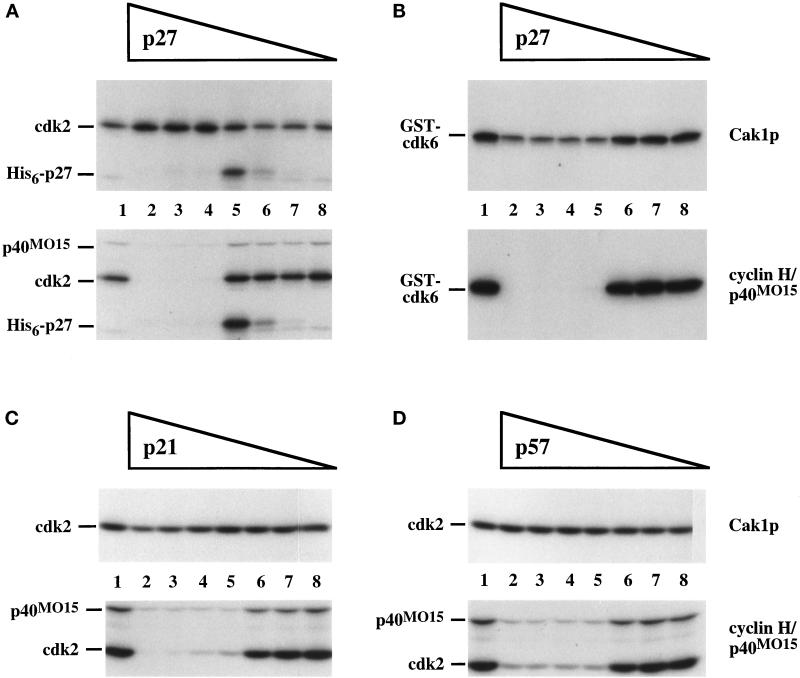
Since cyclins had dramatic effects on cdk phosphorylation by CAKs (see Figure 3), we sought to investigate the influence of other cdk-binding proteins. The p21Cip1/Waf1/p27Kip1 inhibitors have been reported to inhibit cdk2 phosphorylation by p40MO15 but not to decrease p40MO15 activity per se (Kato et al., 1994b; Polyak et al., 1994a; Aprelikova et al., 1995). We investigated the effects of the p21, p27, and p57 members of the CIP/KIP family on cdk phosphorylation by Cak1p and by p40MO15 (Figure 6). To compare the two CAK enzymes under identical conditions, we chose an intermediate cyclin:cdk ratio (0.75:1) at which phosphorylation by each enzyme was efficient (see Figure 3). The inhibitors were preincubated with cdk and cyclin before phosphorylation by Cak1p or p40MO15 in the presence of radiolabeled ATP. None of the inhibitors prevented the phosphorylation of cdk2 or cdk6 by Cak1p (Figure 6A–D, top panels), although p27 caused a minor decrease in the phosphorylation of cdk6 (Figure 6B, top panel, lanes 2–4). In contrast, phosphorylation by p40MO15 was abolished by all three inhibitors (Figure 6A–D, bottom panels). Even in the absence of cyclin, cdk phosphorylation by Cak1p was unaffected by p27 (our unpublished results). p27 also failed to further inhibit phosphorylation by Cak1p of cdk2/cyclin A complexes prepared using a 10-fold excess of cyclin (our unpublished results). Thus, p27 does not block the phosphorylation by Cak1p of either monomeric or cyclin-bound cdk2. In fact, excess p27 slightly diminished the inhibition of cdk2 phosphorylation caused by a large excess of cyclin (our unpublished results), similar to the modest stimulation of cdk2 phosphorylation by Cak1p in the presence of p27 seen in Figure 6A (top panel, lanes 2–4). In all cases, however, the cdk/cyclin complexes phosphorylated in the presence of excess p27 remained inactive (our unpublished results). Intermediate p27 concentrations led to the phosphorylation of p27 by the active subset of cdk2/cyclin A complexes (Figure 6A, lanes 5), presumably on a site known to lead to p27 degradation in vivo (Sheaff et al., 1997; Vlach et al., 1997). The inhibition of phosphorylation by p40MO15 appears to require the binding of p27 to cdk2. p27 forms a hydrogen bond to Asn-132 in cdk2 (Russo et al., 1996a). Phosphorylation by p40MO15 of a cdk2N132A mutant bound to cyclin A173–432 is insensitive to p27 (our unpublished results), presumably because this form of cdk2 no longer binds to p27.
Figure 6.
Cdk inhibitors from the CIP/KIP family block phosphorylation of cdks by p40MO15 but not by Cak1p. Cdk2 (A, C, and D) or GST-cdk6 (B) was phosphorylated directly by GST-Cak1p (upper panels) or by p40MO15/cyclin H (lower panels) in the presence of various concentrations of His6-p27 (A and B), p21 (C), or p57 (D), respectively. Lanes 1 contained no inhibitors. Mass ratios of inhibitor:cdk are 10:1 (lanes 2), 5:1 (lanes 3), 2.5:1 (lanes 4), 1.2:1 (lanes 5), 0.3:1 (lanes 6), 0.09:1 (lanes 7), and 0.03:1 (lanes 8). In all experiments the cdk:cyclin A173–432 ratio was chosen to be 1:0.75. In panel A, the position of His6-p27 that has been phosphorylated by cdk2/cyclin A173–432 is indicated.
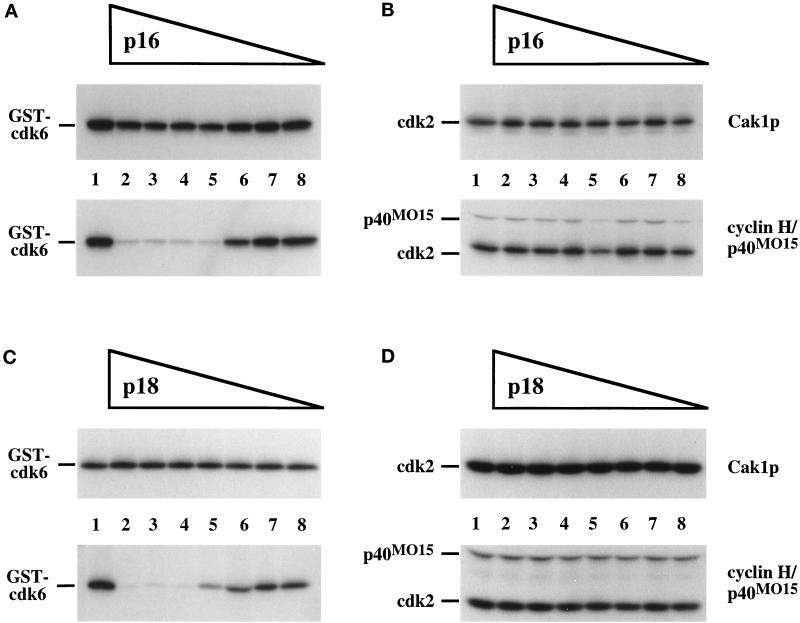
We also tested whether cdk inhibitors of the INK family could block phosphorylation of cdks by the CAKs. Unlike the CIP/KIP family proteins, these CKIs bind predominately to the cdk subunit and are specific for cdk4 and cdk6 (Serrano et al., 1993; Guan et al., 1994; Hannon and Beach, 1994; Chan et al., 1995; Hirai et al., 1995). p18 has been shown previously to inhibit phosphorylation by p40MO15 of cdk6/cyclin D1 but not of cdk2/cyclin A (Aprelikova et al., 1995). We incubated various concentrations of p16 or p18 with cdk6 or cdk2 before phosphorylation by either Cak1p or p40MO15 (Figure 7). Phosphorylation by Cak1p was not affected by the presence of either inhibitor (Figure 7, A–D, top panels). p16 also had no effect on the phosphorylation of cdk6 by Cak1p in the absence of cyclin (our unpublished results). In contrast, phosphorylation of cdk6 by p40MO15 was severely decreased by both p16 and p18 (Figure 7, A and C, bottom panels). p16 and p18 had no effect on the phosphorylation of cdk2 by p40MO15 (Figure 7, B and D, bottom panels), reflecting the lack of binding of these proteins to cdk2 (Serrano et al., 1993; Guan et al., 1994; Hannon and Beach, 1994; Chan et al., 1995; Hirai et al., 1995). Thus, all of the tested cdk inhibitors blocked phosphorylation of cdks by p40MO15 but not by Cak1p.
Figure 7.
Inhibitors of the INK4 family prevent phosphorylation of cdk6 by p40MO15 but not by Cak1p. GST-cdk6 (A and C) or cdk2 (B and D) was phosphorylated directly by GST-Cak1p (upper panels) or by p40MO15/cyclin H (lower panels) in the presence of various concentrations of p16 (A and B) or GST-p18 (C and D). Lanes 1 contained no inhibitors. Mass ratios of cdk:inhibitor are as in Figure 6. In all experiments the cdk:cyclin A173–432 ratio was chosen to be 1:0.75.
DISCUSSION
In this study, we have compared the phosphorylation of cdks by budding yeast Cak1p and by human p40MO15 using an in vitro system with highly purified proteins. Primary sequence analysis had previously revealed that Cak1p and p40MO15 are only distantly related (Espinoza et al., 1996; Kaldis et al., 1996; Thuret et al., 1996). In addition, Cak1p is active as a monomeric enzyme (Kaldis et al., 1996), whereas p40MO15 is found in a complex with cyclin H, MAT1, or even with transcription factor IIH (TFIIH) (see Morgan, 1995). The identification and initial characterization of Cak1p suggested that it had properties distinct from p40MO15. For example, Cak1p was able to phosphorylate monomeric cdk2 and Cdc28p (Espinoza et al., 1996; Kaldis et al., 1996; Thuret et al., 1996), whereas p40MO15 required the presence of a cyclin (Solomon et al., 1993; Fisher and Morgan, 1994). We have now performed a systematic comparison of the substrate specificities of these two CAK enzymes and found that Cak1p preferentially phosphorylates monomeric cdks, whereas p40MO15 prefers cyclin-bound cdks as substrates. Surprisingly, cdk inhibitors prevent phosphorylation of cdks by p40MO15 but not by Cak1p. These results indicate that Cak1p and p40MO15 recognize the same residue in cdks via very different mechanisms. Furthermore, the presence of MAT1 had no effect on the substrate specificity of p40MO15 toward cdk/cyclin complexes, although previous studies have indicated that MAT1 changes the relative substrate specificity of p40MO15 toward the CTD and monomeric GST-cdk2 (Rossignol et al., 1997; Yankulov and Bentley, 1997). These prior studies did not examine the effect of MAT1 on phosphorylation of cyclin-bound cdk2 (a much better substrate for p40MO15).
Although our studies utilized exclusively human proteins as CAK substrates, we believe that the findings are quite general. Since we were interested in identifying differences between Cak1p and p40MO15, it was essential to compare their activities toward common substrates. Human cdk2 is a functional homolog of Cdc28p, demonstrated by the ability of cdk2 to rescue a temperature-sensitive CDC28 mutation in budding yeast (Elledge and Spottswood, 1991; Ninomiya-Tsuji et al., 1991), indicating that cdk2 can be a physiological substrate for Cak1p. Moreover, a diverse array of well characterized human proteins was available for this work. Our previous studies have shown that phosphorylation of the yeast cdk, Cdc28p, by Cak1p is decreased in the presence of a yeast cyclin, Clb2p (Kaldis et al., 1996). Consistent with the current studies we have also found that phosphorylation of cdk2/cyclin A173–432 by p40MO15, but not by Cak1p, is inhibited by a yeast CKI, Sic1p (our unpublished results). These results demonstrate that the major substrate differences we observed here represent general properties of the respective enzymes regardless of the particular substrates employed. In addition, we have recently identified a novel human CAK activity that shares several features with Cak1p (Kaldis and Solomon, unpublished). This partially purified activity elutes at ∼30 kDa from gel filtration, lacks CTD kinase activity, is recognized by antibodies against budding yeast Cak1p but not by antibodies against p40MO15, is insensitive to FSBA, and efficiently phosphorylates monomeric cdks. We predict that this human Cak1p-like CAK will share the properties of Cak1p we have characterized here and that these studies will contribute to our understanding of two very different CAK activities within human cells.
Cak1p Phosphorylates cdks Efficiently in the Absence of Cyclin
We have found that the presence of cyclins has opposite effects on the phosphorylation of cdks by Cak1p and p40MO15. Phosphorylation of both cdk2 and cdk6 by Cak1p was strongly repressed by the presence of cyclin A and cyclin D3, respectively, whereas phosphorylation of these same cdks by p40MO15 was greatly elevated by the presence of cyclins. As a control, cyclin A had little effect on the phosphorylation of cdk6 by Cak1p, reflecting the weak interaction between cyclin A and cdk6. Most likely, cyclin A influences CAK phosphorylation of cdks by binding to the cdks and thereby remodeling the CAK substrate (see below). These results suggest that activation of cdks by Cak1p in vivo may proceed by a pathway distinct from that employed by p40MO15. Monomeric cdks might first become phosphorylated by Cak1p and only subsequently bind to cyclins to yield the active protein kinase. Whether this pathway is actually utilized will depend on a number of parameters, including the binding kinetics and affinity of the cyclin for the unphosphorylated cdk, and the activities of Cak1p and the opposing phosphatase toward the monomeric and cyclin-bound cdks. Perhaps both pathways operate in vivo, one carried out by p40MO15 and the other by Cak1p or by a vertebrate Cak1p-like CAK. We are currently testing this possibility by investigating the activation pathway of yeast Cdc28p in vivo.
Activation of cdk4 and cdk6 by CAK
We extended our investigation of the substrate specificities of CAKs to complexes of cdk4 and cdk6 with D-type cyclins formed either in vitro with purified proteins or in vivo (coinfected in insect cells). Up until now there have been no systematic studies of these CAK substrates. Both Cak1p and p40MO15 specifically phosphorylated cdk6 in the absence of mutation at threonine 177 (Figure 4A) and could activate the Rb kinase activity of cdk6/cyclin D complexes formed in vitro. Cak1p phosphorylated monomeric cdk6 and all cdk6/cyclin D complexes formed in vivo, whereas p40MO15 only phosphorylated cdk6/cyclin D3 complexes (Figure 5), raising the possibility of distinct CAKs for different cdk complexes. Interestingly, cdk6/cyclin D2 complexes assembled in vitro were consistently less active after phosphorylation by CAK than the corresponding cyclin D1 or D3 complexes. Several explanations are possible for this difference: 1) cyclin D2 protein may be less active because of misfolding or aggregation (Blain et al., 1997); 2) the binding affinity of cyclin D2 toward cdk6 may be lower than that of cyclins D1 and D3; or 3) Rb may not be an optimal substrate for cdk6/cyclin D2 complexes, implying some specialization of the cyclin D complexes. Further experiments will be needed to resolve this issue.
We have never observed phosphorylation of cdk4 using purified proteins. These reagents include both Cak1p and p40MO15 (both of which we know to be active toward other substrates) and cdk4 expressed from multiple constructs both in bacteria and in insect cells. On the other hand, we have found that p40MO15 immunoprecipitated from HeLa cell extracts was able to phosphorylate cdk4/cyclin D2 from insect cells (our unpublished results). In reviewing the literature, it appears that phosphorylation of cdk4 has been observed in cell extracts (the most common situation) or when either cdk4 or p40MO15 has been immunoprecipitated (Kato et al., 1994a; Matsuoka et al., 1994; Blain et al., 1997; Diehl and Sherr, 1997; Phelps and Xiong, 1997). We conclude that phosphorylation of cdk4 is strongly dependent on the conditions of the assay. Perhaps highly purified proteins have lost cofactors essential for efficient phosphorylation of cdk4. These cofactors would be present in cell extracts and possibly also in immunoprecipitates.
Phosphorylation of bacterially produced cdk6 by CAK in the presence of cyclin D greatly stimulates its Rb kinase activity (Aprelikova et al., 1995 and this study). In contrast, Cak1p stimulated the Rb kinase activity of in vivo formed cdk6/cyclin D2 complexes purified from insect cells by only 4- to 8-fold (our unpublished results). This is a range of activation similar to that observed by others (Blain et al., 1997), but much less than is observed for cdk2 complexes (Connell-Crowley et al., 1993; see also Figure 1A). Insect cell-produced cdk/cyclin complexes display considerable activity before incubation with CAK in vitro, probably due to activating phosphorylation in vivo. The basal Rb kinase activity of similarly produced cdk4/cyclin D1, D2, or D3 and cdk6/cyclin D1 complexes could not be increased by incubation with CAK in vitro (our unpublished results).
Effects of cdk Inhibitors on Phosphorylation by CAKs
The ability of CAKs to phosphorylate cdks depends not only on the presence of cyclin but also of cdk inhibitors. We have shown that the p21, p27, p57, p16, and p18 CKIs inhibit phosphorylation of cdks by p40MO15 (Figures 6 and 7) but do not prevent phosphorylation by Cak1p. None of the CKIs actually inhibited p40MO15 per se (Aprelikova et al., 1995; and see Figure 7, B and D), suggesting that the CKIs hinder cdk-p40MO15 interactions. Different classes of CKIs appear to inhibit p40MO15 differently. A crystal structure of the p27/cdk2/cyclin A complex indicates that p27 binding would sterically interfere with recognition of the T-loop of the cdk2/cyclin A complex. On the other hand, p16 binds to cdks far from the T-loop, and presumably exerts its effects through indirect conformational alterations in T-loop residues. While CKIs can both block cdk phosphorylation by p40MO15 and directly inhibit the phosphorylated cdks, there is no such “double block” with respect to Cak1p. The in vivo relevance of such a double block for p40MO15 is unclear, however.
Cak1p and p40MO15 Recognize Substrates Differently
Cak1p and p40MO15 recognize their substrates, cdks and ATP, very differently. The effect of cyclin binding to cdks on phosphorylation by the CAKs suggests that each CAK binds to a different orientation of the T-loop, on which the site of activating phosphorylation resides. Based on the crystal structure of monomeric cdk2, the Thr-160 on the T-loop is less accessible (“closed” conformation), blocking substrates from the site of catalysis (De Bondt et al., 1993). This closed conformation appears to be preferred by Cak1p. In contrast, binding of cyclin A induces large motions of the entire T-loop, exposing Thr-160 and permitting substrate access (Jeffery et al., 1995). This “open” conformation seems to be favored by p40MO15. Interestingly, similar activating sites in a MAP kinase are phosphorylated by MEK while in a distinct closed position (Canagarajah et al., 1997), resulting in a very similar open conformation. Thus, there are multiple solutions as to how to achieve activating phosphorylation of protein kinases in which the final conformations of the activated T-loops are similar. An intriguing way in which Cak1p might gain access to Thr-160 in the closed conformation would be by inducing movement of the T-loop into an open or partially open conformation. Perhaps Cak1p and cyclin bind to overlapping sites on cdks, thereby inducing similar conformational changes. Overlapping binding sites would also explain the decreased phosphorylation by Cak1p caused by cyclin binding to the cdk. p40MO15 might not be able to move the T-loop and would therefore only efficiently phosphorylate the cyclin-bound cdks. Alternatively, p40MO15 might bind directly to cyclin A and use it as a docking site to gain access to the activating threonine. Indeed, we have detected complexes of p40MO15 with cyclin A or cdk2/cyclin A but not with monomeric cdk2 by using gel filtration chromatography. We have not observed similar stable binding of monomeric cdk2 to Cak1p under our experimental conditions (our unpublished results).
The differential effect of the cdk inhibitors on phosphorylation by Cak1p and by p40MO15 also indicates that these enzymes approach the site of activating phosphorylation on the T-loop differently. Binding of p27 does not induce a conformational change in the T-loop of cdk2 (Russo et al., 1996a), suggesting that p27 sterically interferes with phosphorylation by p40MO15 rather than causing a repositioning of the Thr-160 on the T-loop to create a poor p40MO15 substrate. Cak1p may approach the T-loop in a way that avoids the bound CKI. We presume that the other cdk inhibitors act similarly. Even though the T-loop is located in a groove between the N-terminal and C-terminal lobes of cdk2 (De Bondt et al., 1993), it appears that there are at least two different ways to gain access to the activating threonine.
Some cdk2 mutations had interesting effects on the phosphorylation of cdk2 by CAKs. The cdk2N132A mutant prevents ATP binding. The effect of the cyclin A173–432 titration on phosphorylation of this mutant (Figure 3E) indicated that binding of cyclin A173–432 was roughly normal, which is consistent with a report that a similar non ATP-binding mutant bound cyclin normally (van den Heuvel and Harlow, 1993). Cdk2N132A is an excellent substrate for p40MO15 even in the absence of cyclin, indicating that the mutation induces a conformational change in cdk2 that makes it a better substrate. Crystal structures of various forms of cdk2 indicate that cyclin binding has little effect on Asn-132 (which interacts with ATP) and that mutation of this residue might not affect the T-loop significantly. The effects of this mutation on the phosphorylation of cdk2 are therefore most likely due to the inability of cdk2N132A to bind ATP. The T-loop may be more sensitive to the presence of cyclin when ATP is bound. Interestingly, the D163N mutation in cdk6 abolished phosphorylation by p40MO15 but had no effect on phosphorylation by Cak1p. We speculate that since the D163N mutation in cdk6 is closer to the activating threonine (Thr-177) than is Asn-132 in cdk2, it could affect the structure of the T-loop more profoundly.
p40MO15 is a typical protein kinase in its mode of ATP binding whereas Cak1p is quite unusual. FSBA covalently modifies an invariant lysine in protein kinases (equivalent to Lys-41 in p40MO15) and thereby inactivates all tested ATPases and protein kinases of which we are aware. This lysine, together with specific glutamic acid and asparagine residues, is involved in the proper positioning of the ATP phosphates (Zheng et al., 1993; Jeffery et al., 1995). p40MO15 is completely inhibited by FSBA (Figure 1C and Solomon et al., 1993), whereas Cak1p is unaffected (Figure 1C). Furthermore, Cak1p shows only minor sensitivity to mutation of Lys-31 [equivalent of Lys-41 in human p40MO15 (Thuret et al., 1996; Chun and Goebl, 1997; Enke, Kaldis, and Solomon, unpublished results) and lacks a canonical glycine-rich loop (GxGxxG motif) that is also involved in positioning the phosphates of ATP (Hemmer et al., 1997). These properties suggest that Cak1p binds ATP quite differently from other protein kinases and raise the possibility of identifying drug inhibitors that are specific for Cak1p-like CAKs. A detailed study of the ATP-binding properties of Cak1p will be published elsewhere (Enke, Holmes, Kaldis, and Solomon).
Conclusion
We have shown that Cak1p and p40MO15 have strikingly different substrate requirements with respect to ATP binding, cyclins, and cdk inhibitors. These differences predict distinct cdk activation pathways involving these CAKs. An important unsolved question is whether both Cak1p and p40MO15 actually function as physiological CAKs in vivo. Two groups have demonstrated that Cak1p is the physiological CAK of budding yeast (Kaldis et al., 1996; Thuret et al., 1996). Our study significantly extends the list of differences between Cak1p and p40MO15. Furthermore, many non-cdk substrates of p40MO15 have been identified, including the CTD, components of the transcription machinery, and p53. The phosphorylation sites of these substrates share no homology to the consensus cdk phosphorylation sites, are not located in T-loop contexts, and share little resemblance to each other. These results indicate that p40MO15 has a relaxed substrate specificity and suggest that it might simply be fortuitous that p40MO15 can also phosphorylate cdk/cyclin complexes. Two recent reports suggest physiological roles for p40MO15 as CAK in Xenopus egg extracts and in Drosophila embryos (Fesquet et al., 1997; Larochelle et al., 1998). Clearly, further studies will be needed to elucidate the in vivo function(s) of p40MO15. We expect that the properties of Cak1p will be shared by our partially purified human Cak1p-like CAK and that our results will thereby affect the understanding of mammalian cdk activation by both classes of CAK.
ACKNOWLEDGMENTS
This study is the result of a fruitful collaboration that started without intention and could not have been completed without the efforts of all authors. We thank Ray Deshaies, Rob Fisher, Nick Grammatikakis, Wade Harper, David Morgan, Rati Verma, and Yue Xiong for providing essential reagents. We gratefully acknowledge the support of Ed Harlow in whose laboratory part of the work was done. We are also grateful to Ken Allen and Carolyn Slayman (Yale University, Department of Genetics) for sharing equipment and for help with the French press and to Beth Egan, Debbie Enke, Jennifer Holmes, Zach Pitluk, and the Solomon laboratory for sharing reagents, discussion, and support. We thank Janet Burton, Barry Elkind, Debbie Enke, Karen Ross, and David Stern for comments on the manuscript. This work was supported by a long-term fellowship from the Swiss National Science Foundation (P.K.), The Patrick and Catherine Weldon Donaghue Medical Research Foundation (P.K.), the National Institutes of Health (grant GM-47830 to M.J.S.), and by the Searle Scholars Program/The Chicago Community Trust (M.J.S.). H.S.C. is a recipient of a Physician Postdoctoral Fellowship Award from the Howard Hughes Medical Institute. M.J.S. is a Leukemia Society of America Scholar.
REFERENCES
- Adamczewski JP, Rossignol M, Tassan J-P, Nigg EA, Moncollin V, Egly J-M. MAT1, cdk7 and cyclin H form a kinase complex which is UV light-sensitive upon association with TFIIH. EMBO J. 1996;15:1877–1884. [PMC free article] [PubMed] [Google Scholar]
- Aprelikova O, Xiong Y, Liu ET. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995;270:18195–18197. doi: 10.1074/jbc.270.31.18195. [DOI] [PubMed] [Google Scholar]
- Blain SW, Montalvo E, Massagué J. Differential interaction of the cyclin-dependent kinase (cdk) inhibitor p27Kip1 with cyclin A-cdk2 and cyclin D2-cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Chan FKM, Zhang J, Chen L, Shapiro DN, Winoto A. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol Cell Biol. 1995;15:2682–2688. doi: 10.1128/mcb.15.5.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 1996a;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I-T, Akamatsu M, Smith ML, Lung FDT, Duba D, Roller PP, Fornace AJ, O’Connor PM. Characterization of p21Cip1/Waf1 peptide domains required for cyclin E/Cdk2 and PCNA interaction. Oncogene. 1996b;12:595–607. [PubMed] [Google Scholar]
- Chun KT, Goebl MG. Mutational analysis of Cak1p, an essential protein kinase that regulates cell cycle progression. Mol Gen Genet. 1997;256:365–375. doi: 10.1007/s004380050580. [DOI] [PubMed] [Google Scholar]
- Cismowski MJ, Laff GM, Solomon MJ, Reed SI. KIN28 encodes a C-terminal domain kinase that controls mRNA transcription in Saccharomyces cerevisiae but lacks cyclin-dependent kinase-activating kinase (CAK) activity. Mol Cell Biol. 1995;15:2983–2992. doi: 10.1128/mcb.15.6.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell-Crowley L, Solomon MJ, Wei N, Harper JW. Phosphorylation independent activation of human cyclin-dependent kinase 2 by cyclin A in vitro. Mol Biol Cell. 1993;4:79–92. doi: 10.1091/mbc.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bondt HL, Rosenblatt J, Jancarik J, Jones HD, Morgan DO, Kim S-H. Crystal structure of cyclin-dependent kinase 2. Nature. 1993;363:595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- Devault A, Martinez A-M, Fesquet D, Labbé J-C, Morin N, Tassan J-P, Nigg EA, Cavadore J-C, Dorée M. MAT1 (’menage à trois’) a new RING finger protein subunit stabilizing cyclin H-cdk7 complexes in starfish and Xenopus CAK. EMBO J. 1995;14:5027–5036. doi: 10.1002/j.1460-2075.1995.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Sherr CJ. A dominant-negative cyclin D1 mutant prevents nuclear import of cyclin-dependent kinase 4 (CDK4) and its phosphorylation by CDK-activating kinase. Mol Cell Biol. 1997;17:7362–7374. doi: 10.1128/mcb.17.12.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer E, Kinzler KW, Vogelstein B. WAF1, a potent mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Elledge SJ, Spottswood MR. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991;10:2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza FH, Farrell A, Erdjument-Bromage H, Tempst P, Morgan DO. A cyclin-dependent kinase-activating kinase (CAK) in budding yeast unrelated to vertebrate CAK. Science. 1996;273:1714–1717. doi: 10.1126/science.273.5282.1714. [DOI] [PubMed] [Google Scholar]
- Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Feaver WJ, Svejstrup JQ, Henry NL, Kornberg RD. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994;79:1103–1109. doi: 10.1016/0092-8674(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Fesquet D, Labbé J-C, Derancourt J, Capony J-P, Galas S, Girard F, Lorca T, Shuttleworth J, Dorée M, Cavadore J-C. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 1993;12:3111–3121. doi: 10.1002/j.1460-2075.1993.tb05980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesquet D, Morin N, Dorée M, Devault A. Is cdk7/cyclin H/MAT1 the genuine cdk activating kinase in cycling Xenopus egg extracts? Oncogene. 1997;15:1303–1307. doi: 10.1038/sj.onc.1201300. [DOI] [PubMed] [Google Scholar]
- Fisher RP, Morgan DO. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994;78:713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Fisher RP, Jin P, Chamberlin HM, Morgan DO. Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell. 1995;83:47–57. doi: 10.1016/0092-8674(95)90233-3. [DOI] [PubMed] [Google Scholar]
- Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Guan K, Jenkins CW, Li Y, Nichols MA, Wu X, O’Keefe CL, Matera AG, Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarski K, Elledge SJ. The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hemmer W, McGlone M, Tsigelny I, Taylor SS. Role of the glycine triad in the ATP-binding site of cAMP-dependent protein kinase. J Biol Chem. 1997;272:16946–16954. doi: 10.1074/jbc.272.27.16946. [DOI] [PubMed] [Google Scholar]
- Hirai H, Roussel MF, Kato J-Y, Ashmun RA, Sherr CJ. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavarone A, Massagué J. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-β in cells lacking the CDK inhibitor p15. Nature. 1997;387:417–422. doi: 10.1038/387417a0. [DOI] [PubMed] [Google Scholar]
- Inamoto S, Segil N, Pan Z-Q, Kimura M, Roeder RG. The cyclin-dependent kinase-activating kinase (CAK) assembly factor, MAT1, targets and enhances CAK activity on the POU domains of octamer transcription factors. J Biol Chem. 1997;272:29852–29858. doi: 10.1074/jbc.272.47.29852. [DOI] [PubMed] [Google Scholar]
- Jeffery PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massagué J, Pavletich NP. Mechanism of cdk activation revealed by the structure of a cyclin A-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Noble MEM, Owen DJ. Active and inactive protein kinases: structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Kaldis P, Sutton A, Solomon MJ. The cdk-activating kinase (CAK) from budding yeast. Cell. 1996;86:553–564. doi: 10.1016/s0092-8674(00)80129-4. [DOI] [PubMed] [Google Scholar]
- Kato J-Y, Matsuoka M, Storm DK, Sherr CJ. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol Cell Biol. 1994a;14:2713–2721. doi: 10.1128/mcb.14.4.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J-Y, Matsuoka M, Polyak K, Massagué J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994b;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters J-M, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- Ko LJ, Shieh S-Y, Chen X, Jayaraman L, Tamai K, Taya Y, Prives C, Pan Z-Q. p53 is phosphorylated by CDK7-cyclin H in a p36MAT1-dependent manner. Mol Cell Biol. 1997;17:7220–7229. doi: 10.1128/mcb.17.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J-C, Martinez A-M, Fesquet D, Capony J-P, Darbon J-M, Derancourt J, Devault A, Morin N, Cavadore J-C, Dorée M. p40MO15 associates with a p36 subunit and requires both nuclear translation and Thr176 phosphorylation to generate cdk-activating kinase activity in Xenopus oocytes. EMBO J. 1994;13:5155–5164. doi: 10.1002/j.1460-2075.1994.tb06845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle S, Pandur J, Fisher RP, Salz HK, Suter B. Cdk7 is essential for mitosis and for in vivo cdk-activating kinase activity. Genes Dev. 1998;12:370–381. doi: 10.1101/gad.12.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Reynisdottir I, Massagué J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Lin J, Reichner C, Wu X, Levine AJ. Analysis of wild-type and mutant p21WAF-1 gene activities. Mol Cell Biol. 1996;16:1786–1793. doi: 10.1128/mcb.16.4.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Fisher RP, Bailey P, Levine AJ. The cdk7-cycH-p36 complex of transcription factor IIH phosphorylates p53, enhancing its sequence-specific DNA binding activity in vitro. Mol Cell Biol. 1997;17:5923–5934. doi: 10.1128/mcb.17.10.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä TP, Tassan J-P, Nigg EA, Frutiger S, Hughes GJ, Weinberg RA. A cyclin associated with the CDK-activating kinase MO15. Nature. 1994;371:254–257. doi: 10.1038/371254a0. [DOI] [PubMed] [Google Scholar]
- Martinez A-M, Afshar M, Martin F, Cavadore J-C, Labbé J-C, Dorée M. Dual phosphorylation of the T-loop in cdk7: its role in controlling cyclin H binding and CAK activity. EMBO J. 1997;16:343–354. doi: 10.1093/emboj/16.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Kato J-Y, Fisher RP, Morgan DO, Sherr CJ. Activation of cyclin-dependent kinase 4 (cdk4) by mouse MO15-associated kinase. Mol Cell Biol. 1994;14:7265–7275. doi: 10.1128/mcb.14.11.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Edwards M, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ. p57KIP2, a structurally distinct member of the p21CIP1 cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Control of the yeast cell cycle by the Cdc28 protein kinase. Curr Opin Cell Biol. 1993;5:166–179. doi: 10.1016/0955-0674(93)90099-c. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Nomoto S, Yasuda H, Reed SI, Matsumoto K. Cloning of a human cDNA encoding a CDC2-related kinase by complementation of a budding yeast cdc28 mutation. Proc Natl Acad Sci USA. 1991;88:9006–9010. doi: 10.1073/pnas.88.20.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps DE, Xiong Y. Assay for activity of mammalian cyclin D-dependent kinases CDK4 and CDK6. Methods Enzymol. 1997;283:194–205. doi: 10.1016/s0076-6879(97)83016-9. [DOI] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995;308:697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Kato J-Y, Solomon MJ, Sherr CJ, Massagué J, Roberts JM, Koff A. p27Kip1, a cyclin-cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994a;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994b;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Poon RYC, Yamashita K, Adamczewski JP, Hunt T, Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993;12:3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon RYC, Yamashita K, Howell M, Ershler MA, Belyavsky A, Hunt T. Cell cycle regulation of the p34cdc2/p33cdk2-activating kinase p40MO15. J Cell Sci. 1994;107:2789–2799. doi: 10.1242/jcs.107.10.2789. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C, Adam S, Rossignol M, Egly J-M, Chambon P. Stimulation of RARα activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell. 1997;90:97–107. doi: 10.1016/s0092-8674(00)80317-7. [DOI] [PubMed] [Google Scholar]
- Rossignol M, Kolb-Cheynel I, Egly J-M. Substrate specificity of the cdk-activating kinase (CAK) is altered upon association with TFIIH. EMBO J. 1997;16:1628–1637. doi: 10.1093/emboj/16.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Adamczewski JP, Seroz T, Vermeulen W, Tassan J-P, Schaeffer L, Nigg EA, Hoeijmakers JHJ, Egly J-M. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell. 1994;79:1093–1101. doi: 10.1016/0092-8674(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Russo AA. Purification and reconstitution of cyclin-dependent kinase 2 in four states of activity. Methods Enzymol. 1997;283:3–12. doi: 10.1016/s0076-6879(97)83003-0. [DOI] [PubMed] [Google Scholar]
- Russo AA, Jeffery PD, Patten AK, Massagué J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996a;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- Russo AA, Jeffrey PD, Pavletich NP. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol. 1996b;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- Serizawa H, Mäkelä TP, Conaway JW, Conaway RC, Weinberg RA, Young RA. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature. 1995;374:280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Shiekhattar R, Mermelstein F, Fisher RP, Drapkin R, Dynlacht B, Wessling HC, Morgan DO, Reinberg D. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature. 1995;374:283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- Solomon MJ, Kaldis P. Regulation of cdks by phosphorylation. In: Pagano M, editor. Results and Problems in Cell Differentiation: Cell cycle control. Vol. 22. Heidelberg: Springer Verlag; 1998. pp. 79–109. [DOI] [PubMed] [Google Scholar]
- Solomon MJ, Glotzer M, Lee TH, Philippe M, Kirschner MW. Cyclin activation of p34cdc2. Cell. 1990;63:1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Solomon MJ, Harper JW, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993;12:3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan J-P, Jaquenoud M, Fry AM, Frutiger S, Hughes GJ, Nigg EA. In vitro assembly of a functional human CDK7-cyclin H complex requires MAT1, a novel 36 kDa RING finger protein. EMBO J. 1995;14:5608–5617. doi: 10.1002/j.1460-2075.1995.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuret J-Y, Valay J-G, Faye G, Mann C. Civ1 (CAK in vivo), a novel Cdk-activating kinase. Cell. 1996;86:565–576. doi: 10.1016/s0092-8674(00)80130-0. [DOI] [PubMed] [Google Scholar]
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankulov KY, Bentley DL. Regulation of CDK7 substrate specificity by MAT1 and TFIIH. EMBO J. 1997;16:1638–1646. doi: 10.1093/emboj/16.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Knighton DR, Ten Eyck LF, Karlsson R, Xuong N-H, Taylor SS, Sowadski JM. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide inhibitor. Biochemistry. 1993;32:2154–2161. doi: 10.1021/bi00060a005. [DOI] [PubMed] [Google Scholar]
- Zoller MJ, Taylor SS. Affinity labeling of the nucleotide binding site of the catalytic subunit of cAMP-dependent protein kinase using p-fluorosulfonyl-[14C]benzoyl 5′-adenosine. J Biol Chem. 1979;254:8363–8368. [PubMed] [Google Scholar]